Abstract
Objective To pilot test a two-session computer-delivered motivational intervention to facilitate adherence among youth with HIV newly prescribed antiretroviral treatment (ART). Methods Youth (N = 76) newly prescribed ART were recruited from 8 sites, and were randomized to the intervention or an active nutrition and physical activity control. Primary outcomes were HIV-1 viral load at baseline, 3 months, and 6 months, and self-reported adherence at 3 and 6 months. Results Satisfaction ratings were high. Effect sizes suggested that the intervention group showed a greater drop than controls in viral load from baseline to 6 months (Cohen’s d = 0.39 at 3 months; d = 0.19 at 6 months), and had greater percent undetectable by 6 months (d = 0.28). Effects sizes were medium to large for 7-day and weekend adherence. Conclusions A brief computer-delivered motivational intervention showed promise for youth starting ART and is ready to be tested in a full-scale clinical trial.
Keywords: adherence, adolescent, eHealth, HIV
Introduction
Adherence to antiretroviral treatment (ART) is a significant predictor of viral suppression and is associated with dramatic reductions in mortality and morbidity for persons with HIV of all ages (Feingold, Rutstein, Meislich, Brown, & Rudy, 2000; Flynn et al., 2004; Rouet et al., 2003). Young people aged 15–24 years represent almost half of new HIV infections, with >5 million youth currently living with HIV (YLH) (UNAIDS, 2008). A potential consequence of suboptimal ART adherence in YLH is a future population of HIV-infected adults who have progressed in immune deficiency, harbor multiple drug-resistant viruses, and suffer from drug- and virus-induced metabolic complications. Conversely, optimal treatment adherence during adolescence decreases the pool of infectious individuals during the risky sexual activity commonly reported among youth living with HIV (Outlaw et al., 2010). The time is now to develop effective adherence interventions for this population not only to improve morbidity and mortality but also to reduce the spread of the disease, as transmission is less likely when ART adherence results in undetectable viral load (Cohen et al., 2011).
Studies show that YLH have the low rates of ART adherence compared with adults (Nachega et al., 2009) or with younger children (Williams et al., 2006). Studies of behaviorally infected YLH suggest adherence patterns that are inadequate to effectively manage the disease (MacDonell, Naar-King, Huszti, & Belzer, 2012; Tanney, Naar-King, Murphy, Parsons, & Janisse, 2010). For example, a prospective study of 120 behaviorally infected YLH reported that only 24% of the original sample had viral suppression after 3 years (Flynn et al., 2004). The most important predictor of viral suppression in this study was self-reported “complete adherence” as defined by no missed clinic visits and no missed medication doses in the 4 days prior to clinic visits.
The newest treatment guidelines (DHHS, 2012) strongly recommend ART for patients with CD4+ T-cell counts below 500 cells/mm3 instead of 350, and recommends consideration of ART for all persons with HIV regardless of CD4. Because young people represent the largest number of new infections (UNAIDS, 2008), YLH are likely to be the largest group of ART initiators. Although a few nonrandomized pilot studies have been published (Dowshen, Kuhns, Johnson, Holoyda, & Garofalo, 2012; Gray, Janicke, Fennell, Driscoll, & Lawrence, 2011; Puccio et al., 2006), there are strikingly few randomized controlled trials (RCTs) targeting medication adherence in YLH. Two pilot studies tested intensive home-based family treatment with primarily perinatally infected younger adolescents (Berrien, Salazar, Reynolds, & Mckay, 2004; Letourneau et al., 2012), but behaviorally infected youth, particularly young adults, may not be living in traditional family structures. Furthermore, the transportability of such intensive interventions into real-world settings is a concern (Glasgow & Emmons, 2007). Only one randomized adherence intervention trial to date included mostly behaviorally infected youth. In a multisite trial, Naar-King et al. (2006, 2009) tested Healthy Choices, a four-session clinic-based Motivational Interviewing (MI) (Miller & Rollnick, 2012) intervention targeting adherence, substance use, and sexual risk. Motivational interviewing is a collaborative goal-oriented counseling style designed to strengthen personal motivation and commitment to change. Youth (aged 16–24 years; 85% behaviorally infected) randomized to Healthy Choices had significantly improved viral load at 6-month follow-up compared to standard care controls, but effects were not maintained at 9-month follow-up. Furthermore, only 50% of youth received all four sessions. Thus, although MI has shown promise and is already embedded in the clinical guidelines for HIV care (Bartlett, Cheever, Johnson, & Paauw, 2004; New York State Department of Health, 2009), existing studies of YLH have not been able to identify an intervention that would be easily transportable to real-world settings.
