Abstract
The extracellular matrix has a crucial role in determining the spatial orientation of epithelial polarity and the formation of lumens in glandular tissues, however the underlying mechanisms remain elusive. By using Cre-Lox deletion we show that β1-integrins are required for normal mammary gland morphogenesis and lumen formation, both in vivo and in a 3D primary culture model where epithelial cells directly contact basement membrane. Downstream of basement membrane-β1-integrins, Rac1 is not involved, however ILK is needed to polarize microtubule plus ends at the basolateral membrane and disrupting each of these components prevents lumen formation. The integrin-microtubule axis is necessary for the endocytic removal of apical proteins from the basement membrane-cell interface and for internal Golgi positioning. We propose that this integrin-signalling network controls the delivery of apical components to the correct surface and thereby governs the orientation of polarity and development of lumens.
Keywords: Mammary, breast, integrin, β1-integrin, integrin linked kinase, microtubules, polarity, acinus, lumen
Cell polarity is a fundamental organizing principle in metazoa that is necessary for cell division, differentiation and morphogenesis. Polarization of epithelia is implicit in the development of lumens, which are essential for glandular tissues to carry out their normal functions, but polarity is disrupted in diseases such as cancer. Understanding mechanisms of polarity will therefore improve future prospects for therapy.
The establishment of epithelial polarity involves a coordinated series of events by several protein complexes, leading to the asymmetric segregation of plasma membranes into apical and basolateral domains. Polarity is accompanied and maintained by asymmetric distribution of intracellular organelles, together with dynamic cytoskeleton and membrane trafficking1-4. However, the mechanisms defining the spatial orientation of polarity are not well understood.
Cell interactions with the extracellular matrix (ECM) contribute to the organisation of the basolateral surface, which creates an apical face on the opposite membrane5-7. Although the formation of apical domains are relatively well understood, little is known about how cells respond to an ECM in order to locate their apical membranes correctly. We hypothesised that an intracellular network regulated by cell-ECM interactions determines the orientation of epithelial polarity and therefore lumen formation.
Signals from the ECM are transmitted through integrins, which connect to the cytoskeleton, and to adaptor proteins within adhesion complexes8, 9. Integrins control cell shape, migration, proliferation and differentiation, and also have a role in the establishment of polarity. For example, β1 integrin ablation resulted in a loss of polarity leading to defective arterial lumen formation and asymmetric cell division in skin epithelia10 11. In kidney epithelia, function-perturbing antibodies revealed that β1-integrins regulate basement membrane (BM) assembly, which is necessary for polarity12. However it is not known how BM-integrins subsequently signal inside the cell to establish the apico-basal polarity axis.
The mammary gland epithelium is organized into a branched network of polarized ducts and acini. Lactating acini are composed of a monolayer of luminal epithelial cells surrounded by a sparse network of myoepithelia, and jointly subtended by a BM. The luminal cells are polarized, with the apical secretory face adjacent to a lumen and the basolateral surface in contact with the BM. This asymmetric orientation of apico-basal membranes ensures that the cells secrete their milk products into the lumen.
We previously showed that β1-integrins determine the differentiated function of mammary glands13. We now demonstrate that β1-integrins also control the orientation of epithelial polarity and thereby the formation of lumens in differentiated acini. Our results reveal the mechanism by which β1-integrins regulate tissue polarity and lumen formation downstream of cell interactions with the BM12.
Results
β1-integrins are required for glandular lumen formation downstream of cell-BM interactions
The role of cell-matrix interactions in lumen formation have been studied in kidney epithelia cultured in collagen I gels. Here, function-blocking anti-β1-integrin antibodies prevent the formation of polarized cysts. This is because integrin signalling via IRSp53-Rac1 induces the cells to deposit and organize a BM around the cyst periphery, which then contributes to lumen formation12, 14, 15. However the intracellular mechanism for lumen formation downstream of BM is unknown.
To distinguish the intracellular function of integrins from their role in BM assembly, we used primary luminal mammary epithelial cells (MECs) cultured within an exogenous BM-matrix, in which the cells form lactational acini. We generated β1fx/fx;CreER™ mice, which permitted β1-integrin gene deletion in MECs using 4-hydroxy-tamoxifen (4OHT)16. Immunofluorescence staining showed that untreated wild type (WT) acini develop lumens with apical f-actin, lateral E-cadherin, and basolateral β1-integrins (Fig. 1a). Treatment with 4OHT at the time of plating cells caused β1-integrin gene deletion (β1-KO), and the acini were unable to develop lumens (Fig. 1a,c). Lumen formation in MECs from non-transgenic ICR mice was unaffected by 4OHT (Fig. 1b,c). Thus, β1-integrins are required for MECs cultured on BM to form hollow acini.
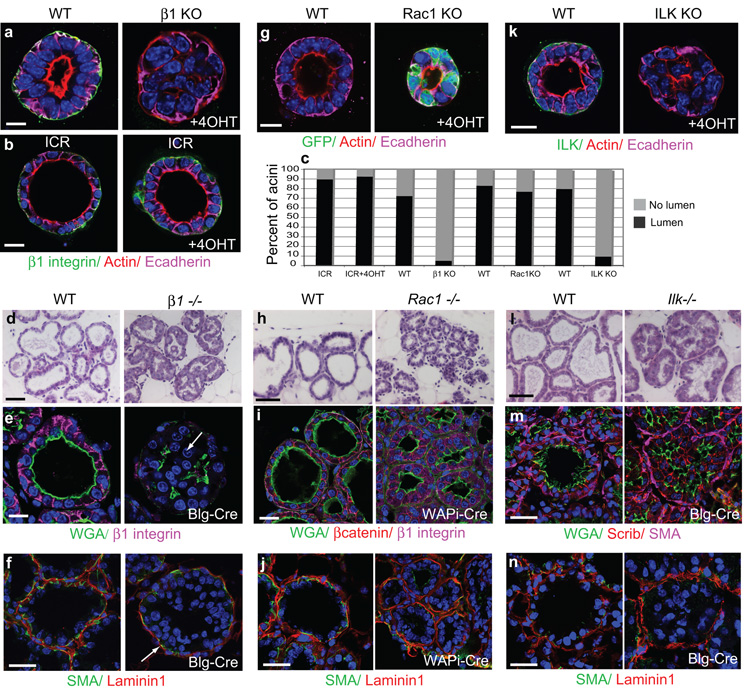
Figure 1. Deletion of β1-integrins or ILK disrupts acinar morphogenesis.
(a) Immunofluorescence staining of MECs isolated from β1fx/fx;CreER™ mice and cultured in 3D on BM-matrix. 4OHT added at the time of plating cells, caused β1-integrin deletion and absence of lumens. Bar: 10μm.
(b) No lumen disruption in acini from non-transgenic ICR mice, treated with 4OHT. Bar: 10μm.
(c) Quantification of ICR, β1-KO, Rac1-KO, ILK-KO acini with lumens, n=100 for each condition, 3 independent experiments.
(d) H+E staining of lactation day 2 (L2) mammary glands isolated from β1−/− mice (β1fx/fx;Blg- Cre) and their WT littermates (β1fx/fx ). Bar: 40 μm.
(e) L2 WT and β1−/− glands, immuno-stained for β1-integrin, and WGA to detect apical surfaces and lumens. Note that cells protrude into the luminal space of β1−/− glands. Bar: 15 μm.
(g) Immunofluorescence staining of MECs isolated from Rac1fx/fx:LSLYFP:CreER™ mice and cultured in 3D on BM-matrix. 4OHT added at the time of plating cells, caused Rac1 deletion but no lumen loss. Bar: 10 μm.
(h) H+E staining of L2 mammary glands isolated from Rac1−/− mice (Rac1fx/fx:LSLYFP:WAPi- Cre) and their WT littermates (Rac1fx/fx ). Bar: 40μm
(i) L2 WT and Rac1−/− glands, immuno-stained for β1-integrin, βcatenin and WGA-488 to detect basolateral and apical surfaces, respectfully. Bar: 30μm.
(k) Immunofluorescence staining of MECs from Ilkfx/fx:CreER™ mice and cultured in 3D on BM-matrix. 4OHT added at the time of plating cells, caused ILK deletion and lumen loss. Bar: 10μm.
