Abstract
HIV-specific T cells that produce interferon-γ (IFN-γ) are present in the genital tract of HIV-infected women although these do not provide protection against genital HIV shedding. Because polyfunctional HIV-specific T cells have been implicated in better HIV control than those with a single function, this study aimed to investigate whether polyfunctional T cells were present at the female genital mucosa. Cervical cytobrush-derived T cells were obtained from chronically HIV-infected women and compared with blood. CD3+ T cells from both compartments were expanded with Dynal anti-CD3/CD28 expander beads for 14 days and flow cytometry was used to evaluate four T-cell functions (CD107a, IFN-γ, tumour necrosis factor-α and macrophage inflammatory protein-1β) from 16 women. The majority of Gag-specific T-cell responses in the female genital tract were monofunctional, although low frequencies of HIV Gag-specific polyfunctional CD8+ T cells were detected at the cervix in 81·3% (13/16) of women. The ability of CD8+ T cells at both the cervix and in blood to express CD107a and to exhibit polyfunctional responses (two or more functions) following Gag stimulation was inversely associated with plasma viral load and positively associated with blood CD4 counts, suggesting that clinical status impacted on the functionality of HIV-specific T cells at the mucosa, in a similar way to blood. HIV Gag-specific cervical T cells were largely monofunctional. Polyfunctional T cells were detected at the cervix in women with high blood CD4 count and low plasma viral load but these did not protect from HIV genital shedding.
Keywords: cervical cytobrushes, HIV, mucosal, polyfunctional, T-cell expansion
Introduction
Virus-specific CD8+ T cells contribute to viral control by directly killing virus-infected cells, secreting antiviral factors, and secreting factors that recruit other cells of the immune system.1–5 Although virus-specific T cells are often measured by limited parameters, such as interferon-γ (IFN-γ) and/or interleukin-2 (IL-2) secretion, T cells are capable of secreting a broad range of cytokines and chemokines; T cells capable of simultaneous secretion of multiple cytokines are referred to as polyfunctional.6 In addition to being able to secrete multiple cytokines and exhibit multiple functions, polyfunctional T cells have been shown to produce more of each individual cytokine per cell than a monofunctional cell7–9 and be associated with better control of HIV in blood.6,10,11 In addition, polyfunctional HIV-specific T cells have been reported at mucosal sites such as bronchoalveolar sites12 and gastrointestinal tract.13–15
Despite recent suggestions that the magnitude of polyfunctional cells correlate positively with HIV clinical status,13 it is unclear whether better clinical status of individuals results in preservation of polyfunctional responses or whether polyfunctional responses result in better clinical outcome.12–15 Moreover, there is little information about T-cell polyfunctionality in the female genital tract and this is critical because it is the major route of infection driving the pandemic worldwide.
Most studies that have investigated ex vivo phenotypic and functional qualities of genital immunity16–18 were hampered by low cell yields, making the development of methods to expand immune subsets in vitro for more in depth studies of mucosal HIV immune responses an important goal.19,20 Cervical cytobrush sampling of the female genital tract classically yields few cells,21,22 making thorough immunological analysis difficult. In addition, T cells derived from the female genital tract are predominantly antigen-experienced and highly differentiated, with effector memory T cells being the most predominant subset at the cervix.23 We have previously explored in vitro polyclonal expansion to improve genital tract cytobrush-derived T-cell yields.23,24
We compare the frequencies of Gag-specific T-cells that are capable of polyfunctional effector functions from expanded cervical and blood-derived T-cell lines from HIV-infected women. Flow cytometry was used to simultaneously assess the frequency of expression of CD107a, IFN-γ, tumour necrosis factor-α (TNF-α) and macrophage imflammatory protein-1 β (MIP-1β) by CD8+ and CD4+ T cells in response to HIV Gag stimulation. The relationship between specific functions and polyfunctionality with protection from HIV shedding in the genital tract and markers of HIV disease progression were evaluated.