Computer-based brief interventions cannot replicate the human elements of traditional interventions. However, computers offer tremendous advantages in terms of transportability, replicability, anonymity, flexibility, and cost and time. Evidence in favor of brief computer-delivered interventions for health-related behaviors is growing (Stinson, Wilson, Gill, Yamada, & Holt, 2009). Computer-based interventions fit easily into the natural ecology of late adolescents and emerging adults who are the largest group of internet users (Smith, 2012). Further, brief interventions targeting YLH before the onset of adherence problems may be more successful than interventions targeting adherence problems after they occur. In its seminal report on mental health, the Surgeon General (1999) declared that preventing a problem from occurring is inherently better than having to treat the problem. There is evidence to suggest that the critical period for adherence is early in treatment when viral suppression is being established (Bangsberg, 2011), and brief interventions during critical periods may have lasting effects on biological outcomes, as the risk of virological failure owing to poor adherence is lower after longer duration of viral suppression (Lima et al., 2010; Rosenblum, Deeks, Van Der Laan, & Bangsberg, 2009). In addition, the recommendation to initiate ART may be a “teachable moment,” one of the transitions or health events that may motivate individuals to adopt new health behaviors (McBride, Emmons, & Lipkus, 2003). Timing interventions to take advantage of these naturally occurring events might increase the effectiveness of low intensity interventions focused on self-change.
Rapoff (2000) presented a prevention model for facilitating adherence in chronic pediatric diseases. In this model, although secondary and tertiary prevention efforts focus on adherence problems, primary prevention of nonadherence focuses on individuals who are recently prescribed a regimen and includes educational and simple behavioral strategies. To date, there have been no published primary nonadherence prevention trials for YLH, and few in other chronic medical conditions affecting adolescents. Given the evidence of face-to-face MI in promoting adolescent behavior change in HIV (Naar-King et al., 2009) as well as other health risk behaviors (Jensen et al., 2011; Seid et al., 2012), this study developed and pilot tested a two-session computer-based MI intervention called Motivational Enhancement System for Adherence (MESA). MESA was designed to prevent adherence problems among for YLH newly prescribed ART. We hypothesized that MESA would be feasible and acceptable to youth and that YLH randomized to MESA would show higher rates of ART adherence and lower viral load at 3- and 6-month follow-up than YLH randomized to a two-session computer-based nutrition and physical activity control condition called Motivational Enhancement System for Health (MESH).
Method
Participants and Procedures
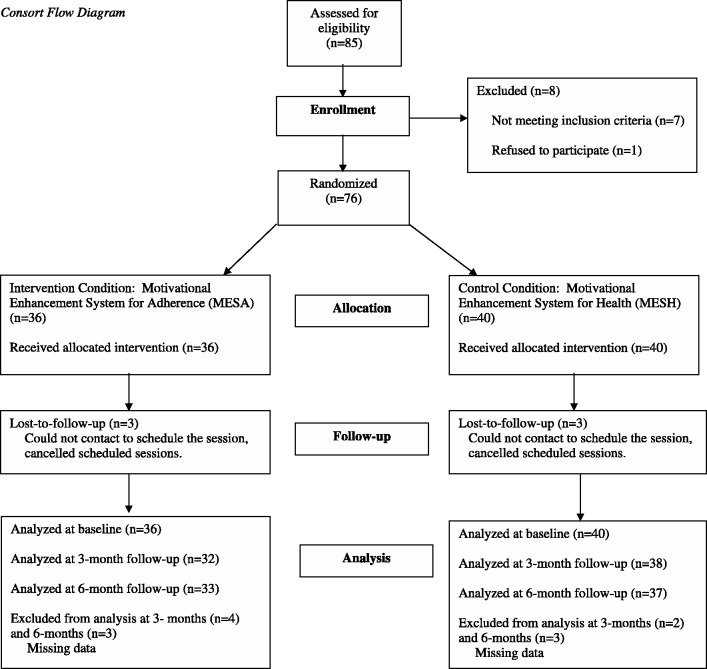
YLH were recruited from eight sites in the National Institutes of Health Adolescent Trials Network for HIV/AIDS (Children’s Hospital of Los Angeles, CA; Children’s National Medical Center, DC; Children’s Hospital of Philadelphia, PA; Stroger Hospital of Cook County, IL; Montefiore Medical Center, NY; Tulane University, Department of Pediatrics/Adolescent Medicine, LA; University of Miami School of Medicine Division of Adolescent Medicine, FL; St. Jude Children’s Research Hospital, TN). All participating sites and the coordinating center received approval from the local Human Investigation Committee. Eligibility criteria included being HIV positive, between the ages of 16 and 24 years 11 months, newly recommended to begin ART at the site no >12 weeks prior to study enrollment, and still treatment naive. Exclusion criteria included known pregnancy, unable to understand written and spoken English, having an active psychiatric disorder that interfered with study participation, or participation in any concurrent adherence intervention trial. Of 85 youth approached, one refused screening and eight were ineligible, yielding a final sample of 76 who provided informed consent or assent (Figure 1). Parental consent was waived for those under 18. After completing measures via Computerized Intervention Authoring Software in confidential setting in the clinic, the computer automatically randomized participants to either MESA (treatment; N = 36) or MESH (control; N = 40); thus, study staff were blind to treatment condition. The uneven cells were due to true randomization for participant assignment rather than random order of an a priori determined sample size. Following randomization, youth immediately completed the intervention component. The second intervention session occurred one month later, consistent with the time that youth beginning medications typically return for medical care. Youth were asked to return for 3-month and 6-month post-intervention data collections, timed with quarterly clinic visits. Two youth were removed from the study owing to pregnancy, one youth was incarcerated, and three youth were lost to follow-up. Participants were compensated $50 for baseline, 3-month and 6-month assessment sessions. Youth received a $10 gift card for 1-month brief assessment and intervention session. The trial was registered in ClinicalTrials.gov under registry number NCT01009749.
