Figure 1.
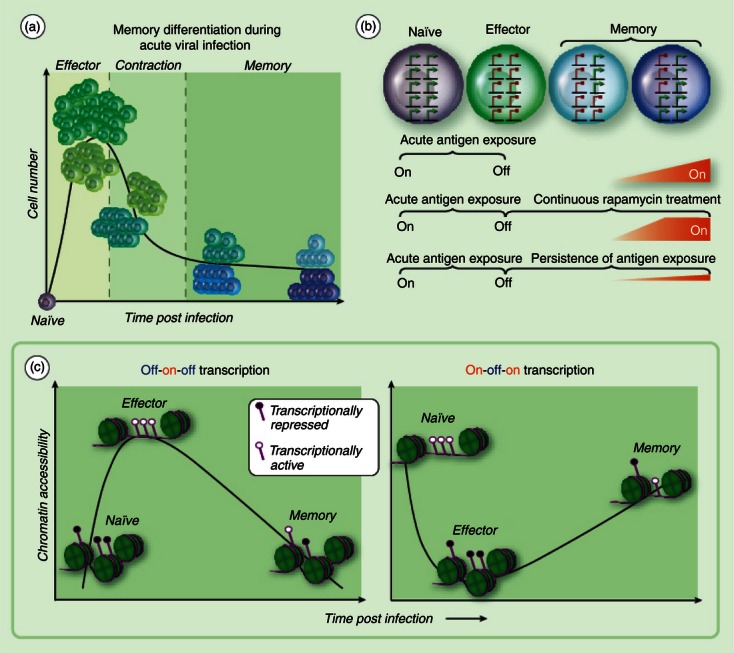
Adaptations in gene regulation during memory T-cell differentiation. (a) Naive antigen-specific CD8 T cells undergo clonal expansion upon presentation with the cognate antigen. The quantity of antigen-specific CD8 T cells at peak of clonal expansion is ∼ 1 × 105 greater than the naive population. Approximately 90–95% of antigen-specific CD8 T cells die during the contraction stage of the immune response. A subset of effector cells progressively modify the transcriptional programme, acquiring homing and pro-survival properties before and into the contraction phase of the response. (b) Longitudinal gene expression profiling studies of total antigen-specific CD8 T cells during acute viral infection have revealed a group of genes that are expressed (green arrows) in naive cells, repressed (red circle) in effector cells, and re-expressed at different memory time points, i.e. on-off-on gene expression. Continuous treatment of mice with rapamycin at the effector stage of an acute immune response enhances the kinetics of gene re-expression in CD8 T cells. Persistence of antigen presentation during chronic viral infection retains the ‘off’ state for several genes in the category of on-off-on expression. (c) Off-on-off gene expression is coupled to the loss of repressive epigenetic transcriptional regulatory programmes at the effector stage of the immune response followed by reacquisition of repressive and poised epigenetic programmes. A hypothetical model for epigenetic regulation of on-off-on gene expression would involve reduction in chromatin accessibility and acquisition of repressive epigenetic programming at the effector stage of the immune response followed by permissive epigenetic programming and chromatin accessibility during the transition to central memory T cells. Acquisition of repressive epigenetic modifications are indicated by a filled circle marker on the purple line (DNA) at transcriptional regulatory regions. Chromatin accessibility is indicated by the absence of green histone octamers.