Materials and methods
Study participants
Sixteen chronically HIV-infected women were recruited from the Nyanga Day Hospital in Nyanga, Cape Town, South Africa for this study. Women who were menstruating at the time of sampling, who were post-menopausal or who had undergone a hysterectomy were excluded from the study. Syndromic management of sexually transmitted infections was followed in this study and all women with vaginal discharge, visible ulcers or genital warts were excluded from further study. All women gave written informed consent, and the Research Ethics Committee of the University of Cape Town approved all aspects of the study.
Collection and processing of specimens
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Histopaque density gradient centrifugation (Sigma-Aldrich, Egham, Runnymede, UK) from 40 ml whole anticoagulated blood using LeucoSep® tubes (Greiner Bio-one, Frickenhausen, Germany). A single Digene cervical cytobrush was collected from the genital tract of each woman under speculum examination.16,21,25,26 Cervical cytobrushes with visible blood contamination were discarded.26 Cells were processed within 4 hr of collection by flushing the cytobrush ∼ 30 times with R10 medium [RPMI-1640 medium, supplemented with 10% human AB serum, 5 mm l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin (GIBCO Invitrogen, Carlsbad, CA) and 2 mg/ml fungin® (Invivogen, San Diego, CA)]. The absolute number of CD3+ T cells in each cytobrush sample was counted using a Guava automated cell counter (Guava Technologies, Hayward, CA).21 Viability of cervical mononuclear cells was determined by Trypan staining (Sigma-Aldrich, Irvine, UK).
Determination of viral load in cervical supernatant and plasma
Viral load was determined in cervical supernatants and plasma samples using Nuclisens Easyq HIV-1 Version 1.2 (Biomerieux Clinical Diagnostics, Marcy I'Etoile, France). The detection limit of this assay was 50 copies/ml. The cervical supernatant fraction was obtained following flushing of the cervical cytobrush 30 times with 3 ml of transport medium and removal of cells by centrifugation (250 g for 10 min).
Polyclonal expansion of cervical and blood-derived T-cell lines
Cervical cytobrush-derived T cells were expanded with Dynal anti-CD3/CD28 (T-cell expander; Invitrogen Dynal, AS, Oslo, Norway)27 in the presence of IL-2, IL-7 and IL-15.23 Dynal magnetic beads (Dynabeads® coated with anti-CD3 and anti-CD28 antibodies) were added to wells at a 1 : 1 bead to cell ratio in a final volume of 200 μl/well (cell suspension + R10 medium). Recombinant IL-2 (200 IU/ml; National Institutes of Health AIDS Reagent Repository, Germantown, MD), IL-7 (20 ng/ml; R&D Biosystems, Minneapolis, MN) and IL-15 (20 ng/ml; R&D Biosystems) were added to cultures. The PBMC were added at 1 × 105 PBMC/well in R10 medium and expansion was performed in triplicate. Cervical cells were resuspended in R10 at 0·5 × 106 to 1 × 106 cells/ml and added to each well at 100 μl/well. Cervical and blood cells were cultured in 5% CO2 at 37° for 14 days and fresh R10 medium with cytokines was replenished every 2 days. When the cell density exceeded 2 × 106 cells/ml, cultures were split to a density of 0·5 × 106 cells/ml. T-cell yields were determined as described above.