(l) H+E staining of L8 mammary glands from Ilk−/− mice (Ilkfx/fx:Blg-Cre) and their WT littermates (Ilkfx/fx ). Note the activation of the Blg-Cre promotor is asynchronous in vivo, thus some lumens may already exist before the Ilk gene was ablated. Bar: 40μm.
(m) L8 WT and Ilk−/− glands, immuno-stained for Scribble, Smooth muscle actin (SMA) to detect myoepithelia, and WGA to detect apical surfaces and lumens. Bar: 20μm.
(f, j, n) β1−/−, Rac1−/− and Ilk−/− glands respectively, stained for SMA and Laminin1. Note Laminin1 assembly around the acini of all transgenic glands. Bar: 20μm.
In this and subsequent figures: a) WT refers to in vivo acini from β1/ ILK/ Rac1fx/fx;Cre-ve mice or cultured acini from β1/ ILK/ Rac1fx/fx;CreER™ MECs with no 4OHT treatment; b) in IF studies, nuclei were detected with Hoechst; c) confocal images of cultured 3D acini were taken through their centres.
See also Supplementary Figs. 1, 2.
To confirm the role of β1-integrins in acinar morphogenesis, we analysed mammary glands from β1fx/fx;Blg-Cre mice in vivo (β1−/−). Cre recombinase driven by both the Blg and Wap promoters is activated in mid-pregnancy, specifically in luminal epithelial cells but not in myoepithelia13. By employing this strategy, myoepithelial cells were still able to make and deposit a BM around the acini, allowing integrin contribution downstream of BM assembly to be analysed (Fig. 1f). At lactation day 2 (L2), β1-integrin gene deletion resulted in defective acinar morphogenesis with epithelial cells filling the luminal space whilst WT acini (β1fx/fx, with no Cre) displayed a single central lumen (Fig. 1d, e).
β1-integrins are thus required for normal mammary lumen formation, both in vivo and in a primary culture model downstream of a BM.
Integrin mediated lumen formation requires ILK but not Rac1
To determine whether Rac1 is required to establish glandular lumens, we generated Rac1fx/fx;LSLYFP;CreER™ mice. In MECs from these mice, 4OHT specifically deleted Rac1 and the cells expressed YFP (Supplementary Fig. 1a-c). Unlike β1-integrin, Rac1 deletion did not prevent mammary acini from developing lumens (Fig. 1c,g). We confirmed this by generating Rac1fx/fx:LSLYFP:WAPiCre mice to delete the Rac1 gene in vivo (Supplementary Fig. 1d-g). Lactating mammary acini were still able to form polarized lumens (Fig. 1h,i,j). These data indicate that Rac1 is not required for lumen formation downstream of a BM-integrin axis and that integrins establish intracellular polarity via a distinct mechanism to the molecular pathway involved in BM assembly.
To identify proximal integrin signalling components controlling lumen formation, we analysed two focal adhesion proteins, integrin-linked kinase (ILK) and focal adhesion kinase (FAK). We reasoned that these proteins might be involved because deletion of β1-integrins in MECs resulted in displacement of ILK from the basal cell surface and dephosphorylation of FAKY397 (Supplementary Fig. 2a). We generated Ilkfx/fx:CreER™ mice and analysed acini after 4OHT treatment to remove the ILK gene (Supplementary Fig. 1h-k). ILK deletion resulted in 90% of acini containing filled lumens (Fig. 1c,k). In contrast, FAK deletion in MECs isolated from FAKfx/fx mice did not inhibit lumen formation (not shown).
To determine whether these integrin effectors control lumen formation in vivo, we generated FAKfx/fx;Blg-Cre and Ilkfx/fx;Blg-Cre mice (Supplementary Fig. 1l,m). ILK deletion resulted in abnormal morphogenesis similar to the β1−/− phenotype, with cells filling the luminal space of acini (Fig. 1l-n). In contrast, lactating FAK-null glands showed no morphogenesis defects17.
These data show that downstream of cell-BM interactions, ILK has a key role in linking integrins with MEC lumen formation, but FAK and Rac1 do not.
Mechanisms not involved in integrin-mediated lumen formation
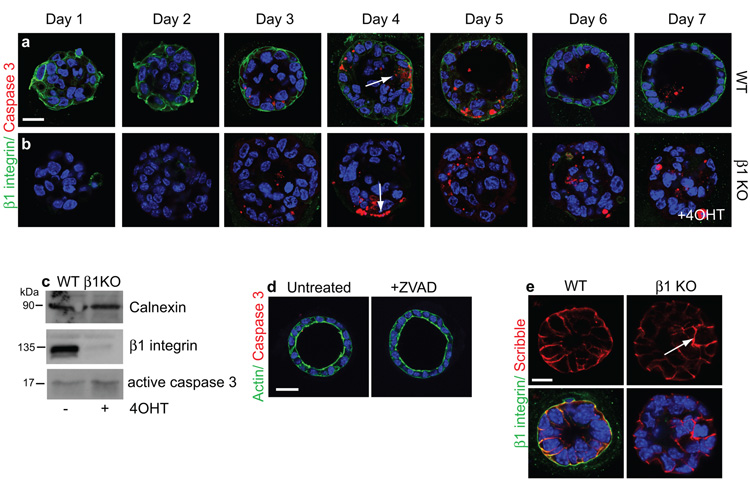
Integrin signalling is instructive in controlling lumen formation and utilizes a distinct pathway to the Rac-dependent pathway for BM assembly in MDCK cells. To identify possible mechanisms, we analysed whether apoptosis of internal cells caused lumen formation18. In WT MECs, low levels of apoptosis were observed over a time-course of acinar morphogenesis both in the internal cells and those contacting the BM (Fig. 2a, b arrows). Lumens developed in the presence of the caspase inhibitor zVAD (Fig. 2d). Thus, as with some other epithelial models, apoptosis is not the mechanism for lumen formation in primary MECs19-22. Moreover, β1-integrin deletion did not alter apoptosis (Fig. 2c).
Figure 2. Luminal filling is not due to a lack of apoptosis.
(a, b) Time course of lumen development over 7 days in WT and β1-KO acini, treated with 4OHT at time of plating. Cells expressing caspase 3 (arrows) were not restricted to the centre of acini. Bar: 10μm.
(c) Immunoblotting shows loss of β1-integrin and a small increase in caspase 3 in 4OHT-treated cells.
(d) zVAD treatment of ICR MECs did not prevent lumen formation. Bar: 10μm.
(e) Scribble staining revealed intact intercellular adhesions in WT and β1-KO acini. Arrows indicate Scribble in internal cells. Bar: 10μm.
See also Supplementary Fig. 3, 8.
We investigated the possibility that β1-integrin ablation disrupted tissue organization by affecting adherens junctions. Scribble and E-cadherin localized to cell-cell junctions of internal cells in β1-KO MEC acini and β1−/− glands (Fig 1a, Fig. 2e, Supplementary Fig. 3,), as well as ILK-deleted glands and acini (Fig. 1k, m). These findings are consistent with other cell types, e.g. E-cadherin localizes normally in β1−/− keratinocytes23.
β1-integrin-null acini are therefore not filled with cells because of defective apoptosis or altered intercellular adhesions.