Figure 1.
Consort flow diagram.
Intervention Condition: MESA
The intervention was created with Computer Intervention Authoring Software (CIAS) developed by Ondersma, Svikis, and Schuster (2007). CIAS is designed to allow easy development and modification of screening, assessment, and intervention components without new programming. It has flexible and authorable options including: (1) colors, narrator, voice, and language; (2) single-choice, multiple choice, visual analog scale, or text input; (3) skips and subgroup formation; (4) eligibility criteria, randomization, and counterbalancing; (5) single or multisession (with recap of previous session); and (6) tailored intervention components such as recap of past responses in natural language, video insertion, image with pointing/explanation, web surfing, normed feedback, and conversation. The MESA intervention is delivered via a web-based server. The software uses realistic interactions with a two-dimensional animated character to mimic the conversational nature of person-delivered brief interventions. The character speaks, moves, points, provides empathic reflections, and displays emotional responses such as surprise, sadness, and thoughtfulness as appropriate. Built on the principles of MI for adolescents (Naar-King & Suarez, 2011), the character not only delivers personalized health feedback, ART information and activities to promote motivation and confidence (e.g., pros and cons, identifying strengths and resources), but also is able to deliver MI strategies by reflecting participants’ responses, providing affirmations based on responses, and allowing for personal choice to support autonomy. For example, reflections are made possible by a feature in the software that allows content developers to enter natural-language reflections that are tied to any one of a given participant’s earlier responses, all of which are provided via single- or multiple-choice from pre-determined answers. For example, if a participant checks a box indicating that it is sometimes hard to remember to take the medication, but also elsewhere checks a box indicating that they believe it is important to do so, the character might respond, “You have a lot on your mind, and sometimes forget to take your medication. But you also know how important it is. You take your own health seriously.”
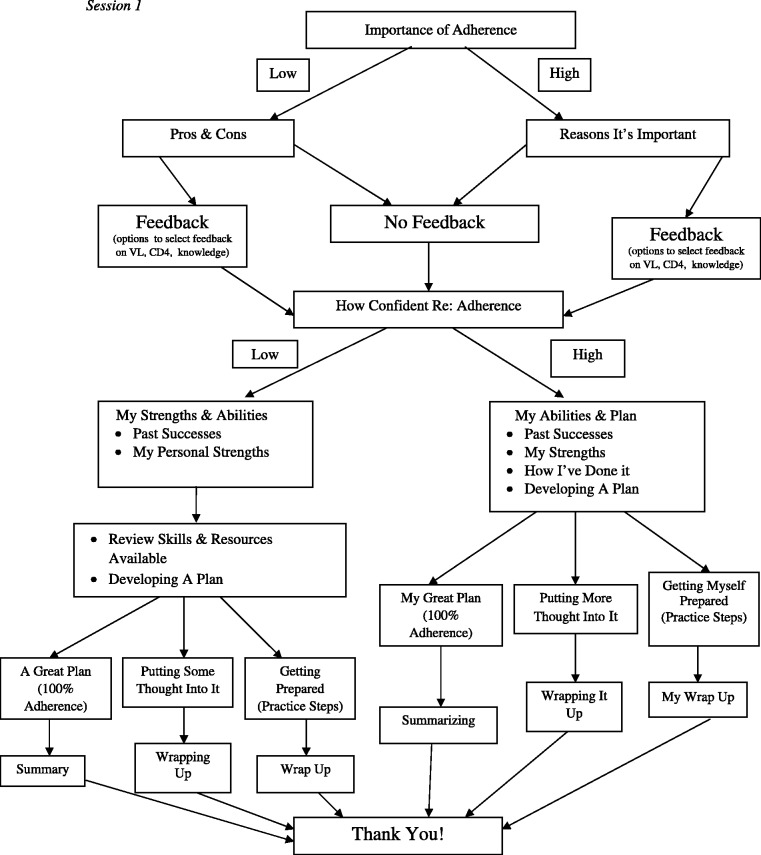
The intervention is tailored in several ways: (1) participants choose one of seven avatars; (2) participants are routed through arms of the program based on their ratings of importance and confidence and choices for goal setting; (3) participants receive personalized feedback and ART information based on their recent medical information and response to an ART knowledge questionnaire; (4) participants may choose to read through the intervention screens or be read to, based on their literacy level and choice; (5) participants have the choice to skip informational components and to pick among a menu of options for goal setting; and (6) all intervention content (including interventionist characters) was reviewed extensively by youth advisory groups as well as providers to ensure appropriate tailoring for the cultural context of adolescent HIV in the United States (e.g., age appropriateness of language; appropriateness for ethnic and sexual minority youth). Session branching is shown in Figure 2. In the second session (1 month), branches are based on whether the youth felt they met the goal (reinforcement and plan for continued success), partially met the goal (identifying plans for overcoming barriers), or did not meet the goal (review of importance and confidence). Youth then may choose to plan for a new goal or continue the same goal. Each intervention session takes 30 min to complete.