Flow cytometry to detect intracellular polyfunctional T-cell responses
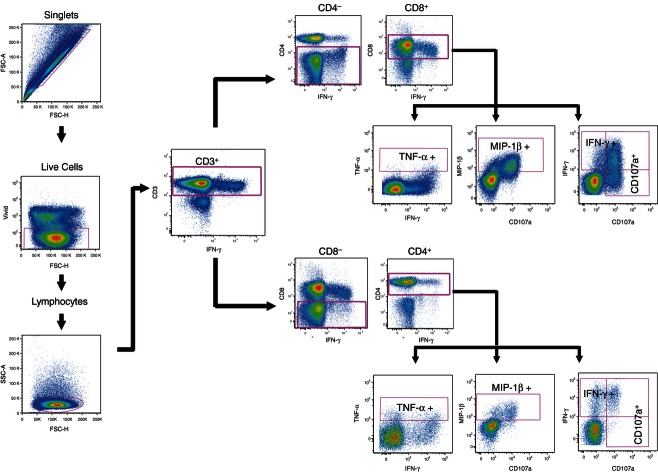
The phenotype and function of Gag-specific T-cell responses was measured using the following antibodies: CD3-allophycocyanin-H7, CD4-Peridinin chlorophyll protein-Cy5.5, CD107-FITC, TNF-α-Cy7-phycoerythrin (PE), MIP-1β-PE, IFN-γ-AlexaFluor 700 (all BD Biosciences, San Diego, CA), CD8-quantum dot (QD) 605 (Invitrogen, Carlsbad, CA), CD14-PacBlue (Dump channel; BD Biosciences San Diego, CA), and CD19-PacBlue (Dump channel; Invitrogen, Carlsbad, CA). ViVid (Invitrogen Molecular Probes, Eugene, OR) was included in the staining protocol. All antibodies were pre-titrated to optimal concentrations. Expanded cervical and blood-derived T-cell lines were deprived of IL-2, IL-7 and IL-15 for 24 hr before stimulation with 121 HIV subtype C Gag peptides (15-mer overlapping by 11 amino acids; provided by the National Institutes of Health AIDS Reagent Repository Program; 1 μg/ml). PMA (0·1 μg/ml)/ionomycin (0·1 μg/ml) and unstimulated cells were used as positive and negative controls, respectively. Stimulations were performed in the presence of DNase I, co-stimulatory factors [anti-CD49d (1 μg/ml), anti-CD28 (1 μg/ml)], brefeldin A (0·5 μg/ml) and monensin (10 μg/ml) (Sigma Aldrich). In addition, anti-CD107a-FITC antibody (5 μl) was included in each stimulation tube. With the exception of PMA, cells were stimulated with antigen for 16 hr at 37° in 5% CO2 (Thermo Electron Corporation, Marietta, OH). For PMA/ionomycin stimulation, cells were incubated for only 4 hr. This was followed by surface staining with anti-CD19-Pacific Blue, anti-CD14-Pacific Blue, anti-CD8-Qdot 605, anti-CD4-Peridinin chlorophyll protein-Cy5.5, as well as ViVid. Cells were then fixed and permeabilized at room temperature for 20 min using Cytofix/Cytoperm buffer (BD Biosciences) and stained intracellularly with anti-CD3-allophycocyanin-H7, anti-IFN-γ-AlexaFluor 700, anti-TNF-α-PE-Cy7 and anti-MIP-1β-PE. Samples were acquired on an LSRII (BD Biosciences) with FACSdiva software version 6.0 (BD Biosciences). The number of events collected ranged between 200 000 and 1 000 000 for both blood and cervix. Data analysis was performed using FlowJo v8.8.6 (Tree Star, Ashland, OR). Dead cells (ViVid), monocytes (CD14+) and B cells (CD19+) were excluded from the analysis. The gating strategy used is shown in Fig. 1. Responses were background-subtracted using unstimulated cells, and are expressed as net percentage response. Fluorescence minus one controls were used to set gates. spice software28 was used to represent polyfunctional data. Polyfunctional responses were defined as the total of 2+, 3+ and 4+ functions in blood and at the cervix of CD8+ T cells.
Figure 1.
