Abstract
Objective To adapt and pilot test a multicomponent motivational intervention that includes family-based contingency management (CM) for adolescents with poorly controlled type 1 diabetes. Methods A total of 17 adolescents, age 12–17 years (M = 14.8, SD = 1.5), with type 1 diabetes (duration M = 6.2 years, SD = 4.5) and mean HbA1c of 11.6% (SD = 2.5%) were enrolled. Adolescents and their parents received 14 weeks of motivational interviewing, clinic-based CM, and parent-directed CM that targeted increased blood glucose monitoring (BGM). Results Adolescents significantly increased their BGM (p < .001) and showed significantly improved HbA1c levels (glycemic control) from pre-to posttreatment (p < .0001). Conclusions The magnitude of improvements in the frequency of BGM and glycemic control in adolescents with type 1 diabetes is encouraging and will be tested in a randomized controlled trial.
Keywords: cognitive behavior therapy, contingency management, motivational interviewing, type 1 diabetes
Diabetes is a leading cause of death in the United States and is associated with significant mortality and economic cost (Centers for Disease Control and Prevention, 2008). Although improved in recent decades, persons with diabetes have mortality rates 5.6 times higher than those in the general population (Secrest, Becker, Kelsey, LaPorte, & Orchard, 2010). The incidence of type 1 diabetes among teens increased significantly over the past 25 years (Vehik et al., 2007), so that ∼1 in 500 adolescents ages 12–19 have type 1 diabetes (Centers for Disease Control and Prevention, 2008; The Writing Group for the SEARCH for Diabetes in Youth Study Group, 2007). Unfortunately, teens, even with intensive insulin regimens, have much poorer glycemic control than adults (Diabetes Control and Complications Trial Research Group, 1994).
Glycemic control (measured via HbA1c levels) is a powerful determinant of diabetes outcomes (Diabetes Control and Complications Trial Research Group, 1994). In the short term, higher HbA1c is directly related to hospitalization and increased costs (Menzin et al., 2010). Blood glucose monitoring (BGM) frequency is a robust predictor of glycemic control (Guilfoyle, Crimmins, & Hood, 2011; Helgeson, Honcharuk, Becker, Escobar, & Siminerio, 2011). BGM adherence is a promising target for teens, whose rates are generally low (Anderson et al., 2009), because daily monitoring can be objectively measured and reinforced. Parental monitoring can also positively affect teen adherence to BGM and other aspects of the medical regimen (Anderson, Ho, Brackett, Finkelstein, & Laffel, 1997; Ellis et al., 2007a; Horton, Berg, Butner, & Wiebe, 2009).
Studies with teens provide some support for the efficacy of individual and family-based treatments that include the goal of increasing BGM for teens (Channon et al., 2007; Ellis et al., 2007b; Franklin, Waller, Pagliari, & Greene, 2006; Nansel et al., 2007; Salamon, Hains, Fleischman, Davies, & Kichler, 2009; Wysocki et al., 2008). However, across these studies, BGM frequency remained low (<3× daily; ≥6× daily is recommended for teens with poor glycemic control) (Diabetes Care, 2010), with treatment gains often lasting <6 months (Alam, Sturt, Lall, & Winkley, 2009). In addition, only small to moderate effect sizes on HbA1c (ES = 0.13–0.35) (Alam et al., 2009) were observed, with mean HbA1c remaining >8.5%, well above the recommended target of <7.5% (Silverstein et al., 2005). Thus, more effective interventions are needed to improve BGM adherence and glycemic control among teens with type 1 diabetes who consistently are not at the American Diabetes Association (ADA) blood glucose target.
To address this problem, motivational interviewing (MI) and cognitive behavior therapy (CBT) were combined with a family-based contingency management (CM) intervention. The MI intervention has been tested with teens with type 1 diabetes in a prior trial (Channon et al., 2007). The MI, CBT, and CM interventions were originally developed to treat adolescent substance abuse (Stanger, Budney, Kamon, & Thostensen, 2009) and were adapted for adolescents with poorly controlled type 1 diabetes to target teen coping skills, BGM frequency, and parental monitoring. Others have also reported success adapting substance use interventions for teens with type 1 diabetes (Channon et al., 2007; Ellis et al., 2005; Raiff & Dallery, 2010). Similar behavior analytic principles can be applied to address the challenging behaviors and enhance motivation to change in teens who are not effectively managing their type 1 diabetes and those who abuse substances. Our parallel intervention for teen substance abuse has been demonstrated to motivate change in teen behavior (promote compliance with substance abstinence goal) in the context of low motivation and poor parental monitoring (Stanger et al., 2009). The current pilot study was conducted to determine the feasibility and outcomes of this MI/CBT + CM intervention for improving the management of poorly controlled type 1 diabetes in adolescents.