Figure 2.
Session 1.
Control Condition: MESH
A two-session attention control intervention used the same platform (CIAS software with an MI-consistent avatar) to target nutrition and physical activity. Youth chose to discuss nutrition or physical activity first. The software followed the same format as the MESA flow including feedback on body mass index (based on heights and weights collected at each session) and knowledge of nutrition or physical activity. However, because of concerns that the modules focusing on confidence might generalize to other behaviors, the second part of the session focused on importance, feedback, and psycho-education for the second behavior instead of confidence. The session ended with goal setting similar to MESA. Session 2 followed a similar format to MESA for both nutrition and physical activity goals.
Measures
HIV-1 Viral Load
Approximately 5 cc of blood was collected for HIV-1 RNA PCR only from participants who did not have a documented HIV-1 viral load measurement obtained close to the study visit (12 weeks for baseline; 2 weeks for 3-month follow-up; 4 weeks for 6-month follow-up). The blood sample was sent to the site’s local laboratory for testing.
Adherence Visual Analog Scale
Self-report of adherence was collected at 3 months and 6 months (Audio Computer-Assisted Self-Interviewing). Because there is no gold standard self-report HIV adherence measure, youth completed multiple adherence measures that had been previously used with YLH. The Visual Analog Scale, VAS (Kalichman et al., 2009), for medication adherence is a single-item asking participants to consider a specific time period (e.g., previous month) and to estimate along a continuum the percentage of medication doses taken from 0 to 100%. As evidence of validity, the developers reported moderate correlations with unannounced pill counts (r = .48) and self-reported recall (r = .58) in adults. A similar scale was used in the Healthy Choices study (MacDonell, Naar-King, Murphy, Parsons, & Harper, 2010) and correlated with viral load (r = −.54).
Adherence Recall
Participants also completed recall measures used within the Adolescent Trials Network (MacDonnel et al., in press). YLH were asked the total number of doses per day of their HIV medication they were currently prescribed and were asked to report number of doses of HIV medication they had missed in the last 7 days. Percentage of doses of HIV medication missed in the last 7 days was calculated as number of doses missed/(daily dosage × 7). Participants were also asked to report the number of doses of HIV medication they missed last weekend (Friday, Saturday, and Sunday). Percentage of doses of HIV medication missed last weekend was calculated as the number of doses missed/(weekend dosage × *3). However, to be consistent with the VAS percent doses taken, percent adherence in Table II is reported as 100 minus percent doses missed for these two measures. Youth who had not yet initiated ART were instructed to report 0% adherence or missing all doses.
Table II.
Comparison of Reductions in HIV Viral Loads and Comparison of Adherence for MESA (Intervention) and MESH (Control Condition) Participants
| 3-Month viral load change |
6-Month viral load change |
|||||
|---|---|---|---|---|---|---|
| MESA | MESH | Effect size | MESA | MESH | Effect size | |
| n = 32 | n = 38 | n = 33 | n = 37 | |||
| Change in log 10 viral load from baseline (mean, SD) | 1.32 (1.10) | 0.90 (1.07) | 0.39 | 1.84 (1.33) | 1.60 (1.16) | 0.19 |
| Viral load categories (n, %) | ||||||
| Detectable | 26 (81.25) | 32 (84.21) | 16 (48.48) | 23 (62.16) | ||
| Below detectable | 6 (18.75) | 6 (15.79) | 0.09 | 17 (51.52) | 14 (37.84) | 0.28 |
| 3 Months |
6 Months |
|||||
|---|---|---|---|---|---|---|
| MESA | MESH | Effect size | MESA | MESH | Effect size | |
| n = 32 | n = 37 | n = 33 | n = 36 | |||
| Adherence—VAS (mean, SD) | 71.22 (42.19) | 63.95 (44.07) | 0.17 | 70.27 (41.31) | 66.61 (40.35) | 0.09 |
| Adherence—% past week (mean, SD) (past 7 days) | 90.60 (22.13) | 87.78 (25.34) | 0.12 | 97.10 (10.56) | 87.55 (25.42) | 0.49 |
| Adherence—% past weekend (mean, SD) (Friday, Saturday, and Sunday) | 89.37 (26.25) | 81.53 (34.65) | 0.26 | 97.92 (11.79) | 83.80 (28.03) | 0.65 |
Note. VAS = Visual Analog Scale.
Satisfaction
Immediately following each intervention session, participants completed the widely used Client Satisfaction Questionnaire, CSQ-8 (Larsen, Attkisson, Hargreaves, & Nguyen, 1979). Eight items are rated on a 4-point scale from 1 (poor) to 4 (excellent). For example, “How would you rate the quality of today’s computer intervention session?”