Gating scheme used for polychromatic flow cytometry of HIV Gag-specific T-cell responses in the female genital tract and blood. Cells were first gated on singlets, live cells and then lymphocytes. From this gate, CD3+ cytokine-producing T cells were gated, and then further gated on CD4+ and CD8+ T cells. Gates for each of the four functions were set based on fluorescence minus one and the negative control and were kept constant for all samples analysed. Data analysis was performed using FlowJo v8 8 6.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5® (GraphPad Software, San Diego, CA). Mann–Whitney U-test was applied for independent sample comparison, the Wilcoxon Ranks test was used for matched non-parametric comparisons and Spearman Ranks correlation was applied for correlation comparisons. The false discovery rate step-down procedure was used to reduce false-positive results when performing multiple comparisons.29 P-values ≤ 0·05 were considered significant.
Results
Sixteen chronically HIV-infected women were included in this study (Table 1). Thirteen women were naive to anti-retroviral therapy whereas three had recently started taking highly active anti-retroviral therapy (HAART). Their median age was 35 years [interquartile range (IQR) 33–37] and their median CD4 cell count was 311 cells/μl (IQR 262–442). Women had a median log plasma viral load of 3·9 RNA copies/ml (IQR 2·9–5·0). The majority of women were also shedding HIV in their genital secretions with 13/16 (81·3%) having detectable HIV RNA [3·6 log RNA copies/ml in cervical secretions (IQR 1·1–4·1)]. HIV load in plasma was found to significantly predict the amount of HIV detected in genital secretions in this study (ρ = 0·75, P = 0·0008).
Table 1.
Clinical status
| PID | Age | CD4 count | Viral load (Log RNA copies/ml) genital tract | Plasma |
|---|---|---|---|---|
| 1 | 39 | 290 | 3·7 | 3·9 |
| 2 | 37 | 242 | 4·2 | 5·0 |
| 3 | 38 | 428 | 2·2 | 2·7 |
| 4 | 35 | 200 | 3·4 | 5·2 |
| 5 | 32 | 512 | 1·7 | 1·7 |
| 6 | 37 | 277 | 2·8 | 3·3 |
| 7 | 33 | 332 | 4·3 | 3·8 |
| 8 | 38 | 442 | 4·1 | 3·9 |
| 9 | 33 | 599 | 1·7 | 1·7 |
| 10 | 36 | 263 | 2·1 | 3·7 |
| 11 | 28 | 407 | 3·5 | 4·1 |
| 12 | 27 | 442 | 4·1 | 5·0 |
| 13 | 34 | 258 | 4·1 | 5·1 |
| 14 | 33 | 265 | 4·7 | 5·2 |
| 15 | 35 | 534 | 1·7 | 1·7 |
| 16 | 37 | 172 | 4·0 | 4·2 |
| Median (IQR) | 35 (33–37) | 311 (262–442) | 3·6 (1·1–4·1) | 3·9 (2·9–5·0) |
Polyclonal expansion of HIV-specific T cells from the female genitals
Cervical T cells were expanded for 14 days with Dynal beads in the presence of IL-2, IL-7 and IL-15 to improve mucosal CD3+ T-cell numbers.23 While a median of 0·11 × 106 cervical mononuclear cells was obtained ex vivo (Table 2), this number increased 15-fold following in vitro expansion, to yield a median of 1·8 × 106 cells after 14 days. The PBMC lines showed a significantly greater fold increase in T-cell numbers in comparison to matched cervical lines after 14 days of expansion (29-fold versus 15-fold; P = 0·0006) (Table 2). Ex vivo cervical mononuclear cell viability ranged from 88 to 92% (with a median of 90%) whereas PBMC viability ranged from 98 to 100% (median of 99%). Following expansion, the median viability of cervical lymphocytes was 85% (ranging from 80 to 90%) whereas expanded blood median viability was 92% (ranging from 88 to 97%).
Table 2.