Motivational Interviewing/Cognitive Behavior Therapy (MI/CBT)
Motivational interviewing (MI) has targeted a broad range of health behaviors, including adherence in teens with diabetes (Channon et al., 2007; Rollnick, Mason, & Butler, 1999). In a randomized trial, the MI intervention selected for the current study resulted in small, but significant, improvements among adolescents with type 1 diabetes in HbA1c and quality-of-life measures up to 12 months later (Channon et al., 2007). In the current study, the Channon MI intervention was supplemented with CBT skills adapted from an evidence-based curriculum developed for teens with substance use problems (Webb, Scudder, Kaminer, & Kadden, 2001). This CBT curriculum includes several general coping skills designed to improve decision making. The combination of MI and CBT has been shown to be more effective than CBT alone for adults with diabetes, supporting the potential utility of combined MI/CBT interventions for teens with diabetes (Ismail et al., 2008). Further, in the substance abuse literature, MI and CBT have been frequently combined with CM, and this combination of study treatments has repeatedly been found to improve long-term outcomes relative to single-modality interventions (Budney, Moore, Rocha, & Higgins, 2006; Higgins, Silverman, & Heil, 2008).
Incentives/Contingency Management (CM)
CM involves the systematic reinforcement of desired behaviors (e.g., BGM). Ten of 11 randomized trials showed that incentives led to greater medical adherence than tested alternatives for blood pressure control, appointment attendance, and immunization rates (Giuffrida & Torgenson, 1997). Tangible incentives have also been effective in improving healthy habits such as losing weight (Volpp et al., 2008a), lowering cholesterol (Bloch et al., 2006), adhering to daily medication (Volpp et al., 2008b), and promoting tobacco, alcohol, and drug abstinence (Higgins et al., 2008). The proposed incentive intervention rearranges the consequences of BGM by providing immediate rewards for monitoring, and immediate negative consequences for not monitoring. The use of incentives to increase BGM among teens with type 1 diabetes has been reported in one case series (Raiff & Dallery, 2010). In this study, four teens increased daily monitoring from an average 1.7 times daily to 5.7 times daily over 5 days, when monetary incentives were available for submitting videos over the internet that documented BGM. A recent study offered adults with type 2 diabetes increasing incentives for reducing HbA1c by 1 or 2 percentage points (or to 6.5%) at a 6-month follow up assessment, and showed mean reductions of 0.45 percentage points, which was not significant relative to usual care (Long, Jahnle, Richardson, Loewenstein, & Volpp, 2012). These results suggest that more frequent incentives may be necessary as well as targeting a specific self-care behavior that might lead to improved glycemic control (e.g., BGM).
Teaching parents to use incentives (contingency contracting) is a common approach used in behavioral family therapy to treat a wide range of child and adolescent behaviors (Eyberg, Nelson, & Boggs, 2008) and has been used to improve adherence among adolescents with diabetes (Carroll, DiMeglio, Stein, & Marrero, 2011; Schafer, Glasgow, & McCaul, 1982). A randomized trial using similar incentive procedures demonstrated the efficacy of this approach in substance using teens (Stanger et al., 2009). In the current study, we hypothesized that the multicomponent intervention would lead to significant increases in BGM, and secondary improvements in parent and teen reports of diabetes self-care behaviors and in HbA1c.
Method
Participants
Adolescents were recruited from the Arkansas Children’s Hospital Endocrinology Clinic. A total of 17 adolescents, (5 males) ages 12–17 years (M = 14.8; SD = 1.5), and their parent(s) were enrolled. Twelve teens were non-Hispanic, Caucasian, four were African American, and one was multiracial. Inclusion criteria were a diagnosis of type 1 diabetes, duration of disease >18 months, and poor glycemic control operationalized as HbA1c ≥8% for the past 6 months (mean of two values) and most recent HbA1c ≥8%. Exclusion criteria were pregnancy/breast feeding, active psychosis, and/or severe medical or psychiatric illness that would limit participation (no participants were excluded). Mean pretreatment HbA1c was 11.6% (SD = 2.5%; range = 8.4%–16.8%; see Table I for HbA1c values for each subject). Mean time since diagnosis was 6.2 years (SD = 4.5, range = 1.5–14.4). Families had a mean Hollingshead (1975) 9-step socioeconomic status (SES) based on parental occupation of 5.4 (SD = 2.0, range = 3–9), which is equivalent to jobs such as bank clerk/teller, cashier, clerical worker, dental/medical assistant, and sales (retail), and 47% of teens had public insurance. The primary participating parent had on average 13.4 years of education (SD = 2.1, range = 8–17 years), and 70.6% were two-parent families. Insulin administration methods were multiple daily injections (n = 7), pump (n = 9), and injections plus pump (n = 1).
Table I.