Results
The mean age of participants was 20 years (Table I), with majority non-Hispanic black/African Americans (71%) and male (80%). Over half considered themselves as gay or lesbian (58%). Most (95%) had a high school degree or equivalent. No significant differences were found statistically between MESA and MESH participants on these characteristics. Mean satisfaction ratings for MESA participants were 3.7 out of 4 for the first session and 3.65 out of 4 for the second session.
Table I.
Sample Sociodemographic Characteristics at Baseline
| Overall n = 76 | MESA n = 36 | MESH n = 40 | p-value | |
|---|---|---|---|---|
| Age (mean, SD) | 20.32 (2.13) | 20.11 (1.97) | 20.51 (2.27) | .36 |
| Race/ethnicity (n, %) | ||||
| White (non-hispanic) | 2 (2.63) | 2 (5.56) | 0 (0.00) | .16 |
| Black/African American (non-hispanic) | 54 (71.05) | 27 (75.00) | 27 (67.50) | |
| Hispanic (Spanish) or Latino | 17 (22.37) | 5 (13.89) | 12 (30.00) | |
| Other/mixed race (non-hispanic) | 3 (3.95) | 2 (5.56) | 1 (2.50) | |
| Gender (n, %) | ||||
| Male | 61 (80.26) | 29 (80.56) | 32 (80.00) | 1.00 |
| Female | 15 (19.74) | 7 (19.44) | 8 (20.00) | |
| Sexual orientation (n, %) | ||||
| Straight/heterosexual | 17 (22.37) | 8 (22.22) | 9 (22.50) | .44 |
| Bisexual | 15 (19.74) | 5 (13.89) | 10 (25.00) | |
| Gay/lesbian | 44 (57.89) | 23 (63.89) | 21 (52.50) | |
| Income (mean, SD) | 566.97 (583.68) | 426.85 (499.26) | 747.12 (644.98) | .06 |
| Biological children? (n, %) | ||||
| Yes | 6 (7.89) | 2 (5.56) | 4 (10.00) | .68 |
| No | 70 (92.11) | 34 (94.44) | 36 (90.00) | |
| Education (n, %) | ||||
| <High school | 4 (5.26) | 1 (2.78) | 3 (7.50) | .79 |
| High school graduate | 17 (22.37) | 7 (19.44) | 10 (25.00) | |
| GED | 29 (38.16) | 15 (41.67) | 14 (35.00) | |
| > High school or GED | 26 (34.21) | 13 (36.11) | 13 (32.50) | |
| Baseline log 10 viral load (mean, SD) | 4.24 (0.76) | 4.33 (0.67) | 4.16 (0.83) | .62 |
Because the pilot study was not powered for significance testing, effect sizes were calculated using Cohen’s d. Table II summarizes the change in log10 transformed viral load (transformed to manage skew) from baseline to each study visit. The results indicate an overall decreasing trend in magnitude from study entry to 3 months and study entry to 6 months for both groups, but the magnitude of change was larger for the MESA group compared with MESH (Cohen’s d = 0.39 at 3 months; d = 0.19 at 6 months). Viral load was also transformed to a dichotomous variable indicating whether viral load was undetectable or detectable. In the analysis of viral suppression, the proportion of suppressing viral load was increasing from entry to month 6 within each study group (from 0 to 52% for MESH group and from 0 to 38% for MESA group). The viral load suppression rate in the MESA group appeared to be larger in magnitude when compared with the MESH group (Cohen’s d = 0.09 at 3 months; d = 0.28 at 6 months).
Adherence measures were analyzed at 3 months and 6 months, as youth were not prescribed medications at baseline. The two-sample Wilcoxon test was used to examine the difference of the measures between MESA and MESH at each visit. The MESA group reported greater adherence (Table II) than the MESH control group on two of three adherence measures at 6 months with medium to large effect sizes (d = 0.49 for 7-day adherence; d = 0.66 for weekend adherence). Despite small sample size, these effect sizes were large enough to reach statistical significance using the two-sample Wilcoxon test (p < .05 for 7-day; p < .01 for weekend adherence).
Discussion
YLH are the largest group of ART initiators, and yet there is a dearth of intervention studies to improve adherence or prevent the development of nonadherence in this group. The current pilot study demonstrated that a two-session computer-delivered MI adherence intervention was feasible and acceptable to YLH as evidenced by high recruitment and retention rates and high satisfaction scores. Consistent with findings of a recent meta-analysis that technology-based interventions with behavioral components are more efficacious for children and youth than information-only interventions (Cushing & Steele, 2010), the MESA program used an MI approach to support autonomous motivation, build self-efficacy, provide feedback, and engage in goal-setting. All differences favored the MESA condition, with effect sizes similar to those seen in Cushing and Steele’s meta-analyses. Effect sizes were comparable with or even better than more intensive and costly HIV adherence interventions in the adult literature (Braithwaite et al., 2010; Simoni, Frick, & Huang, 2006). Further, these effects were obtained in a study in which youth received an active intervention designed to promote health, as well as ongoing and intensive assessment of their adherence to ART. A growing literature in the area of brief alcohol interventions suggests that assessment and other study-related activities may have small, but clear, effects on behavior (McCambridge & Kypri, 2011).