β1 integrins orient epithelial polarity via ILK
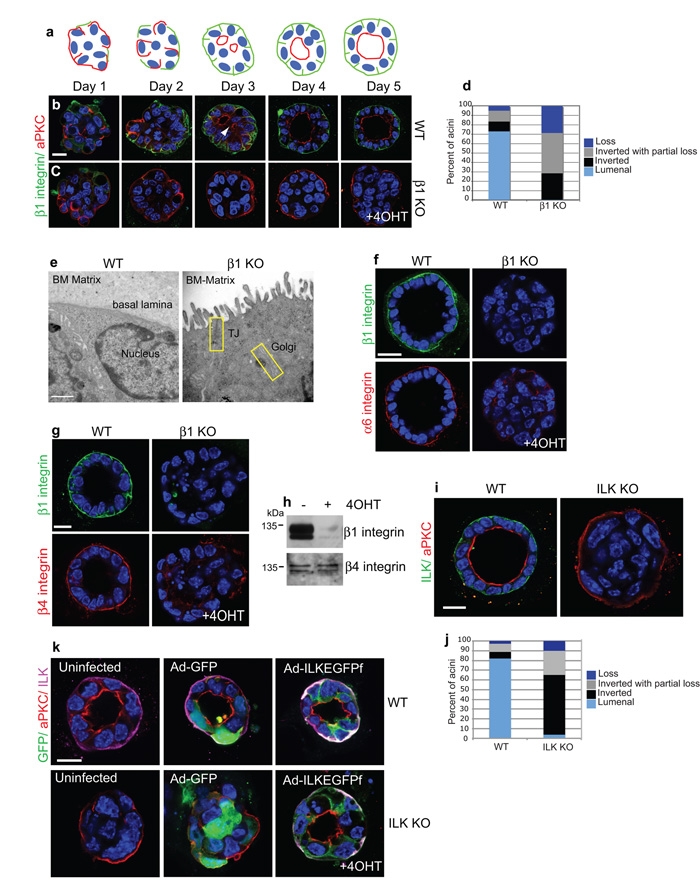
Lumen formation requires apical polarity which is typically controlled by the PAR, Scribble and Crumbs complexes24. We asked whether integrins are needed to establish apico-basal polarity. A time-course of MEC lumen development revealed that 1 day after plating, acini lacked lumens, and aPKC was unpolarized (Fig. 3a,b,d). During β1-integrin engagement with BM, aPKC was displaced away from the basolateral membrane and multiple aPKC-lined lumens developed, progressively forming a single lumen. However in the absence of β1-integrins, aPKC, ZO-1 and PAR3 did not relocalize but instead were inverted at the outer membrane (Fig. 3c,d Supplementary Fig. 4a, Movies S1, S2). Electron microscopy revealed a proper apical membrane containing microvilli and tight junctions bordering the BM in ®1-KO acini (Fig. 3e, Supplementary Fig. 4b). Transferrin receptors were mislocalised, indicating lost basolateral polarity, and the ability of MECs to internalize transferrin-488 from the media was diminished (Supplementary Fig. 4c,d). These results suggest that apical domain assembly is not integrin-independent. Rather, ®1-integrins are required to form the epithelial basolateral surface and to separate it topologically from the apical domain.
Figure 3. Apical polarity is inverted in β-integrin and ILK-KO acini.
(a,b,c) Time-course of polarity and lumen development in (b) WT and (c) β1-KO acini. Staining for aPKC shows apical polarity inversion upon integrin deletion. (a) The schematic shows redistribution of β1-integrin (green) and aPKC (red) in WT controls. Note that mammary acini become depolarized following the enzymatic digestion required to isolate them from tissue. Bar: 17 μm.
(d) Histogram represents (%) of acini with either luminal or inverted polarity. Loss indicates acini with no apical polarity.
(e) Electron micrographs of the outer edges of 3D acini cultured on BM-matrix. Note that β1-integrin deletion resulted in microvilli and tight junctions on the periphery next to the ECM. Bar: 500nm.
(f) β1-integrin deletion in acini results in redistribution of α6 integrin from the basal cell surface. Scale bar: 10μm
(g) WT and β1-KO acini stained for β1- and β4-integrin. β4-integrin redistributed from the basal domain following β1-integrin deletion. Bar: 10μm.
(h) β4-integrin levels were not affected after β1-integrin deletion.
(i) WT and ILK-KO acini from Ilkfx/fx:CreER™ mice. The inverted polarity phenocopied that of β1-null acini. Bar: 10μm.
(j) Histogram represents (%) of Ilkfx/fx:CreER™ acini with either luminal or inverted polarity. Loss indicates acini with no apical polarity.
(k) WT or ILK-KO MECs infected with Ad-GFP or Ad-ILKEGFPf and plated onto 3D BM-matrix. Only the Ad-ILKEGFPf expression rescued polarity and lumens (89% of acini). Bar: 10μm.
See also Supplementary Fig. 4, 8, Movies S1 and S2
®1-integrin deletion caused polarity inversion despite culturing acini on a BM suggesting that other laminin-binding ®-integrins were unable to compensate for β1-integrin loss. Analysis of the laminin-binding α6- and ®4-integrins showed they were juxtaposed to the BM in WT acini, but redistributed from the basal domain in ®1-null acini (Fig 3f-h). In previous studies, genetic deletion of α6- and ®4-integrins had no observable effect on mammary acinar morphogenesis in vivo25.
We investigated whether integrin polarity signals require ILK. 90% of acini lacking ILK had inverted apical polarity and filled lumens (Fig. 3i,j). Moreover, adenoviral expression of ILKEGFPf reverted polarity and lumens in 89% of ILK-KO acini (Fig. 3k).
β1-integrins therefore orient apical polarity away from the cell-BM interface by establishing BM-cell interactions and by controlling intracellular signalling via ILK. This determines the location of the apical domain and thereby the position of the lumen.
β1-integrins and ILK control internal organelle polarity
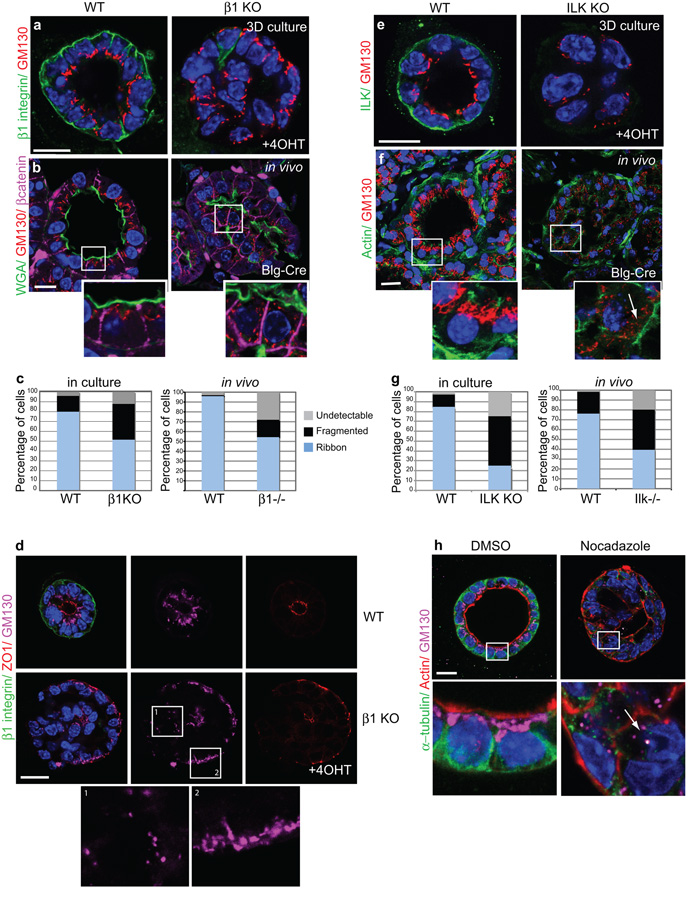
Trafficking of newly synthesized proteins from the trans Golgi network (TGN) is essential for cell polarization26. We investigated whether reduced β1-integrin signalling alters Golgi positioning. WT acini contained sub-apical Golgi (GM130) (80% of cells, Fig. 4a). However,®1-integrin deletion resulted in either redistribution of Golgi to the periphery (51.1%) or Golgi ribbon fragmentation (35.9%) (Fig. 4c, left). Fragmentation of Golgi correlated with a loss of apical membrane polarity (Fig 4d). In vivo, WT acini displayed apical Golgi (95% of cells), which were scattered or undetectable in β1−/− glands (Fig. 4b,c, right). ILK deletion reproduced the effects of β1-integrin deletion (Fig. 4e-g).
Figure 4. β1 integrins and ILK control internal Golgi polarity.
(a) WT and β1-KO cultured acini, stained for β1-integrin and GM130.
(b) WT and β1−/− in vivo acini, stained for WGA488, GM130 and β-catenin. Note the repositioning or fragmentation of Golgi following β1-integrin deletion. Bar: 15μm.
(c) Histograms represents average Golgi positioning (%) in β1fx/fx:CreER™ cultured acini or from 9 areas imaged within each β1fx/fx:Blg-Cre gland: ‘Ribbon’ Golgi are those accumulated halfway perinuclear towards the apical surface, but they are ‘Fragmented’ if localized more than half way around the nucleus. In some cells Golgi were undetectable by GM130 immunostaining and scored as ‘Undetectable’.