Blood Glucose Monitoring (BGM) and HbA1c Pre- and PostTreatment
| Subject | BGM times per day |
HbA1c % |
|||
|---|---|---|---|---|---|
| First week of treatment | Last week of treatment | Intake | End of treatment | 3 months after treatment | |
| 01 | 1.2 | 6.3 | 10.9 | 8.7 | 11.1 |
| 02 | 6.7 | 10.0 | 8.4 | 8.4 | 8.1 |
| 03 | 0.8 | 3.3 | 13.6 | 10.2 | 12.3 |
| 04 | 2.4 | 7.1 | 10.2 | 8.8 | 7.9 |
| 05 | 3.7a | 11.9 | 10.4 | 9.2 | |
| 06 | 4.4 | 6.7 | 8.5 | 9.0b | 8.5 |
| 07 | 7.0 | 6.7 | 14.0c | 10.6 | 10.3 |
| 08 | 3.6 | 4.4d | 11.1 | 10.1 | |
| 09 | 3.7 | 6.6 | 14.0c | 8.8 | 11.3 |
| 10 | 3.7 | 5.9e | 9.5 | 8.6 | 9.3 |
| 11 | 0.6a | 14.0c | 8.9 | 8.5 | |
| 12 | 5.9 | 1.3e | 14.0c | 10.3 | |
| 13 | 5.7 | 7.6 | 16.8b | 9.1 | 9.9 |
| 14 | 4.6 | 6.1 | 9.9 | 7.9 | |
| 15 | 4.5 | 7.6 | 8.6 | 8.0 | 8.2 |
| 16 | 4.1 | 7.7 | 12.2 | 7.7 | 11.6 |
| 17 | 4.0 | 6.9 | 9.9 | 9.4b | 10.3b |
| M (SD) | 4.1 (1.9) | 6.3 (2.0) | 11.6 (2.5) | 9.1 (0.9) | 9.8 (1.4) |
aThese two participants did not complete treatment and did not provide meter data at the end of treatment assessment.
bValue obtained via laboratory blood test.
cValues of “>14.0” using point of care testing were scored as 14.0 for analyses.
dThis participant moved away unexpectedly in week 9 of treatment and did not have access to a computer. This participant’s week 9 data were used above as the end of treatment value.
eThese two participants did not complete treatment but did provide meter data at the end of treatment assessment.
Procedures
Families were screened by their treating endocrinologist at a quarterly appointment and referred to the research program. Pretreatment HbA1c obtained clinically at this visit was used in analyses. All teens meeting the study inclusion criteria were offered the opportunity to participate. Parents and teens provided consent/assent, and procedures were approved by the IRB at the University of Arkansas for Medical Sciences. HbA1c results were obtained with consent from the medical record, and teens received a study blood glucose meter (Bayer Contour), and test strips as necessary throughout the intervention. On average, intake appointments were 14.7 days (SD = 7.8, range = 1–29) after the pretreatment HbA1c date. End of treatment HbA1c was obtained on average 102.7 days (SD = 26.3, range = 69–162) after the first session (91 day target), and 3-month follow-up (94 day target) HbA1c was obtained on average 120.6 days (SD = 56.0, range = 59–258) after the end of treatment.
Adolescents and their parents received 14 weekly, 1-hr sessions of MI/CBT, clinic-based CM, and parent-directed CM as described below. Therapists were masters’ level clinicians. Therapists met weekly with the first author for supervision and case review. Therapists completed structured adherence checklists after each session, documenting their completion of treatment components.
Intervention Components
MI/CBT
Teens received weekly individual MI/CBT. MI included a menu of intervention components (Channon, Huws-Thomas, Gregory, & Rollnick, 2005). Therapists reviewed BGM and other self-care behaviors using MI exercises designed to build awareness of costs and benefits of change, identify and weigh alternatives, choose alternative behaviors, and set goals while avoiding confrontation. CBT components (adapted from Webb et al., 2001) included functional analysis (identify antecedents/consequences of missed/skipped BGM and other self-care behaviors), increasing social support, effective communication, problem solving, mood management, and anger management.
Contingency Management
Teens were rewarded for a BGM frequency of ≥6 times/day increasing gradually up to ≥5 days per week. The goal of having teens test ≥6 times daily is based on standard recommendations (Diabetes Care, 2010), which specify that monitoring should occur a minimum of 3 times a day (i.e., before meals), plus those on multiple injection therapy or pumps or who do not meet glycemic targets (i.e., the target population) are to add monitoring after meals (Diabetes Care, 2010). Bedtime and/or nocturnal monitoring are recommended if a hypoglycemia risk exists.
In weeks 1 and 2, teens earned $10 weekly for bringing their blood glucose meters to the session for download. Incentives were paid in gift cards or certificates from local merchants chosen by teens. Starting in week 3, teen incentives were contingent on meeting a personalized weekly monitoring goal. Incentives were earned for BGM ≥6 times a day (tests spaced > 1 hour apart) on one day more than the prior week, up to a maximum of 5 days per week. Unmet goals remained unchanged for the next week. The first week a goal was met was worth $10. The incentive value increased by $5 for each consecutive week a goal was met. To further increase the likelihood of continuous monitoring at targeted levels, a $10 bonus was earned for each week in which the teen exceeded the monitoring goal. If the monitoring goal was not met incentives were reset to the initial level ($10), where they escalated again under the same schedule. Missed counseling sessions did not result in loss of incentives, provided the meter was uploaded (this could be done over the internet) or brought in to the clinic. Maximum teen earnings were $590. This reinforcement schedule was identical to that used in our teen substance abuse trials (Stanger et al., 2009).