Expansion kinetics and viability of cervical cytobrush and blood-derived T-cell lines using Dynal anti-CD3/28 T-cell expander beads
| PID | Cervical T cells | Blood T cells | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ex vivo recovery (×106 cells) | Ex vivo viability (%) | Expanded recovery (×106 cells) | Ex vivo viability (%) | Fold expansion (Day 14:Day 0) | Ex vivo recovery (×106 cells) | Ex vivo viability (%) | Expanded recovery (×106 cells) | Ex vivo viability (%) | Fold expansion (Day 14:Day 0) | |
| 1 | 0·17 | 88 | 2·92 | 90 | 17·4 | 0·1 | 98 | 5·05 | 98 | 50·5 |
| 2 | 0·08 | 88 | 1·24 | 78 | 15·7 | 0·1 | 100 | 3·35 | 88 | 23·5 |
| 3 | 0·11 | 85 | 1·58 | 94 | 14·8 | 0·1 | 98 | 3·35 | 98 | 23·5 |
| 4 | 0·09 | 92 | 1·27 | 80 | 14·3 | 0·1 | 100 | 3·12 | 92 | 21·2 |
| 5 | 0·13 | 98 | 1·31 | 88 | 10·3 | 0·1 | 100 | 3·55 | 89 | 25·5 |
| 6 | 0·19 | 90 | 2·87 | 85 | 15·0 | 0·1 | 98 | 5·50 | 84 | 65·0 |
| 7 | 0·14 | 92 | 2·69 | 94 | 19·2 | 0·1 | 96 | 3·37 | 96 | 33·7 |
| 8 | 0·10 | 98 | 1·29 | 80 | 12·5 | 0·1 | 98 | 4·89 | 98 | 28·9 |
| 9 | 0·12 | 96 | 2·09 | 80 | 17·3 | 0·1 | 100 | 3·89 | 92 | 28·9 |
| 10 | 0·11 | 80 | 1·45 | 80 | 12·9 | 0·1 | 97 | 3·61 | 86 | 36·1 |
| 11 | 0·13 | 89 | 2·69 | 90 | 20·5 | 0·1 | 100 | 3·16 | 100 | 31·6 |
| 12 | 0·11 | 90 | 1·45 | 90 | 13·3 | 0·1 | 96 | 3·26 | 94 | 32·6 |
| 13 | 0·09 | 90 | 1·20 | 76 | 13·0 | 0·1 | 99 | 3·60 | 89 | 26·0 |
| 14 | 0·12 | 85 | 2·10 | 78 | 16·8 | 0·1 | 99 | 3·74 | 88 | 37·4 |
| 15 | 0·12 | 98 | 1·94 | 84 | 16·7 | 0·1 | 99 | 3·91 | 88 | 29·1 |
| 16 | 0·08 | 90 | 1·94 | 94 | 25·5 | 0·1 | 99 | 2·91 | 92 | 19·1 |
| Meadian | 0·11 | 90 | 1·76 | 84·5 | 15·4 | 0·1 | 99 | 3·57 | 92 | 29·0 |
| IQR | 0·10 | 88 | 1·31 | 80 | 13·2 | 0·1 | 98 | 3·32 | 88 | 25·0 |
| IQR | 0·13 | 93 | 2·25 | 90 | 17·3 | 0·1 | 100 | 3·90 | 96·5 | 34·3 |
Impact of expansion on the frequency and functional profile of Gag-specific T cells
We have previously shown that short-term in vitro expansion with Dynal beads improves T-cell yields but does not significantly alter either the magnitude of Gag-specific IFN-γ responses, or the Gag regions targeted.23 Because polyclonal expansion of T cells may introduce expansion bias with certain T-cell functional clonotypes potentially out-competing others, the effect of short-term expansion with Dynal anti-CD3/28 T-cell expander beads following stimulation with HIV peptides on the range of T-cell functions to be studied was evaluated before and after expansion in matched PBMC (see Supplementary material, Fig. S1). The total magnitude (sum of all four functions) of CD8+ and CD4+ T-cell responses to Gag was stable before and after short-term expansion. CD8+ T cells in blood predominantly expressed CD107a in response to Gag stimulation (Fig. S1), irrespective of whether they had been expanded or not. In contrast, MIP-1β production dominated HIV-specific CD4+ T cells before expansion, whereas IFN-γ was the major cytokine produced after expansion. The net frequency of CD107a+ responses by expanded CD8+ T cells in blood was significantly lower than responses detected before expansion (P = 0·009), although CD107a continued to dominate CD8+ T-cell responses to Gag after expansion and frequencies of CD8+ T cells expressing CD107a in response to Gag were significantly positively correlated before and after expansion (ρ = 0·55; P = 0·03; Spearman Rank test). None of the other cytokines (IFN-γ, MIP-1β and TNF-α) produced in responses to Gag were significantly different before and after expansion of matched PBMC.