(d) WT and β1-KO acini stained for β1-integrin, ZO1 and GM130. Note that regions of inverted polarity and no polarity are found in the same β1-KO. In these cases, ribbon Golgi distribution to peripheral edges in β1-KO acini correlates with intact apical polarity (box 2) whilst fragmented Golgi are evident in cells that have lost apical polarity (box 1). Bar: 10 μm
(e,f) GM130 staining shows sub-apical Golgi in WT (e) cultured and (f) in vivo acini and fragmentation upon ILK depletion. Arrow indicates fragmented Golgi. Bar: 15μm.
(g) Histogram represents average Golgi positioning (%) in Ilkfx/fx:CreER™ cultured acini or from 9 areas imaged within each Ilkfx/fx:Blg-Cre gland (as in Fig 4b).
(h) ICR acini, treated with DMSO or Nocodazole (24h). Arrow indicates Golgi dispersal after MT disruption. Bar: 10μm.
Golgi positioning at the pericentrosome is principally controlled by the microtubule (MT) cytoskeleton, a process driven by dynein and dynactin motors, and treatments with MT depolymerising reagents (Nocodazole) disperse Golgi into ministacks27. In polarized acini cultured from non-transgenic ICR MECs, Nocodazole caused Golgi fragmentation similar to the integrin/ILK knockouts (Fig. 4h).
Although individual Golgi units are functional for membrane trafficking 27, Golgi scattering in β1-integrin and ILK null epithelial cells correlated with the loss of polarized trafficking. This could additionally contribute to abnormal lumen development.
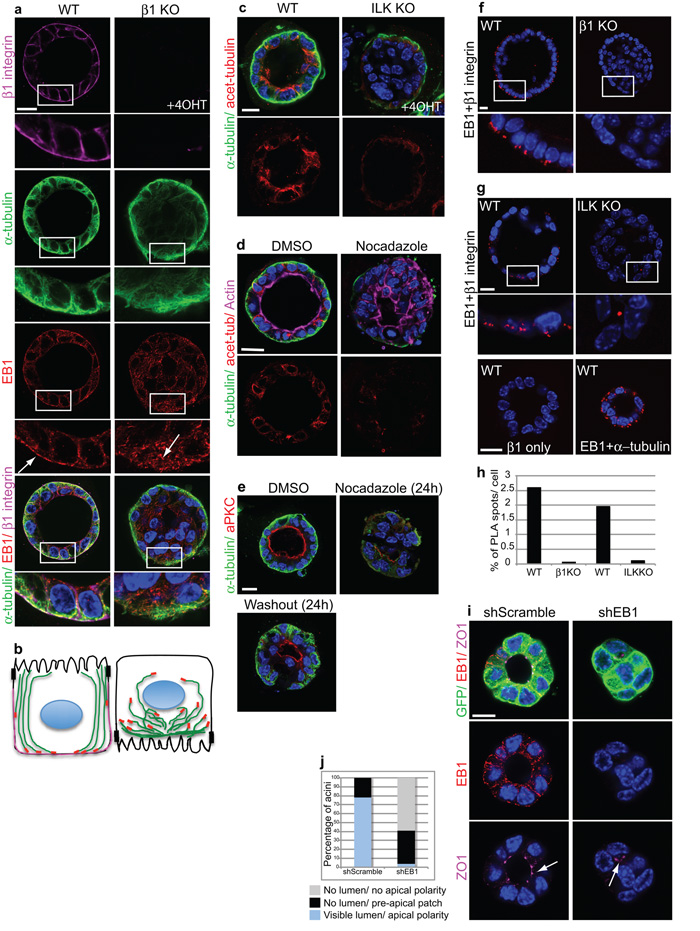
β1-integrin adhesions control the spatial orientation of microtubules
Since ®1-integrins and ILK deletion caused Golgi to redistribute or disperse into ministacks, we reasoned that integrin adhesion might control polarity by organizing MTs. We examined microtubule orientation by analysing their plus tips. In WT acini, MTs aligned along the apico-basal polarity axis with EB1 positioned towards the basolateral membrane (Fig. 5a,b, left panel). Over the time-course of acinar polarization, integrin reorganisation coincided with recruitment of EB1 towards the basolateral membrane (Supplementary Fig. 5). By contrast, in the KO MECs, MTs were not able to form a discrete apical-basal orientation and EB1 was scattered throughout the cell, suggesting the presence of short unstable MTs (Fig. 5a,b, right panel). This was confirmed by immunostaining acetylated MTs which were enriched near the apical cortex in WT acini, but destabilized in the absence of ILK (Fig. 5c). Moreover, WT acini showed reduced MT acetylation at the basolateral pole suggesting that they are more dynamic here and undergo temporary stabilizations only. Destabilizing MTs with Nocodazole treatment of polarized acini also disrupted polarity and lumens, but this effect was reversed when the drug was washed out (Fig. 5d,e).
Figure 5. β1-integrins and ILK control polarity and lumens through polarization of microtubules.
(a) WT and β1-KO acini stained for α-tubulin, EB1 and β1-integrin. Integrin deletion prevented plus-end MT orientation and alignment along the apicobasal polarity axis. Bar: 10 μm.
(b) Schematic of (a) showing MT orientation (green) and location of EB1 (red) and β1 integrin (magenta) in WT and β1-KO acini.
(c) WT and ILK-KO acini stained for α-tubulin and acetylated α-tubulin. Bar 10μm.
(d) Polarized ICR MEC acini were treated with DMSO or Nocodazole (1.5h), fixed and stained with antibodies to α-tubulin, acetylated tubulin and Alexa 647 phalloidin. Bar: 10μm.
(e) Polarized ICR acini were treated with DMSO or Nocodazole (24h), then either harvested or the drug was washed out and cells cultured for a further 24 h. MT disruption depolarized acini, but polarity was rescued after the washout. Bar: 10μm.
(f,g) WT and β1-KO (f), or ILK-KO (g) acini were stained with β1 integrin and EB1 antibodies, followed by proximity ligation assay (PLA) to detect complex formation between these two proteins. Each PLA spot represents a point of interaction between β1-integrin and EB1. Note in the absence of ILK, β1 integrin-EB1 complexes do not form. EB1 and α-tubulin were used as a positive control for interaction, showing PLA spots throughout the cells. β1-integrin alone was used as a negative control for the PLA. Bar: 10μm.
(h) Histogram represents the average number (%) of PLA spots per cell within acini.
(i) ShRNA lentiviral knock down of EB1 in MECs resulted in abnormal lumens. Arrow indicates a visible lumen with apical ZO1 in shScrambled acini but only a small pre-apical patch and no lumen in shEB1 acini. Bar: 7μm
(j) Quantification of scrambled or EB1 knockdown acini with polarized lumens.
See also Supplementary Figs. 5, 6.
The results suggest that integrin adhesions recruit EB1, which then establishes apico-basal MT orientation and cell polarity. We therefore determined whether EB1 is physically associated with integrin adhesions. In situ Proximity Ligation Assays showed that β1-integrin adhesions formed a complex with EB1 specifically at the basal surface of polarized WT acini (Fig. 5f). Notably this complex was disrupted in the absence of ILK, indicating that ILK links integrin adhesions with EB1 (Fig. 5g). To determine if EB1 is required for apical lumens, we depleted it from MECs using an shRNA lentivirus (Supplementary Fig. 6). EB1 knockdown resulted in defective formation of mammary acini, with fewer than 95% forming discernable lumens (Fig. 5h, i).
These findings reveal that integrin-ILK complexes anchor MT plus ends to the basolateral cell surface via EB1 thereby providing a mechanism for MT orientation. A possible outcome is that integrin anchoring of MT plus ends may temporarily stabilize MTs, thus allowing deposition of basolateral trafficking cargo at this membrane. It follows that some of this cargo may trigger the removal of apical proteins from the outer membrane, thereby re-orienting polarity.