Parent-directed CM involved establishing a daily BGM contract to increase the adolescent’s BGM. The parent was directed to review their adolescent’s BG meter daily. Parents met weekly with a therapist to learn to develop and implement a blood glucose monitoring contingency contract (BGMC). The BGMC, which focused on daily BGM frequency, specified positive and negative consequences the parent(s) would implement in response to teen monitoring. Families selected diverse incentives and consequences they felt were appropriate, provided they could be used daily. Incentives and consequences sometimes changed over the course of the intervention based on family needs, teen preferences, and efficacy. Examples of daily rewards used by families included later bedtimes, healthy snack foods, and small amounts of money (e.g., $2 a day toward a cell phone purchase). Examples of consequences used included household chores and restricted access to TV, internet, and cell phone.
Parents participated in an incentive system similar to that for teens because parental adherence can be similarly difficult to achieve and maintain. Parental adherence to monitoring and consistently rewarding positive teen behavior is expected to sustain teen adherence after treatment ends. Parents were asked to provide a daily report to the clinic (voice mail, text message, or e-mail) that was stamped to identify delivery time. In week 1, parents received $10 for attendance if at least one parent and teen were present and the teen brought his/her meter. In weeks 2 and 3, parents received $10 for sending a daily message to the clinic on >5 days documenting daily BGM frequency. Starting in treatment week 4 (coincides with start of home enforcement of BGMC), parent incentives were contingent on reporting BGM frequency >5/7 days per week and detailing the daily incentive or consequence provided to the teen, consistent with the BGMC, with a $5 weekly bonus for calling >5/7 days. After week 4, parent incentives increased by $5 a week for each consecutive week of parent compliance with BGM monitoring and contract enforcement. If weekly parent monitoring and contract implementation goals were not met, incentives were reset to the initial level ($10), where they escalated again under the same schedule. Maximum parent earnings were $470. We have used similar incentive procedures with parents in our teen substance use trials (Stanger et al., 2009) and in a prevention trial showing that daily calling significantly improved treatment outcomes relative to parenting intervention alone (Stanger, Ryan, Fu, & Budney, 2011).
Outcome Measures
The primary outcome variable, BGM frequency, was downloaded weekly from the study-provided glucometer during the 14 weeks of treatment. The mean frequency of BGM per day and the number of days per week with BGM ≥6 times per day during the first and last week of treatment were used in analyses. Teen adherence to diabetes treatment recommendations was assessed pre- and posttreatment with the Self-Care Inventory (SCI), a 15-item self- and parent-report measure that includes items focusing on BGM, insulin and food regulation, exercise, and emergency precautions (Lewin et al., 2009). Scores on the 15 items are averaged and converted to a 0- to 100-point scale (Parent α = .72; Teen α = .80). In addition, glycemic control was assessed using the glycated hemoglobin test (HbA1c), which measures the non-enzymatic glycation status of hemoglobin over the previous 2–3 months, with about half of the value reflecting past month blood glucose. HbA1c was obtained from medical records pretreatment, at the end of treatment, and 3 months posttreatment.
Analyses
Descriptive statistics regarding treatment completion are reported as an index of teen and parent acceptance of MI/CBT + CM. Linear repeated measures mixed models with random intercepts and fixed treatment effects (operationalized as the effect of time) were used to test improvement in BGM, SCI scores, and HbA1c. The Tukey method was used to adjust p-values for HbA1c due to multiple comparisons. A repeated measures effect size is reported for each comparison (drm =
trm/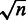 ), where trm was the t-statistic comparing least square means from the mixed model and n was the pairwise n across time points for each measure (Rosenthal, 1991). Pre–post pairwise ns were as follows: BGM (n = 15), teen SCI (n = 15), parent SCI (n = 16), HbA1c (n = 17). In addition, pairwise ns for comparisons for HbA1c at 3 months were n = 14.
), where trm was the t-statistic comparing least square means from the mixed model and n was the pairwise n across time points for each measure (Rosenthal, 1991). Pre–post pairwise ns were as follows: BGM (n = 15), teen SCI (n = 15), parent SCI (n = 16), HbA1c (n = 17). In addition, pairwise ns for comparisons for HbA1c at 3 months were n = 14.
Results
Twelve of 17 teens completed the 14-week program; mean attendance was 12.2 weeks (SD = 3.4), with >80% of teens attending >75% of weeks. Families were allowed up to 18 weeks to complete the 14 counseling sessions to allow for therapist- or client-missed sessions owing to illness, vacations, or other reasons. On average, families completed the 14 sessions in 15.7 weeks (SD = 1.3, range = 14–18). Parents made calls on average 6.3 (SD = 0.3) days per week over the 14 weeks of treatment. Teens earned an average of $389 (SD = $213) of $590 possible, and parents earned an average of $352 (SD = $162) of $470 possible.