The impact of expansion on the frequency of polyfunctional T-cell responses to Gag was investigated. HIV Gag-specific CD8+ and CD4+ T-cell responses in blood from these chronically HIV-infected women were predominantly monofunctional with a median of 22% of CD8+ T cells and 4% of CD4+ T cells exhibiting more than one functional response to Gag and three of 16 (18·8%) women exhibiting four functional T-cell responses (data not shown). In vitro expansion did significantly alter the magnitude or functionality of responses detected compared with unexpanded responses.
Comparison of cervical and blood functional responses to Gag
To investigate how local responses within the female genital tract compare with those found in blood, the overall functionality of Gag-specific CD8+ and CD4+ T-cell responses detected at the cervix after expansion of T cells were compared with those detected in matched blood T-cell lines (Fig. 2). The total magnitude of CD8+ Gag-specific T-cell responses detected at the cervix was significantly higher than those detected in matching blood T-cell lines (P = 0·004). Of the individual functions measured, CD107a expression was the predominant response detected in Gag-specific CD8+ T-cell responses from both compartments (Fig. 2b), although higher frequencies of cervical CD8+ T cells expressed CD107a than blood CD8+ T cells (Fig. 2a; P = 0·007). Similarly, the frequency of MIP-1β expression by Gag-specific CD8+ T cells at the cervix was significantly higher than that detected in blood (P = 0·03). Although the frequency of cervical Gag-specific CD8+ T-cell responses was higher than in blood, both blood and cervix showed similar cytokine response profiles (Fig. 2B). In addition to expressing CD107a and producing IFN-γ and MIP-1β, Gag-specific CD4+ T cells at the cervix and in blood also produced TNF-α (Fig. 2c,d). Furthermore, blood and cervical CD4+ T cells responded similarly to Gag with all four functions being detected (Fig. 2d), and IFN-γ being the predominant cytokine detected in both compartments.
Figure 2.
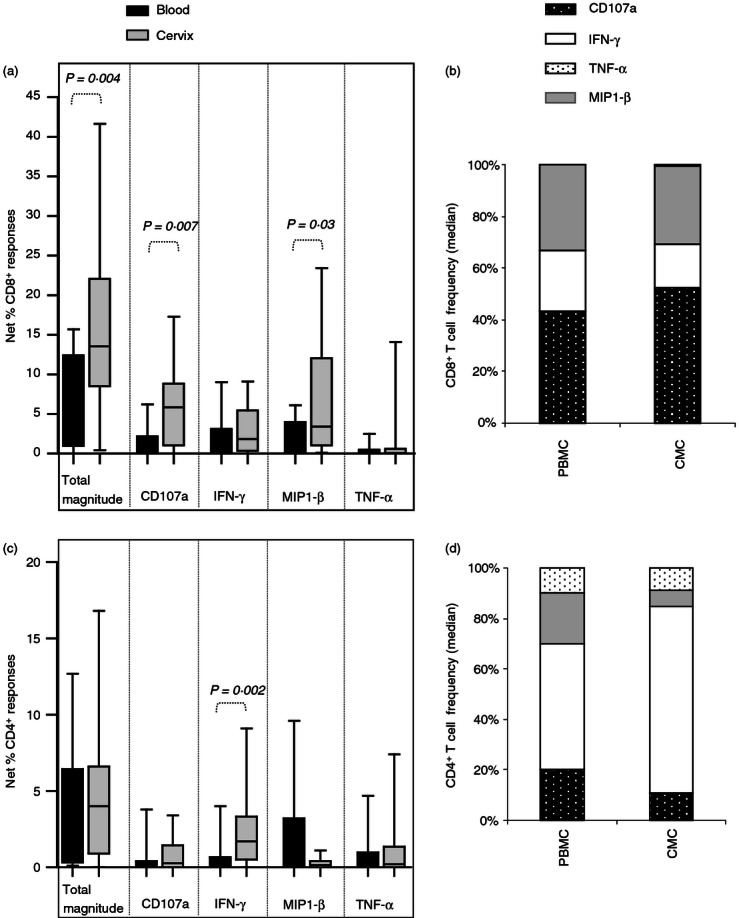