First introduced by Abrams et al. (1996) then reinforced by Glasgow’s RE-AIM model (Glasgow, Vogt, & Boles, 1999), public health impact is a function of reach (percent of the population receiving the intervention) times its efficacy (I = R × E). Thus, technology-based interventions such as MESA have the potential for high impact even in the context of small effect sizes due to the potential reach of this flexible easily adaptable technology-based intervention. Though the current intervention was clinic-based, future efforts to adapt the system to smart phone technology may further enhance reach, as data suggest that low-income minority individuals are more likely to access the internet via phone versus computer (Smith, 2012).
Limitations include a small sample size, though the multisite design of this pilot study is a strength. Because self-report may overestimate adherence, future studies should consider more objective measures of adherence such as pill counts (S. C. Kalichman et al., 2008), hair sample analysis (Gandhi et al., 2009), or Medication Electronic Monitoring Caps. Furthermore, self-report of ART prescription was not confirmed by chart review owing to staffing constraints and should be confirmed in future studies. Maintenance of adherence behaviors and perhaps more importantly, an understanding of who may need more intensive intervention to support adherence following such a prevention intervention, requires further study. Effects were stronger at 6 months than at 3 months. This may be due to a delayed intervention effect on adherence or to an increase in the number of youth in the intervention group initiating ART over time. This study did not disentangle youth who did not initiate ART at all (0% adherent) from youth who initiated ART but were then poorly adherent. Future studies with larger samples could assess the effect of the intervention on ART initiation. Also, the purported mediators of treatment effects such as intrinsic motivation and self-efficacy should be assessed in future studies. Meta-analyses have shown that interventions based on MI have promoted change across many different behaviors and that that effect sizes may be even larger in health disparity populations (Hettema, Steele, & Miller, 2005; Lundahl, Kunz, Brownell, Tollefson, & Burke, 2010). A computer-delivered MI intervention shows promise in promoting adherence and improving health outcomes in a primarily minority youth population and is ready to be tested in a full-scale clinical trial.
Funding
The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) supported this work (U01-HD040533 from the National Institutes of Health through the National Institute of Child Health and Human Development, with supplemental funding from the National Institutes on Drug Abuse and Mental Health).
Conflicts of interest: None declared.
Acknowledgments
This work was supported by The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health [U01 HD 040533 and U01 HD 040474] through the National Institute of Child Health and Human Development (B. Kapogiannis, R. Hazra, C. Worrell), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (S. Allison). The ATN’s Behavioral Leadership Group scientifically reviewed the study. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at the University of Alabama at Birmingham. The ATN Data and Operations Center at Westat, Inc. (K. Vellala, M. Saar, J. Xu) provided network operations and data management support. We acknowledge the contribution of the investigators and staff at the following ATN 072 sites that participated in this study: Children’s Hospital of Los Angeles, Los Angeles, CA (Belzer, Tucker, Salata); Children’s Hospital of Philadelphia (Douglas, Rudy, Tanney, DiBenedetto, Aagenes, Seth); Children’s National Medical Center, Washington, DC (D’Angelo, Trexler, Hagler, Klamberg); John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center, Chicago, IL (Martinez, Henry-Reid, Bojan, Jackson); Montefiore Medical Center, Bronx, NY (Futterman, Enriquez-Bruce, Campos); St. Jude Children’s Research Hospital, Memphis, TN (Flynn, Stender, Dillard, McKinley); Tulane University Health Sciences Center, New Orleans, LA (Abdalian, Baker, Kozina); University of Miami School of Medicine (Friedman, Maturo, Major-Wilson); University of South Florida, Tampa, FL (Emmanuel, Lujan-Zilbermann, Straub, Callejas, Julian). We sincerely thank the members of the local youth Community Advisory Boards for their insight and counsel and are particularly indebted to the youth who participated in this study.
References
- Abrams D B, Orleans C T, Niaura R S, Goldstein M G, Prochaska J O, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: A combined stepped-care and matching model. Annals of Behavioral Medicine. 1996;18:290–304. doi: 10.1007/BF02895291. [DOI] [PubMed] [Google Scholar]
- Bangsberg D R. Perspectives on adherence and resistance to ART; Paper presented at the 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. [Google Scholar]
- Bartlett J G, Cheever L W, Johnson M P, Paauw D S. A guide to primary care of people with HIV/AIDS. 2004. Retrieved from www.hab.hrsa.gov/tools/primarycareguide.