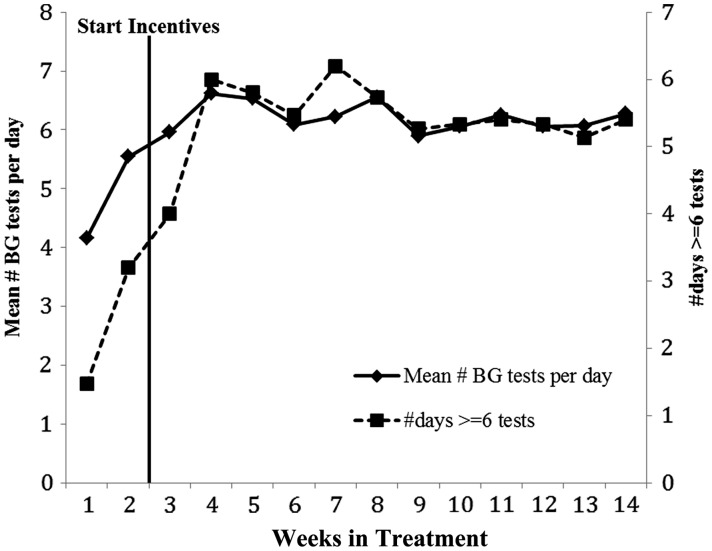
The mean number of BGM tests per week and mean number of days per week with ≥6 tests per day increased significantly over the course of treatment (see Figure 1). Teens increased their monitoring frequency from week 1 of treatment [Least Square Mean (LSM) (95% Confidence Interval (95% CI)) = 3.92 (2.90–4.94)] to week 14 [LSM (95% CI) = 6.20 (5.13–7.28), t(14) = −3.87, p = 0.002, drm = −1.00]. Number of days per week with 6 or more tests increased from LSM (95% CI) = 1.35 (0.30–2.40) to LSM (95% CI) = 5.40 (4.28–6.52), t(14) = −5.63, p < .0001, drm = −1.45. Effects remained significant when the week 1 values were imputed for the two participants with missing week 14 values. In addition, parent and teen reports on the SCI showed significant improvements from pre- to posttreatment: SCI Parent LSM (95% CI) Pre = 51.47 (43.66–59.28); Post = 61.90 (54.02–69.79), t(15) = −4.83, p = 0.0002, drm = −1.21; SCI Teen LSM (95% CI) Pre = 56.51 (50.00–63.02); Post = 61.90 (55.18–68.61), t(14) = −2.26, p = 0.041, drm = −0.58.
Figure 1.
Mean number of blood glucose monitoring (BGM) times per day and mean number of days per week with ≥6 times per day across the 14 treatment weeks. Note: N = 15; one participant moved away unexpectedly in week 9 of treatment and did not have access to a computer. This participant’s 9 week data were used in calculating the means displayed for weeks 10–14.
HbA1c was significantly lower at the end of treatment compared with pretreatment [Pre LSM (95% CI) = 11.62% (10.75%–12.48%), Post = 9.11% (8.25%–9.98%); t(29) = 5.15, adjusted p < 0.0001, drm = 1.25]. Note that four pretreatment HbA1c values were estimated conservatively at 14% because the point-of-care testing method has a maximum value of “>14%”. Thus the true improvement in HbA1c was likely underestimated. Although mean HbA1c was higher at the 3-month follow-up than at the end of treatment, this difference was not significant [3-month LSM (95% CI) = 9.77% (8.83%–10.71%), t(29) = −1.27, adjusted p = 0.42, drm = −0.34]. The mean 3-month HbA1c, however, remained significantly lower than the pretreatment mean [t(29) = 3.56, adjusted p = 0.004, drm = 0.95].
Discussion
This pilot study of MI/CBT + CM for teens with poor control of their type 1 diabetes showed significant improvements in frequency of daily BGM. These results support the potential efficacy of this adapted intervention, consistent with other adaptations of substance abuse treatments. Potential common mechanisms for these behavioral interventions seeking to change adolescent health behavior include increasing motivation, using skills training and principles of reinforcement to increase compliance (e.g., drug abstinence or adherence to testing), and parental monitoring. It is important to note that the upward trend in BGM began prior to the onset of incentives in week 3, and that we cannot determine whether this BGM increase would have continued or been sustained without the contingent incentives. However, teens were informed at the intake, in the Consent form, and in sessions 1 and 2 that the goal of the program was to increase BGM frequency to ≥6 times per day on ≥6 days per week. Teens were encouraged to increase their BGM frequency immediately to increase their chances of earning clinic and home incentives for meeting the monitoring goal in week 3.