Comparison between female genital tract and blood CD8+ and CD4+ T-cell responses to HIV Gag in T-cell lines expanded with Dynal beads from HIV-infected women. (a) Total and individual frequencies of CD8+ responses detected in blood (black bars) and cervix (grey bars) from Dynal bead expanded lines. (b) Cumulative frequencies of CD8+ CD107a (dotted black bar), interferon-γ (IFN-γ; open bar bar), macrophage inflammatory protein-1β (MIP-1β; grey bar) and tumour necrosis factor-α (TNF-α; dotted bar) T-cell responses in peripheral blood mononuclear cells (PBMC) and cervix (CMC). (c) Total and individual frequencies of CD4+ responses detected in blood (black bars) and cervix (grey bars) from Dynal bead expanded lines. (d) Cumulative frequencies of CD4+ CD107a (dotted black bar), IFN-γ (open bar bar), MIP-1β (grey bar) and TNF-α (dotted bar) T-cell responses in PBMC and CMC. Each box and whisker plot shows the median (central line), interquartile range (IQR; outer lines of box) and 10–90% range (error bars) of 16 HIV-infected individuals. Wilcoxon Rank Test was used to compare blood and cervical responses and a P < 0·05 indicates a significant difference.
Impact of HIV clinical status on T-cell cytokine responses in blood and at the cervix
We then went on to evaluate whether plasma viral loads were associated with dominance of any particular function in blood or at the cervix. We found that CD107a expression in blood significantly predicts CD107a at the cervix (P = 0·002 for CD8 and P = 0·003 for CD4 T cells). Furthermore, we found that plasma viral load was negatively associated with the frequencies of HIV-specific CD8+ T cells producing CD107a in blood (ρ = − 0·53, P = 0·04) and at the cervix (ρ = − 0·64, P = 0·03; Fig. 3), suggesting that women with better control of plasma viraemia had preserved CD107a responses in both compartments. None of the other functions were found to be associated with markers of clinical status in these HIV-infected women.
Figure 3.
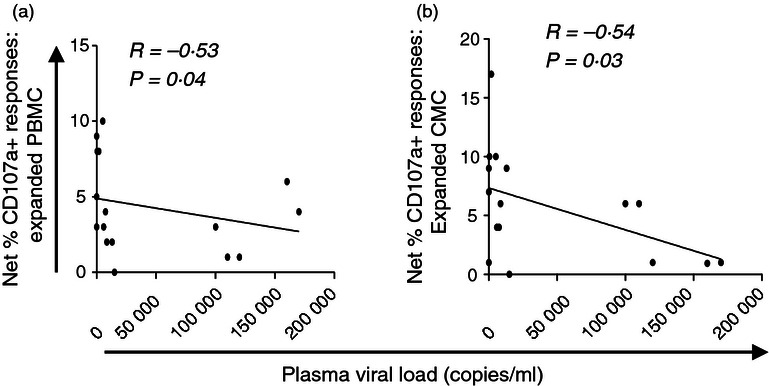
Relationship between HIV plasma viral load and blood or cervical CD8+ T-cell CD107a responses to Gag in expanded T-cell lines. Relationship between expanded blood (a) and cervical (b) CD8+ T-cell responses (shown as percentages of cells producing CD107a) and plasma viral load. Spearman Rank Test was applied to test correlations and P-values < 0·05 were considered significant. Spearman Rho scores are shown on each plot.