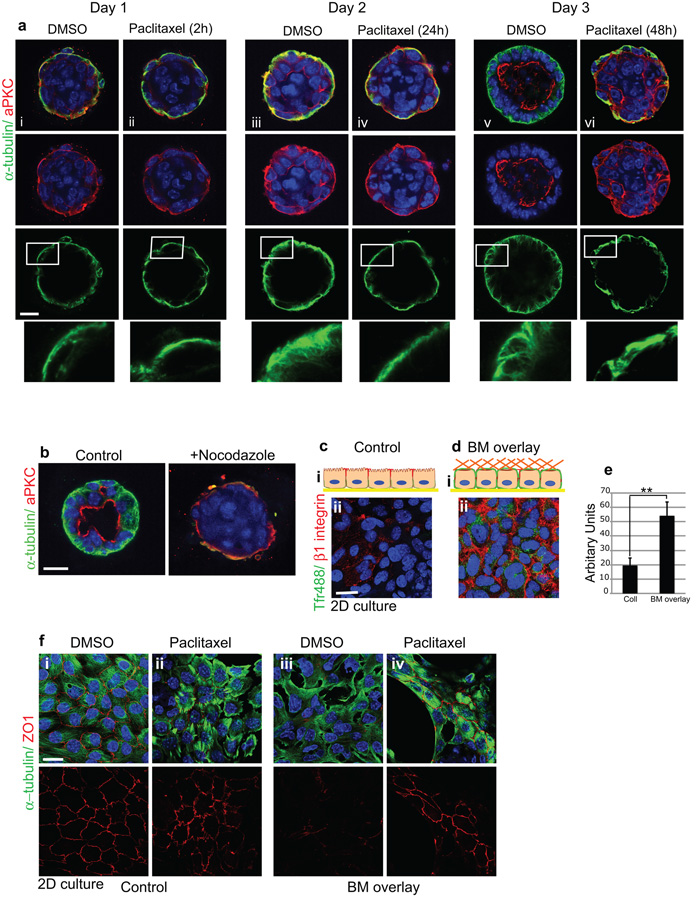
Microtubules are necessary for the orientation of apico-basal polarity
To address the role of MT dynamics in polarity re-orientation, we tested the effects of MT disrupting drugs on the formation of mammary acini. In time-course studies, paclitaxel stabilization prevented MT reorientation along the apicobasal polarity axis, and simultaneously inhibited aPKC redistribution from the cell-BM interface to the luminal surface. The acini failed to form lumens (Fig. 6a). Similarly, Nocodazole inhibited lumen formation (Fig. 6b).
Figure 6. Dynamic MTs are required for apical relocation of aPKC and lumen formation.
(a) Time-course of polarity and lumen formation in 3D ICR acini embedded within BM-matrix and treated with paclitaxel (100 nM). Untreated acini (i, iii, v) developed apical lumens normally and MTs became orientated apico-basally. In contrast, MT stabilization (ii, iv, vi) impaired aPKC reorientation and lumen formation. Bar: 15μm.
(b) Nocodazole prevents lumen formation.
(ci) Schematic Z-view of polarized MECs in monolayer with apical tight junctions and basolateral integrins. (ii) Integrins are absent from top surface (extracellular domain antibody to β1; red) and apical membrane cannot internalize Tfr-488 from the media.
(d) MECs overlaid with BM matrix display β1-integrins at the top (i) schematic Z-view (ii) confocal view and TfR-488 internalization from the media. Bar: 8μm.
(e) Tfr488 uptake was quantified using a fluorescence plate reader. Histogram shows a single experiment representative of n=3. **p < 0.02; ***p< 0.05.
(f) Confluent monolayers of ICR MECs displaying apical tight junctions (i) were treated with DMSO (i, iii) or with paclitaxel (ii, iv) for 1h prior to BM-overlay (iii, iv). BM-overlay induced ZO1 tight junction disruption in DMSO treated cells but paclitaxel treatment prevented the disruption. Bar: 8μm.
This result suggests that the outer membrane of acini remodels when it contacts a BM, and that MTs might be important to actively direct apical proteins away from the cell-BM interface. To test this hypothesis, we used MEC monolayers in order to be able to experimentally manipulate the apical cell surface, by adding BM proteins to the culture media to form a new cell-BM interface (BM-overlay).
Monolayer cultures of MECs were polarized, with ZO1 at apical tight junctions (Fig. 6fi). ®1-integrins were absent from the top surface of the monolayer (Fig 6cii), but BM-overlay caused integrins to locate at the top surface (Fig 6dii) and ZO1 was removed (Fig 6f iii). Under these conditions, exogenous Tfr488 uptake increased, indicating that the top surface of the cells was functioning as a basolateral membrane (Fig 6c-e). MT stabilization prevented the BM-induced removal of ZO1 (Fig 6fiv).
MTs are therefore needed to remove apical proteins away from the cell-BM interface and to orient apico-basal polarity.
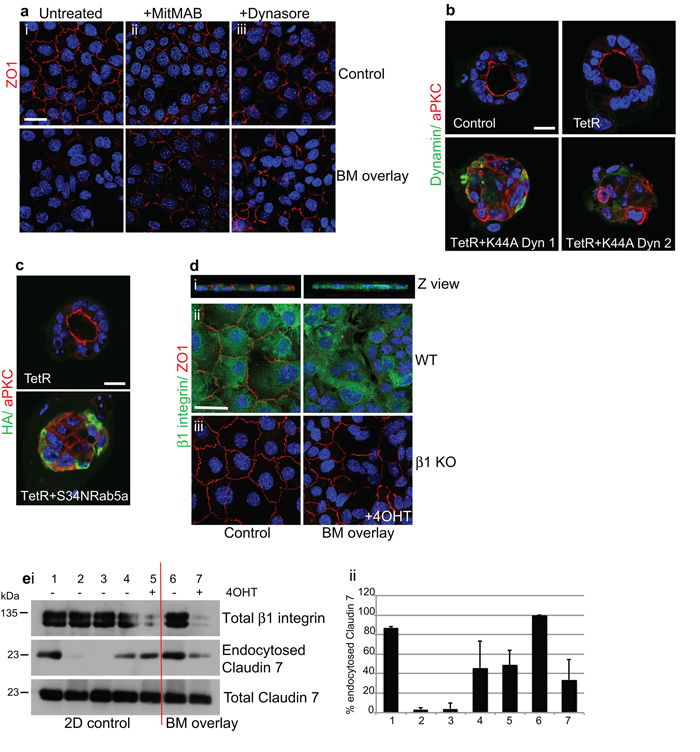
β1-integrins orient epithelial polarity via endocytosis of apical components from the cell-BM interface
To investigate the mechanism for BM-matrix induced apical protein removal, we tested whether endocytosis is involved by examining the requirement of the endocytic trafficking dynamin GTPase. Dynamin inhibitors, MiTMAB and dynasore, blocked endocytosis in MECs because they inhibited Tfr488 and cholera toxin-B uptake from the media (Supplementary Fig. 7). Both inhibitors, as well as adenoviral expression of dominant-negative K44A-dynamin 1 and 2, prevented BM-induced removal of ZO1 (Fig. 7a and not shown). Moreover, K44A-dynamin 1 and 2 prevented polarity establishment and lumen formation in mammary acini (Fig. 7b). To confirm the role of early endosomal trafficking in polarity, we expressed dominant-negative S34NRab5a in MECs, which also prevented lumen formation (Fig. 7c).
Figure 7. β1-integrins orient polarity via an endocytic mechanism.
(a) Confocal images of monolayer ICR MECs (i) untreated or pre-treated for 1 h with (ii) MitMAB or (iii) dynasore prior to 6 h BM-overlay. Blocking dynamin prevented ZO1-internalization by the BM-overlay. Note that dynamin inhibition by itself slightly reduced apical tight junctions compared to untreated controls, possibly as a result of decreased vesicle budding from the Golgi 53, but no further loss occurred in response to the BM-overlay. Bar: 8μm.
(b) MECs infected with adenoviruses expressing TetR alone, TetR + K44A-dynamin1 or TetR + K44A-dynamin2, then cultured in 3D on BM-matrix. DN-dynamin prevented relocation of aPKC to the internal luminal surface and polarization of acini. Note that these acini resemble day 1 of the developmental time course (cf Fig. 3a) Bar: 10μm.
(c) MECs infected with adenoviruses expressing TetR alone, TetR + S34NRab5a and then cultured in 3D on BM-matrix. DN-Rab5a prevented relocation of aPKC to the internal luminal surface and polarization of acini. Bar: 10μm.