- Berrien V M, Salazar J C, Reynolds E, Mckay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care and STDs. 2004;18:355–363. doi: 10.1089/1087291041444078. doi:10.1089/1087291041444078. [DOI] [PubMed] [Google Scholar]
- Braithwaite R S, Fiellin D A, Nucifora K, Bryant K, Roberts M, Kim N, Justice A C. Evaluating interventions to improve antiretroviral adherence: How much of an effect is required for favorable value? Value in Health. 2010;13:535–542. doi: 10.1111/j.1524-4733.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M S, Chen Y Q, McCauley M, Gamble T, Hosseinipour M C, Kumarasamy N, Hakim J G, Kumwenda J, Grinsztejn B, Pilotto J H, Godbole SV, Mehendale S, Chariyalertsak S, Santos B R, Mayer KH, Hoffman IF, Eshleman S H, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha T E, Nielsen-Saines K, Celentano D, Essex M, Fleming T R for the HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. doi:10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing C C, Steele R G. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. Journal of Pediatric Psychology. 2010;35:937–949. doi: 10.1093/jpepsy/jsq023. [DOI] [PubMed] [Google Scholar]
- DHHS. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. 2012. Department of Health and Human Services. Retrieved from http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- Dowshen N, Kuhns L M, Johnson A, Holoyda B J, Garofalo R. Improving adherence to antiretroviral therapy for youth living with HIV/AIDS: A pilot study using personalized, interactive, daily text message reminders. Journal of Medical Internet Research. 2012;14:e51. doi: 10.2196/jmir.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A R, Rutstein R M, Meislich D, Brown T, Rudy B J. Protease inhibitor therapy in HIV-infected children. AIDS Patient Care and STDs. 2000;14:589–593. doi: 10.1089/10872910050193761. doi:10.1089/10872910050193761. [DOI] [PubMed] [Google Scholar]
- Flynn P, Rudy B, Douglas S, Lathey J, Spector S, Martinez J, Silio M, Belzer M, Friedman L, D'Angelo L, McNamara J, Hodge J, Hughes MD, Lindsey J Pediatric AIDS Clinical Trial Group 381 Study Team. Virologic and immunologic outcomes after 24 weeks in HIV type 1 - infected adolescents receiving highly active antiretroviral therapy. The Journal of Infectious Diseases. 2004;190:271–279. doi: 10.1086/421521. doi:10.1086/421521. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Ameli N, Bacchetti P, Gange S J, Anastos K, Levine A, Hyman CL, Cohen M, Young M, Huang Y Women's Interagency HIV Study (WIHS) Protease inhibitor levels in hair strongly predict virologic response to treatment. Acquired Immune Deficiency Syndrome. 2009;23:471–478. doi: 10.1097/QAD.0b013e328325a4a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R E, Emmons K M. How can we increase translation of research into practice? Types of evidence needed. Annual Review of Public Health. 2007;28:413–433. doi: 10.1146/annurev.publhealth.28.021406.144145. [DOI] [PubMed] [Google Scholar]
- Glasgow R E, Vogt T M, Boles S M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89:1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W N, Janicke D M, Fennell E B, Driscoll D C, Lawrence R M. Piloting behavioral family systems therapy to improve adherence among adolescents with HIV: A case series intervention study. Journal of Health Psychology. 2011;16:828–842. doi: 10.1177/1359105310394230. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller W R. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Jensen C D, Cushing C C, Aylward B S, Craig J T, Sorell D M, Steele R G. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2011;79:433–440. doi: 10.1037/a0023992. [DOI] [PubMed] [Google Scholar]
- Kalichman S C, Amaral C M, Cherry C, Flanagan J, Pope H, Eaton L, Kalichman M O, Cain D, Detorio M, Caliendo A, Schinazi RF. Monitoring medication adherence by unannounced pill counts conducted by telephone: Reliability and criterion-related validity. HIV Clinical Trials. 2008;9:298–308. doi: 10.1310/hct0905-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S C, Amaral C M, Swetzes C, Jones M, Macy R, Kalichman M O, Cherry C. A simple single-item rating scale to measure medication adherence: Further evidence for convergent validity. Journal of the International Association of Physicians in AIDS Care. 2009;8:367–374. doi: 10.1177/1545109709352884. doi:10.1177/1545109709352884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen D L, Attkisson C C, Hargreaves W A, Nguyen T D. Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. doi:10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- Letourneau E J, Ellis D A, Naar-King S, Chapman J E, Cunningham P B, Fowler S. Multisystemic therapy for poorly adherent youth with HIV: Results from a pilot randomized controlled trial. AIDS Care. 2012 doi: 10.1080/09540121.2012.715134. Advance online publication. doi:10.1080/09540121.2012.715134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima V D, Bangsberg D R, Harrigan P R, Deeks S G, Yip B, Hogg R S, Montaner J S G. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. Journal of Acquired Immune Deficiency Syndromes. 2010;55:460. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl B W, Kunz C, Brownell C, Tollefson D, Burke B L. A meta-analysis of motivational interviewing: Twenty-five years of empirical studies. Research on Social Work Practice. 2010;20:137–160. doi:10.1177/1049731509347850. [Google Scholar]