We also found significant improvements in parent and teen reports of the teen’s self-care, and observed significant improvements in HbA1c that were maintained 3 months after the end of treatment. Pre- to posttreatment changes in HbA1c were large, decreasing from 11.6% to 9.1%, with the largest changes generally observed among teens with initial HbA1c > 9.5%. However, those with HbA1c < 9.5% at the start of treatment did show robust gains in BGM, which may have long-term benefits if those gains are maintained. Such improvement in glycemic control has high clinical significance because every 10% reduction in HbA1c has been shown to result in 25% to 35% reductions in sustained retinopathy progression and microalbuminuria, and clinical neuropathy (The Diabetes Control and Complications Trial Research Group, 1996). We hypothesize that increased BGM is the mechanism by which self-care and HbA1c improve. Despite the large observed decreases in HbA1c that persisted 3 months posttreatment, most teens still had HbA1c > 8% at the end of the 14-week treatment program (15/17 teens). This finding suggests that additional intervention components and/or longer duration intervention may be necessary to further increase and maintain high levels of BGM and other self-care behaviors that could further reduce HbA1c to the American Diabetes Association (ADA) target (7.5%).
The outcomes achieved with this multicomponent treatment compare favorably with those reported in prior behavioral trials targeting adherence among teens with type 1 diabetes that generally observed smaller changes in BGM and HbA1c (Channon et al., 2007; Ellis et al., 2007b; Franklin et al., 2006; Nansel et al., 2007; Salamon et al., 2009; Wysocki et al., 2008). For example, a study using the same MI used in this study (Channon et al., 2007) observed reductions in HbA1c from 9.3% to 8.7% 24 months after intake, with no change in the comparison condition. Another trial compared an intensive counseling intervention (an average of 48 sessions delivered over 6 months) with remaining in usual medical care (Ellis et al., 2007b). BGM improved significantly among treated teens from 1.8 times per day pretreatment to 2.6 times per day post intervention. HbA1c in the treatment group improved significantly (11.4% to 10.7%), but showed no change among teens in usual care. However, six months after the end of the intervention, there was no significant difference in HbA1c between treated and usual care teens. Despite the limited change in HbA1c, the intervention condition showed significant reductions in hospitalization for ketoacidosis (Ellis et al., 2008). On average, teens in the usual care condition were hospitalized 1.28 times over 24 months, compared with 0.67 times in the intervention condition, suggesting that intervention costs can be at least partially offset by short-term reductions in medical costs among these high-risk teens. If the larger changes in BGM and HbA1c observed in the present study are replicated and can be maintained over time, this multicomponent intervention would have high potential impact on the health and health care costs of teens with type 1 diabetes.
Cost effectiveness is an important issue that will impact dissemination for the type of intervention tested in this study. A strong economic argument for using financial incentives to motivate teens with poorly controlled diabetes to achieve better control could be made if potential cost savings from good control offset the price of the incentives. Cost saving mechanisms can include reduced current and future health care costs and increased parent and future teen productivity. Sharing some of these savings up-front with patients to help motivate better glycemic control (i.e., providing incentives) could be a prudent method to achieve cost-effective improvements in health. Of note, the Affordable Care Act raises the percentage of employer premiums that can be used for outcome-based wellness incentives from 20% to 30% of total premiums and may lead to ongoing use of incentive-based programs (Volpp, Asch, Galvin, & Loewenstein, 2011). Public cost savings are also possible by applying incentives for healthy behaviors in the Medicaid system (American College of Physicians, 2010). However, little research is available to guide effective use of reimbursements as incentives (Marteau, Ashcroft, & Oliver, 2009), and it will be important for future studies to address that gap.
Limitations
This study involved a single condition without a control group. Thus, it is not possible to draw conclusions about the efficacy of this intervention, or to test potential moderators such as age, gender, ethnicity, family status, or initial HbA1c or mechanisms or secondary outcomes such as parenting, family conflict, or barriers to self-care. In addition, HbA1c values should be obtained using the same measure. However, because we relied on clinical testing for this pilot study, that was not possible for all tests (for 4/48 tests, a laboratory blood test was used). Also, the intervention is intensive, requiring weekly clinic visits over an extended period of time. Finally, HbA1c did not reach the ADA target, and it will be important to demonstrate maintenance of positive effects over time.
Conclusion
Results of this pilot study suggest that combining MI/CBT and contingency management may lead to large improvements in BGM as well as in HbA1c. The intervention focused on the daily frequency of BGM as the primary target to improve glycemic control. We hypothesize that increased monitoring frequency is the mechanism by which the intervention led to improvements in diabetes self-care and glycemic control. Frequent monitoring likely provides teens, parents, and the medical team with information that can help teens improve their daily self-care of diabetes. Improvements in family conflict and parenting are additional potential mechanisms addressed in this intervention. We are following up on these promising results in a randomized trial that includes modifications designed to further enhance efficacy by targeting working memory, increasing the duration of treatment to 6 months, fading the use of incentives over time, and providing the counseling in the family’s home over the internet.