(d) Left panels: confluent monolayers of WT MECs displayed apical tight junctions (ZO1) and basolateral β1-integrin. (i) Z-section (ii, iii) confocal view. Right panels: BM-overlay induced ZO1 disruption in WT but not β1-KO MECs. Confocal plan views were merged from the top and middle to show both ZO1 and nuclei. Bar: 8μm
(e) Surface biotinylation of cells followed by 6 h BM-overlay then blotting of endocytosed Claudin 7. i) (1-5) Monolayer controls, (6,7) BM-overlay. (1,2) Efficiency of biotin stripping from the surface at 4°C: (1) unstripped, and (2) stripped monolayers. (3-7) To induce endocytosis, cells were switched to 37°C after biotin labelling: (3) no biotin, (4) monolayer, (5) monolayer +4OHT, (6) BM-overlay, (7) BM-overlay +4OHT. ii) ImageJ64 quantification of endocytosed Claudin7 immunoblots. Histogram represents mean values +/− s.e.m for error bars of n=3 experiments. Note that BM-overlay increased Claudin 7 endocytosis (cf 4,6), which was prevented by β1-integrin deletion (cf 6,7).
See also Supplementary Figs 7, 8.
These results suggest that apical proteins are removed from the cell-BM interface by endocytosis. We investigated the involvement of ®1-integrins using the BM-overlay assay. Whilst BM removed ZO1 from the apical surface of WT, it was unable to do so after β1-integrin gene deletion (Fig. 7d). To confirm the role of ®1-integrins in apical protein endocytosis, we tracked the internalization of a transmembrane tight junction protein, Claudin 7, in response to 6 h BM-overlay using surface biotinylation. An increased pool of endocytosed Claudin 7 was detected in response to BM-overlay (Fig. 7e, compare lanes 4 with 6) but this was reduced after deleting β1-integrins (Fig. 7e, lane 7).
Our results show that the engagement of BM proteins with ®1-integrins coordinate MTs to trigger the endocytic removal of apical proteins. This remodels the BM-cell interface into a basal domain and creates an apical domain on the opposing membrane, allowing lumen development. In the absence of ®1-integrins or MT dynamics, the apical components remain on the basolateral surface.
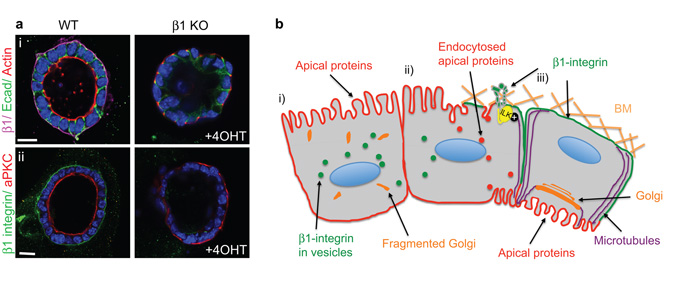
β1-integrins maintain apical polarity
We also investigated whether ®1-integrins maintain the polarized orientation of apical proteins. Deleting the ®1-integrin gene in β1fx/fx;CreER™ MECs after the acini had fully developed caused inversion of the apical components, actin and aPKC, to the outer membrane (Fig. 8a). Maintenance of polarity is therefore an active process that is governed by ®1-integrins, and requires the continuous endocytic removal of apical components from the basolateral membrane.
Figure 8. β1 integrin signalling specifies the orientation and maintenance of epithelial polarity and the formation of lumens.
(a) β1-integrin was ablated in MECs after acini had polarized and developed lumens. Note the inversion of polarity detected by (i) TRITC phalloidin and (ii) aPKC. Bar: 10 μm.
(b) Temporal sequence of how BM-integrin engagement remodel a neo-basolateral surface. i) Initially, cells are unpolarized with fragmented Golgi and apical markers at the membrane. ii) Engagement of β1-integrins with the BM first recruits ILK to instruct the orientation of polarity. Integrin/ILK then polarize MTs along the apico-basal axis by interacting with their plus ends followed by endocytosis of apical components from this surface. iii) Integrins/ ILK regulate internal cell polarity by positioning the Golgi apparatus sub-apically which further aids polarized trafficking to the membrane. A new apical face is established on the membrane opposing the BM, which subsequently leads to lumen formation.
Discussion
In this paper, we provide mechanistic insights into how integrins control apico-basal polarity in epithelia, leading to the formation of lumens and functional glandular tissues. BM interactions with epithelia provide a microenvironmental cue to govern the spatial orientation of cell polarity. However, the intracellular pathways by which integrin-BM interactions contribute to polarity was previously unknown.
Using genetic deletion in a primary 3D mammary culture model, we have shown that ®1-integrins function through ILK in organising MTs, which then promote the establishment of polarity at multiple levels. ®1-integrins and MTs collaborate in the endocytic removal of tight junction proteins from the outer membrane, thereby defining a basolateral surface. The integrin-ILK-MT network also positions the Golgi sub-apically in order that they can govern polarized trafficking of proteins and create a new apical face on the opposing membrane (Fig. 8b).
β1-integrins are not involved in establishing an apical domain, however, active BM-®1-integrin interactions are required to maintain an apical surface adjacent to the lumen, because without ®1-integrins, luminal tight junctions disrupt and reassemble on the surface next to the BM. A possible mechanism is that apical cargo is initially targeted to the basolateral surface, internalized and then transcytosed to the apical domain. This mode of protein transport is utilized by a number of epithelial cell types, e.g. hepatocytes, intestinal epithelia and MDCK cells 4. Thus a loss of integrin signalling in MECs may inhibit endocytosis at the basolateral membrane, resulting in an accumulation of apical polarity proteins and thereby inversion of polarity.
A model emerges in which β1-integrins recruit ILK and capture EB1 plus tips in order to orient MTs. Consistent with this model, mass spectrometry approaches have identified integrin adhesion connections to MT plus ends, e.g. ILK interacts with α and β-tubulin, with IQGAP1 and mDia, and fibronectin-β1-integrin adhesions contain EB1 28, 29 30. Since ILK deletion affects MT stability, integrin-ILK adhesions may bind MT plus ends to organize and stabilise them. Indeed MT stability at the apical cortex was reduced in the absence of ILK suggesting that plus-end anchoring of MTs at the basolateral membrane may serve to redirect apical components along stabilised MTs towards the apical membrane. Similarly, in colonic epithelia, the kinesin KIF17 stabilizes MTs by interacting with EB1, which contributes to epithelial polarization31.
MTs serve as tracks for polarized vesicular transport by orienting their minus ends towards the apical domain and plus ends towards the basal surface 32, 33. One possibility is that β1-integrin-EB1 connections may deliver factors to the basolateral membrane that facilitate the endocytosis of apical components. Caveolae might have a key role here, because ®1-integrin depletion blocks caveolar endocytosis in skin fibroblasts and caveolae are involved with endocytosis of tight junctions in both gut and brain endothelia 34-36. Moreover in keratinocytes, MT stabilisation by ILK is essential for targeting and insertion of caveolae at the plasma membrane30. This suggests that depletion of ®1-integrins/ILK, or inhibiting MT dynamics, may perturb caveolar trafficking to the MEC cell surface, thereby preventing the internalisation of apical proteins.
We have shown that ®1-integrins are required for endocytosing apical proteins and that inhibiting early endosomal trafficking with DN-Rab5a blocks lumen formation. This suggests that some endocytosed apical components may be recycled to generate a new luminal membrane. Consistent with our studies, in MDCK cells, Rab11 positive recycling vesicles transport the polarity complexes, Par3-aPKC and Crumbs3-Pals1-PatJ to early lumens, but the full contribution of synthesis vs recycling is yet to be established 37, 38.
The work presented here identifies a mechanism by which cell-matrix interactions provide orientation cues for organising cell polarity within tissues. Future investigations with live cell imaging will characterize in more detail the roles of these integrin adhesion complexes in the morphogenesis of the mammary gland.
Online Methods
Mouse strains
β1fx/fx; Blg-Cre mice, Ilkfx/fx; Blg-Cre mice, FAKfx/fx; Blg-Cre mice and β1fx/fxCreER™ mice have been described previously 13, 17. The Ilkfx/fx or Rac1fx/fx :LSLRosaYFP 39 and CreER™ lines were crossed to produce the ILKfx/fx;CreER™ or Rac1fx/fx:LSLYFP;CreER™ mice. For the Rac1 in vivo analysis, Cre-mediated specific Rac1 gene deletion in luminal mammary epithelial cells was achieved by crossing Rac1fx/fx :LSLRosaYFP mice with WapiCreTg/• mice to produce Rac1fx/fxYFP :WapiCreTg/• (Rac1−/−) mice. To avoid problems in feeding of pups by mothers with potentially defective mammary glands, only the male mice of the breeding pairs carried the Cre transgene. Rac1fx/fx:LSLYFP mice that lacked the Cre gene were used as wild type (WT) controls. Genotyping was performed using DNA prepared from ear punches39-41. Note that the mammary specific Blg-Cre and WAPiCre promotors were used to conditionally delete the β1 integrinfx/fx gene in luminal MECs but not in myoepithelia or stromal cells 13. BlgCre is activated in nulliparous mice between 8 and 12 weeks whereas WAPiCre is activated during pregnancy. In some experiments, non-transgenic ICR mice were used as the source of primary MECs. Mice were housed and maintained according to the University of Manchester and UK Home Office guidelines for animal research.