- MacDonell K E, Naar-King S, Huszti H, Belzer M. Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV. AIDS and Behavior. 2012 doi: 10.1007/s10461-012-0364-1. Advance online publication. doi: 10.1007/s10461-012-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell K E, Naar-King S, Murphy D A, Parsons J T, Harper G W. Predictors of medication adherence in high risk youth of color living with HIV. Journal of Pediatric Psychology. 2010;35:593–601. doi: 10.1093/jpepsy/jsp080. doi:10.1093/jpepsy/jsp080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C M, Emmons K M, Lipkus I M. Understanding the potential of teachable moments: The case of smoking cessation. Health Education Research. 2003;18:156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023748. e23748. doi:10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W R, Rollnick S. Motivational interviewing: Helping people change. New York, NY: Guilford Press; 2012. [Google Scholar]
- Naar-King S, Parsons J T, Murphy D A, Chen X, Harris D R, Belzer M E. Improving health outcomes for youth living with the human immunodeficiency virus: A multisite randomized trial of a motivational intervention targeting multiple risk behaviors. The Archives of Pediatrics & Adolescent Medicine. 2009;163(12):1092–1098. doi: 10.1001/archpediatrics.2009.212. doi:10.1001/archpediatrics.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York, NY: Guilford Press; 2011. [Google Scholar]
- Naar-King S, Wright K, Parsons J, Frey M, Templin T, Lam P, Murphy D. Healthy Choices: Motivational enhancement therapy for health risk behaviors in HIV+ Youth. AIDS Education and Prevention. 2006;18:1–11. doi: 10.1521/aeap.2006.18.1.1. [DOI] [PubMed] [Google Scholar]
- Nachega J B, Hislop M, Nguyen H, Dowdy D W, Chaisson R E, Regensberg L, Cotton M, Maartens G. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. Journal of Acquired Immune Deficiency Syndromes. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Health. Substance use and dependence among HIV-infected adolescents and young adults. New York, NY: State Department of Health; 2009. [Google Scholar]
- Ondersma S J, Svikis D S, Schuster C R. Computer-based brief intervention: A randomized trial with postpartum women. American Journal of Preventive Medicine. 2007;32:231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw A Y, Naar-King S, Parsons J T, Green-Jones M, Janisse H, Secord E. Using motivational interviewing in HIV field outreach with young African American men who have sex with men: A randomized clinical trial. American Journal of Public Health. 2010;100:S146–S151. doi: 10.2105/AJPH.2009.166991. doi:10.2105/ajph.2009.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccio J A, Belzer M, Olson J, Martinez M, Salata C, Tucker D, Tanaka D. The use of cell phone reminder calls for assisting HIV-infected adolescents and young adults to adhere to highly active antiretroviral therapy: A pilot study. AIDS Patient Care & STDs. 2006;20:438–444. doi: 10.1089/apc.2006.20.438. [DOI] [PubMed] [Google Scholar]
- Rapoff M A. Facilitating adherence to medical regimens for pediatric rheumatic diseases: Primary, secondary, and tertiary prevention. In: Drotar D, editor. Promoting adherence to medical treatment in chronic childhood illness: Concepts, methods, and interventions. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 329–346. [Google Scholar]
- Rosenblum M, Deeks S G, Van Der Laan M, Bangsberg D R. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One. 2009;4:e7196. doi: 10.1371/journal.pone.0007196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouet F, Sakarovitch C, Msellati P, Elenga N, Montcho C, Viho I, Blanche S, Rouzioux C, Dabis F, Leroy V Abidjan ANRS 049 Ditrame Study Group. Pediatric Viral Human Immunodeficiency Virus Type 1 RNA levels, timing of infection, and disease progression in African HIV-1-infected children. Pediatrics. 2003;112:e289–e297. doi: 10.1542/peds.112.4.e289. doi:10.1542/peds.112.4.e289. [DOI] [PubMed] [Google Scholar]
- Seid M, D'Amico E J, Varni J W, Munafo J K, Britto M T, Kercsmar C M, Drotar D, King EC, Darbie L. The in vivo adherence intervention for at risk adolescents with asthma: Report of a randomized pilot trial. Journal of Pediatric Psychology. 2012;37:390–403. doi: 10.1093/jpepsy/jsr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni J M, Frick P A, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV- positive men and women on antiretroviral therapy. Health Psychology. 2006;25:74–81. doi: 10.1037/0278-6133.25.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. 2012 August. 17% of cell phone owners do most of their online browsing on their phone, rather than a computer or other device. Retrieved from http://pewinternet.org/Reports/2012/Cell-Internet-Use-2012.aspx. [Google Scholar]
- Stinson J, Wilson R, Gill N, Yamada J, Holt J. A systematic review of internet-based self-management interventions for youth with health conditions. Journal of Pediatric Psychology. 2009;34:495–510. doi: 10.1093/jpepsy/jsn115. [DOI] [PubMed] [Google Scholar]
- Surgeon General. Mental health: A report of the surgeon general. Bethesda, MD: Surgeon General; 1999. [Google Scholar]
- Tanney M R, Naar-King S, Murphy D A, Parsons J T, Janisse H. Multiple risk behaviors among youth living with Human Immunodeficiency Virus in five U.S. cities. Journal of Adolescent Health. 2010;46:11–16. doi: 10.1016/j.jadohealth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. 2008 Report on the global AIDS epidemic. 2008. Retrieved from http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp.
- Williams P L, Storm D, Montepiedra G, Nichols S, Kammerer B, Sirois P A, Farley J, Malee K, 219C Team PACTG. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118:e1745–e1757. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]