Funding
This work was supported by grants from the National Institutes of Health (grant numbers DA022981, HD076602, UL1RR029884) and from the Sturgis Foundation.
Conflicts of interest: None declared.
References
- Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialists and generalist clinicians on glycaemic control and on psychological status. Patient Education and Counseling. 2009;75:25–36. doi: 10.1016/j.pec.2008.08.026. [DOI] [PubMed] [Google Scholar]
- American College of Physicians. Ethical considerations for the use of patient incentives to promote personal responsibility for health: West Virginia Medicaid and Beyond. Philadelphia, PA: American College of Physicians; 2010. [Google Scholar]
- Anderson B J, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- Anderson B J, Holmbeck G, Iannotti R J, McKay S V, Lochrie A, Volkening L K, Laffel L. Dyadic measures of the parent-child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Families, Systems, and Health. 2009;27:141–152. doi: 10.1037/a0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M J, Armstrong D S, Dettling L, Hardy A, Caterino K, Barrie S. Partners in lowering cholesterol: Comparison of a multidisciplinary educational program, monetary incentives, or usual care in the treatment of dyslipidemia identified among employees. Journal of Occupational and Environmental Medicine. 2006;48:675–681. doi: 10.1097/01.jom.0000205997.18143.6c. [DOI] [PubMed] [Google Scholar]
- Budney A J, Moore B A, Rocha H L, Higgins S T. Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting and Clinical Psychology. 2006;74:307–316. doi: 10.1037/0022-006X.4.2.307. [DOI] [PubMed] [Google Scholar]
- Carroll A E, DiMeglio L A, Stein S, Marrero D G. Contracting and monitoring relationships for adolescents with type 1 diabetes: A pilot study. Diabetes Technology and Therapeutics. 2011;13:543–549. doi: 10.1089/dia.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: General information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. 2008. [Google Scholar]
- Channon S J, Huws-Thomas M V, Gregory J W, Rollnick S. Motivational interviewing with teenagers with diabetes. Clinical Child Psychology and Psychiatry. 2005;10:43–51. [Google Scholar]
- Channon S J, Huws-Thomas M V, Rollnick S, Hood K, Cannings-John R L, Rogers C, Gregory J W. A multicenter randomized controlled trial of motivational interviewing in teenagers with diabetes. Diabetes Care. 2007;30:1390–1395. doi: 10.2337/dc06-2260. [DOI] [PubMed] [Google Scholar]
- Diabetes Care. Executive summary: Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S4–S10. doi: 10.2337/dc10-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Journal of Pediatrics. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: The perspective of the diabetes control and complications trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- Ellis D A, Frey M A, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: A randomized controlled trial. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- Ellis D A, Naar-King S, Templin T, Frey M, Cunningham P, Sheidow A, Cakan N, Idalski A. Multisystemic therapy for adolescents with poorly controlled type 1 diabetes: Reduced diabetic ketoacidosis admissions and related costs over 24 months. Diabetes Care. 2008;31:1746–1747. doi: 10.2337/dc07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D A, Podolski C L, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007a;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Ellis D A, Templin T, Naar-King S, Frey M A, Cunningham P B, Podolski C L, Cakan N. Multisystemic therapy for adolescents with poorly controlled type I diabetes: Stability of treatment effects in a randomized controlled trial. Journal of Consulting and Clinical Psychology. 2007b;75:168–174. doi: 10.1037/0022-006X.75.1.168. [DOI] [PubMed] [Google Scholar]
- Eyberg S M, Nelson M M, Boggs S R. Evidence-based psychosocial treatments for children and adolescents with disruptive behavior. Journal of Clinical Child and Adolescent Psychology. 2008;37:215–237. doi: 10.1080/15374410701820117. [DOI] [PubMed] [Google Scholar]
- Franklin V L, Waller A, Pagliari C, Greene S A. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabetic Medicine. 2006;23:1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Torgenson D J. Should we pay the patient: Review of financial incentives to enhance patient compliance. British Medical Journal. 1997;315:703–707. doi: 10.1136/bmj.315.7110.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle S M, Crimmins N A, Hood K K. Blood glucose monitoring and glycemic control in adolescents with type 1 diabetes: Meter downloads versus self-report. Pediatric Diabetes. 2011;12:560–566. doi: 10.1111/j.1399-5448.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- Helgeson V S, Honcharuk E, Becker D, Escobar O, Siminerio L. A focus on blood glucose monitoring: Relation to glycemic control and determinants of frequency. Pediatric Diabetes. 2011;12:25–30. doi: 10.1111/j.1399-5448.2010.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S T, Silverman K, Heil S H. Contingency management in substance abuse treatment. vol. xviii. New York, NY: The Guilford Press; 2008. [Google Scholar]