Primary cell culture and gene deletion
Primary MECs were harvested from 15.5-17.5 day pregnant mice and cultured as described in 42. Cre-mediated deletion of β1-integrin or ILK or Rac1 in primary MEC cultures was achieved by harvesting the MECs from β1fx/fx:CreER™ or Ilkfx/fx;CreER™ mice, or Rac1fx/fx:LSLYFP:CreER™ and treating with 100 nM 4-hydroxytamoxifen dissolved in ethanol. β1-integrin gene deletion after acini had polarized was performed by addition of 4OHT to 4-5 day old cultures.
Cells were plated onto Collagen 1 for monolayer cultures, BM-matrix (Matrigel; BD Biosciences) to form acini and cultured in growth media (Ham’s F12 medium (Sigma) containing 5 μg/ml insulin, 1 μg/ml hydrocortisone (Sigma), 3 ng/ml epidermal growth factor (EGF), 10% fetal calf serum (Biowittaker), 50 U/ml Penicillin/Streptomycin, 0.25 μg/ml fungizone and 50 μg/ml gentamycin).
For BM-overlay assays, monolayer MECs were overlaid with diluted BM-matrix (1:50) in DMEM:F12 medium (containing 5 μg/ml insulin, 1 μg/ml hydrocortisone, 3 ng/ml EGF, 50 U/ml Penicillin/Streptomycin, 0.25 μg/ml fungizone and 50 μg/ml gentamycin) for 48h, or 1:25 for 1-6h 43.
Inhibitors: In some experiments cells were treated with 10 μM zVAD; dynamin inhibitors 40 μM MitMAB (Calbiochem), 100 μM Dynasore (Sigma); or microtubule inhibitors 200 ng/ml Nocodazole, 100 nM or 1 μM paclitaxel (Sigma).
Genomic PCR
Genomic DNA was isolated from mammary tissue or cultured Ilkfx/fx:CreER™ or Rac1fx/fx:LSL- YFP:CreER™ MECs following 4OHT addition (24h) and analysed by PCR. DNA was extracted with Direct PCR lysis reagent (Viagen Botech, LA) containing 10 μg/ml proteinase K. The position of PCR was carried out as described 44. The PCR reaction products were 2.1 kB (Ilk flox) and 230bp (Ilk flox recombined and Ilk null), or 333bp (Rac1 flox) and 175bp (Rac1 flox recombined and Rac1 null).
Adenovirus infection
Primary cells were infected in suspension, and then replated onto BM-matrix for 48h to form acini 45. Greater than 80% infection was achieved. HA tagged K44A Dynamin 1, K44A Dynamin 2, S34NRab5a and Tetracyclin regulator (TetR) adenoviruses 46 47. To avoid overexpression of viruses, cells were cultured in the presence of 5 ng/ml doxycycline. Ad-GFP and Ad-ILKEGFPf (wild type ILK with farnesylated GFP) have been previously described 17. For rescue experiments β1 integrin or ILK was depleted first in monolayer with 4OHT. Cells were trypsinized, infected in suspension with Ad-GFP or Ad-ILKEGFPf and plated onto BM-matrix as previously described 17. Cells were harvested 48h later for immunofluorescence.
Lentiviral ShRNA knockdown
The mouse EB1 and scrambled shRNA sequences were as described 48. Double stranded oligonucleotides were cloned into shRNA transfer vectors pLVTHM (Tronolab). ShRNA constructs were transfected into Swiss 3T3 fibroblasts using Lipofectamine plus (invitrogen) to verify EB1 knockdown. Lentivirus production in 293T cells was as previously described 49. EPH4 mammary epithelial cells were cultured in DMEM;F12 containing 5% fetal calf serum, 5 μg/ml insulin and 50 U/ml Penicillin/Streptomycin. Lentivirus infection of EPH4 MECs was performed by adding lentiviral particles to 50% confluent monolayers in the presence of polybrene. Greater that 80% infection was achieved. Media was replaced after 12h and infected cells were cultured for 3-4 days. Cells were replated onto BM-matrix for a further 4 days to form acini.
Immunostaining
Expression and distribution of proteins were visualized by indirect immunofluorescence as in 45. For visualising EB1, cells were fixed for 10 min in 90% methanol/ 3% (w/v) paraformaldehyde/ 5mM sodium carbonate (pH 9). F-actin was detected by incubating cells with TRITC or FITC-phalloidin, (Sigma) or Alexa 647 phalloidin (Molecular Probes), for 1 h at RT, and nuclei were stained using 4 μg/ml Hoechst 33258 (Sigma) for 5 min at RT, followed by mounting in prolong gold antifade (Molecular Probes). Acini were visualized by confocal imaging. Images were collected on a Leica TCS SP5 AOBS inverted confocal using a 63x Plan Fluotar objective. The confocal settings were as follows, pinhole 1 airy unit, scan speed 1000Hz unidirectional, format 1024 × 1024. For Z stacks, 0.2μm sections were taken and Leica software was used to determine the optimal number of Z sections.
Images, 3D rendering and movies were developed with Volocity software (Perkin-Elmer) and Image J64. Quantification of acini was scored by analyzing 100 acini for each condition. Where approximately half the cells within an acinus displayed a change in morphology, these acini were scored as positive for that change. Non-biased cell counts were performed by concealing the identity of each slide.
For some experiments cells were immunostained with primary antibodies followed by in situ Duolink ™ proximity ligation assays to detect protein interactions, performed according to the manufacturer’s instructions (Olink Biosciences, Sweden).
Immunofluorescence of mammary tissue was performed on paraffin-embedded tissue or cryosections (7μm)17 and luminal surface was detected with wheat germ agglutinin-488 (Invitrogen) and imaged using confocal microscopy. Primary antibodies used for immunofluorescence have been described in Supplementary table 1. Alexa Flour 488-conjugated wheat germ agglutinin (Molecular Probes), secondary antibodies conjugated to Cy2, Rhodamine-RX and Cy5 (Jackson Immunoresearch).
Histological analysis
Mammary tissue sections were stained with haematoxylin and eosin (H&E) and imaged as in 17.
TEM
Acini were fixed in 1.5% Glutaraldehyde (in 0.1M cacodylate buffer) for 2h, washed 3x in 0.1M sodium cacodylate buffer and embedded in a serum plug 50. After several washes in 0.1M sodium cacodylate buffer containing 3mM calcium chloride (Agar Scientific Ltd, Stansted, UK), the cell plug was diced into small pieces and post-fixed in 1% osmium tetroxide (Agar Scientific Ltd) in 0.05M sodium cacodylate buffer pH 7.3 for 1h at 4°C followed by a rinse in buffer. Cells were dehydrated in an ascending alcohol series, treated twice with propylene oxide (15 mins each) then left in a 1:1 mix of propylene oxide and TAAB epoxy resin (Taab Laboratories Equipment Ltd., Aldermaston, UK) for 1h at RT followed by rotating overnight at 4°C in a mixture of 1:3 propylene oxide and epoxy resin. The cell plug was given two changes of fresh resin at 45°C for 1h each before being embedded in gelatin capsules and polymerized for 72h at 60°C.
Ultrathin sections were prepared with a diamond knife, mounted on copper grids and stained with uranyl acetate and lead citrate. Images were captured on a Philips CM10 electron microscope at an accelerating voltage of 80kV, with a Deben camera.
Cell fractionation
Acini were isolated using Matrisperse (BD Biosciences), lysed in hypotonic lysis buffer (10mM Tris pH7.5, 1.5mM magnesium chloride, 10mM sodium chloride 10μg/ml leupeptin, 10μg/ml aprotinin, 1mM sodium fluoride, 1mM sodium orthovanadate, 1mM PMSF) and cells were fractionated into cytosolic and membrane fractions 51. Alternate fractions were separated by SDS-PAGE and immunoblotted with indicated antibodies.
Surface biotinylation
Assays for surface biotinylation were as described in 52. In brief, confluent MEC monolayers on 100 mm dishes were labelled with 0.5mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford) for 30 min on ice with rocking. Free biotin was quenched using 50 mM ammonium chloride. Cells were left untreated or overlaid with BM-matrix (1:25) and incubated at 37°C for 6 hrs to induce endocytosis of surface apical proteins. To remove biotin on the cell surface, cells were stripped three times with 100mM MESNA buffer (pH 8.6) for 20 min each at 4°C, followed by three 5min incubations with 5mg/ml iodoacetamide at 4°C to quench free SH groups. To detect efficient stripping of the surface, some cells were kept at 4°C throughout the procedure to block endocytosis and stripped as above with MESNA or treated with the same buffer without MESNA. Cells were lysed in 1x Nonidet P40 buffer (10% w/v glycerol, 50 mM Tris-HCl pH7.5, 100 mM NaCl, 1% w/v Nonidet-P40, 2 mM MgCl2 and fresh protease/phosphatase inhibitors) and biotinylated proteins were isolated with NeutrAvidin Agarose.
Protein analysis
Proteins were extracted and immunoblotted as in 45. Equal amounts of proteins were used and equivalent loading assessed by referral to controls, such as Calnexin (Bioquote).
Clathrin and caveolar endocytic assays
Cells were incubated with 25 μg/ml labelled transferrin (Tfr488; Invitrogen) for 30 min or 10 μg/ml cholera toxin B (CtxB-FITC; Sigma) for 15 min at 37°C to internalize fluorescent markers. Cells were washed in PBS and incubated with an acid-salt wash buffer (0.2 M HAc, 0.5 M NaCl) for 10 min at 4°C to strip surface-bound fluorescent marker, followed by PBS wash, and fixed in 4% formaldehyde for 10 min. Internalized fluorescent markers were either analysed by confocal microscopy or quantitated in black 24 well clear bottom plates using a Berthold fluorescent plate reader. P values were determined using the Student’s t test. *p < 0.007; **p < 0.02; ***p < 0.05.
Supplementary Material
Acknowledgements
We thank Carolyn Jones for doing the electron microscopy, Sandra Schmid and Brian Ceresa for dynamin and Rab5a adenoviruses, Cary Wu for anti-ILK monoclonal, Martin Lowe for GM130 polyclonal, and Peter March for confocal microscope training. The FLS Bioimaging Facility microscopes were purchased with grants from BBSRC, Wellcome and the University of Manchester Strategic Fund. Thanks to Pat Caswell, Tim Hardingham, Martin Humphries, Martin Lowe and Paul Lu and for critical appraisal of the manuscript. This work was supported by the Wellcome Trust [#081203/Z/06/Z]. The Wellcome Trust Centre for Cell-Matrix Research is supported by core funding from the Wellcome Trust [#088785/Z/09/Z].
Footnotes
Author contributions NA conceived ideas, performed experiments, analysed and interpreted the data and wrote the manuscript.
CHS conceived ideas and wrote the manuscript.
References
- 1.Carmosino M, Valenti G, Caplan M, Svelto M. Polarized traffic towards the cell surface: how to find the route. Biol Cell. 102:75–91. doi: 10.1042/BC20090134. [DOI] [PubMed] [Google Scholar]
- 2.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Musch A. Microtubule organization and function in epithelial cells. Traffic. 2004;5:1–9. doi: 10.1111/j.1600-0854.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 5.Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107(Pt 3):561–576. [PubMed] [Google Scholar]
- 6.Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- 7.Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. II. Disassembly and assembly of plasma membrane domains during reversal of epithelial cell polarity in multicellular epithelial (MDCK) cysts. J Cell Sci. 1990;95(Pt 1):153–165. doi: 10.1242/jcs.95.1.153. [DOI] [PubMed] [Google Scholar]
- 8.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 10.Zovein AC, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu W, et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor MJ, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Brien LE, et al. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol. 2001;3:831–838. doi: 10.1038/ncb0901-831. [DOI] [PubMed] [Google Scholar]
- 15.Cohen D, Fernandez D, Lazaro-Dieguez F, Musch A. The serine/threonine kinase Par1b regulates epithelial lumen polarity via IRSp53-mediated cell-ECM signaling. J Cell Biol. 2011;192:525–540. doi: 10.1083/jcb.201007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeanes AI, et al. Specific beta-containing Integrins Exert Differential Control on Proliferation and Two-dimensional Collective Cell Migration in Mammary Epithelial Cells. J Biol Chem. 287:24103–24112. doi: 10.1074/jbc.M112.360834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhtar N, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027. doi: 10.1242/dev.028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–633. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jechlinger M, Podsypanina K, Varmus H. Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev. 2009;23:1677–1688. doi: 10.1101/gad.1801809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin Y, Meisen WH, Hao Y, Macara IG. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol. 2010;189:661–669. doi: 10.1083/jcb.201002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson JF, Hughes S, Chambers K, Lang SH. Polarized fluid movement and not cell death, creates luminal spaces in adult prostate epithelium. Cell Death Differ. 2009;16:475–482. doi: 10.1038/cdd.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Klinowska TC, et al. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol. 2001;233:449–467. doi: 10.1006/dbio.2001.0204. [DOI] [PubMed] [Google Scholar]
- 26.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan VJ, Thompson HM, McNiven MA. Motoring around the Golgi. Nat Cell Biol. 2002;4:E236–242. doi: 10.1038/ncb1002-e236. [DOI] [PubMed] [Google Scholar]
- 28.Humphries JD, et al. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol. 2008;180:681–689. doi: 10.1083/jcb.200710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickstrom SA, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010;19:574–588. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 190:443–460. doi: 10.1083/jcb.201006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacallao R, et al. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bre MH, et al. Regulation of microtubule dynamics and nucleation during polarization in MDCK II cells. J Cell Biol. 1990;111:3013–3021. doi: 10.1083/jcb.111.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284:19053–19066. doi: 10.1074/jbc.M109.000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh RD, et al. Inhibition of caveolar uptake, SV40 infection, and beta1-integrin signaling by a nonnatural glycosphingolipid stereoisomer. J Cell Biol. 2007;176:895–901. doi: 10.1083/jcb.200609149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schluter MA, et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–4663. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walmsley MJ, et al. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 40.Terpstra L, et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 42.Pullan S, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109(Pt 3):631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- 43.Streuli CH, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graus-Porta D, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 45.Akhtar N, Streuli CH. Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J Cell Biol. 2006;173:781–793. doi: 10.1083/jcb.200601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altschuler Y, et al. Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J Cell Biol. 1998;143:1871–1881. doi: 10.1083/jcb.143.7.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceresa BP, Lotscher M, Schmid SL. Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(q79l)-induced changes in endosome geometry. J Biol Chem. 2001;276:9649–9654. doi: 10.1074/jbc.M010387200. [DOI] [PubMed] [Google Scholar]
- 48.Komarova Y, et al. EB1 and EB3 control CLIP dissociation from the ends of growing microtubules. Mol Biol Cell. 2005;16:5334–5345. doi: 10.1091/mbc.E05-07-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang P, Ballestrem C, Streuli CH. The C terminus of talin links integrins to cell cycle progression. J Cell Biol. 195:499–513. doi: 10.1083/jcb.201104128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Payne CM, Satterfield VF. A simple procedure for the preparation of rosetted cells for electron microscopy. J Clin Pathol. 1980;33:505–508. doi: 10.1136/jcp.33.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valentijn AJ, Metcalfe AD, Kott J, Streuli CH, Gilmore AP. Spatial and temporal changes in Bax subcellular localization during anoikis. J Cell Biol. 2003;162:599–612. doi: 10.1083/jcb.200302154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura N, Sasaki T. Cell-surface biotinylation to study endocytosis and recycling of occludin. Methods Mol Biol. 2008;440:89–96. doi: 10.1007/978-1-59745-178-9_7. [DOI] [PubMed] [Google Scholar]
- 53.Edwards GM, Streuli CH. Preparing a polyclonal antibody to mouse beta 1 integrin with function-blocking activity. Methods Mol Biol. 1999;129:135–152. doi: 10.1385/1-59259-249-X:135. [DOI] [PubMed] [Google Scholar]
- 54.Klinowska TC, et al. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.