- Hollingshead A B. Four Factor Index of social status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- Horton D, Berg C A, Butner J, Wiebe D J. The role of parental monitoring in metabolic control: Effect on adherence and externalizing behaviors during adolescence. Journal of Pediatric Psychology. 2009;34:1008–1018. doi: 10.1093/jpepsy/jsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail K, Thomas S, Maissi E, Chalder T, Schmidt U, Bartlett J, Patel A, Dickens C M, Creed F, Treasure J. Motivational enhancement therapy with and without cognitive behaviour therapy to treat type 1 diabetes: A randomized trial. Annual of Internal Medicine. 2008;149:708–719. doi: 10.7326/0003-4819-149-10-200811180-00005. [DOI] [PubMed] [Google Scholar]
- Lewin A B, LaGreca A M, Geffken G R, Williams L B, Duke D C, Storch E A, Silverstein J H. Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI) Journal of Pediatric Psychology. 2009;34:999–1007. doi: 10.1093/jpepsy/jsp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J A, Jahnle E C, Richardson D M, Loewenstein G, Volpp K G. Peer mentoring and financial incentives to improve glucose control in African American veterans: A randomized trial. Ann Intern Med. 2012;156:416–424. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau T M, Ashcroft R E, Oliver A. Using financial incentives to achieve healthy behaviour. British Medical Journal. 2009;338:b1415. doi: 10.1136/bmj.b1415. [DOI] [PubMed] [Google Scholar]
- Menzin J, Korn J R, Cohen J, Lobo F, Zhang B, Friedman M, Neumann P J. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. Journal of Managed Care Pharmacy. 2010;16:264–275. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nansel T R, Iannotti R J, Simons-Morton B G, Cox C, Plotnick L P, Clark L M, Zeitzoff L. Diabetes personal trainer outcomes: Short-term and 1-year outcomes of a diabetes personal trainer intervention among youth with type 1 diabetes. Diabetes Care. 2007;30: 2471–2477. doi: 10.2337/dc06-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff B R, Dallery J. Internet-based contingency management to improve adherence with blood glucose testing recommendations for teens diagnosed with type 1 diabetes. Journal of Applied Behavior Analysis. 2010;43:487–491. doi: 10.1901/jaba.2010.43-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Mason P, Butler C. Health behavior change: A guide for practitioners. London: Churchill Livingstone; 1999. [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. 1991. (Revised Edition ed.). Newbury Park, CA: Sage. [Google Scholar]
- Salamon K S, Hains A A, Fleischman K M, Davies W H, Kichler J. Improving adherence in social situations for adolescents with type 1 diabetes mellitus (T1DM): A pilot study. Primary Care Diabetes. 2009;4:47–55. doi: 10.1016/j.pcd.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schafer L C, Glasgow R E, McCaul K D. Increasing the adherence of diabetic adolescents. Journal of Behavioral Medicine. 1982;5:353–362. doi: 10.1007/BF00846162. [DOI] [PubMed] [Google Scholar]
- Secrest A M, Becker D J, Kelsey S F, LaPorte R E, Orchard T J. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: The Allegheny county type 1 diabetes registry. Diabetes Care. 2010;33:2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister L A, Clark N. doi: 10.2337/diacare.28.1.186. & American Diabetes Association (2005). Care of children and adolescents with type 1 diabetes. Diabetes Care, 28, 186–212. [DOI] [PubMed] [Google Scholar]
- Stanger C, Budney A J, Kamon J L, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and Alcohol Dependence. 2009;105:240–247. doi: 10.1016/j.drugalcdep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger C, Ryan S R, Fu H, Budney A J. Parent training plus contingency management for substance abusing families: A Complier Average Causal Effects (CACE) analysis. Drug and Alcohol Dependence. 2011;118:119–126. doi: 10.1016/j.drugalcdep.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehik K, Hamman R F, Lezotte D, Norris J M, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- Volpp K G, Asch D A, Galvin R, Loewenstein G. Redesigning employee health incentives-lessons form behavioral economics. New England Journal of Medicine. 2011;365:388–390. doi: 10.1056/NEJMp1105966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp K G, John L K, Troxel A B, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: A randomized trial. Journal of the American Medical Association. 2008a;300:2631–2637. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp K G, Loewenstein G, Troxel A B, Doshi J, Price M, Laskin M, Kimmel S E. A test of financial incentives to improve warfarin adherence. BMC Health Services Research. 2008b;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb C, Scudder M, Kaminer Y, Kadden R. The Motivational Enhancement Therapy and Cognitive Behavioral Therapy for Adolescent Cannabis Users. vol. 2. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2001. [Google Scholar]
- The Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. Journal of the American Medical Association. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris M A, Buckloh L M, Mertlich D, Lochrie A S, Taylor A, Sadler M, White N H. Randomized, controlled trial of behavioral family systems therapy for diabetes: Maintenance and generalization of effects on parent-adolescent communication. Behavior Therapy. 2008;39:33–46. doi: 10.1016/j.beth.2007.04.001. [DOI] [PubMed] [Google Scholar]