In addition, we found that women not shedding HIV in their genital secretions (n = 3/16) had significantly higher cervical CD107a CD8+ T-cell responses to Gag than those shedding HIV (n = 13/16; P = 0·02, data not shown). In addition, CD4+ T cells producing TNF-α in the genital tract of non-shedders were found at significantly higher frequencies than in shedders (P = 0·02, data not shown).
Comparison of functional Gag-specific T-cell responses in blood and at the cervix
Thirteen of 16 individuals exhibited more than one functional response to Gag in their cervical compartment, while 11/16 showed this in blood (Fig. 4a,b). We found that polyfunctional CD8+ T-cell responses to Gag (two or more functions) in blood significantly predicted similarly polyfunctional CD8+ T-cell responses at the cervix; P = 0·03; data not shown). No significant relationship was observed in polyfunctional frequencies between compartments for CD4+ T cells. HIV Gag-specific CD8+ and CD4+ T-cell responses in cervical mucosa and blood from these chronically HIV-infected women were predominantly monofunctional with > 80% of CD8+ and CD4+ T-cell responses to Gag being represented by one function.
Figure 4.
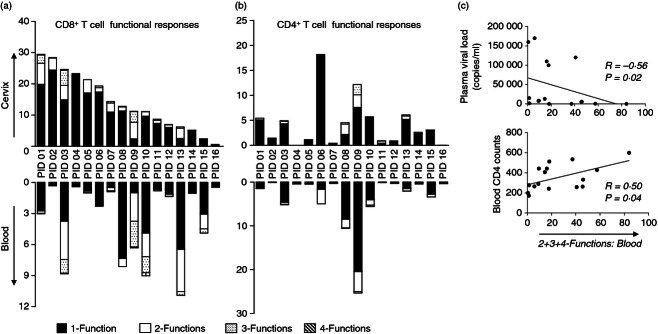
Comparison of HIV-specific T-cell responses between cervix and blood from HIV-infected women and impact of HIV clinical status. (a) Frequency of CD8+ responses within each functional category at the cervix (top graph) were compared with those found in blood (bottom graph). Polyfunctional responses were defined as the total of 2+, 3+ and 4+ functions in blood and at the cervix of CD8+ T cells. (b) Frequency of CD4+ T-cell responses within each functional category at the cervix (top graph) were compared with those found in blood (bottom graph). Responses were considered four-functional (grey) if all four of the markers included in the polyfunctional panel were detected: interferon-γ (IFN-γ), macrophage inflammatory protein 1β (MIP-1β), tumour necrosis factor-α (TNF-α) and CD107a. Responses were considered three (dotted), two (open bar) or one functional if any combinations of three, two or one of the four functions investigated were detected, respectively. The bars on the plots represent median functional response after background subtraction. (c) Relationship between polyfunctional (two or more functions) CD8+ T-cell responses to Gag and markers of HIV clinical status. (top graph) Plasma viral load and (bottom graph) blood CD4 T-cell counts associated with polyfunctional CD8+ T-cell responses (defined as two or more responses). Spearman Rank Test was applied to test correlations and P-values < 0·05 were considered significant. Spearman Rho scores are shown on each plot.
Impact of clinical status on polyfunctional responses in blood and at the cervix
We finally evaluated whether the ability of T cells from blood and cervix to mount polyfunctional responses was influenced by HIV clinical status. The frequency of blood and cervical Gag-specific CD8+ T-cell polyfunctional responses (two or more responses) was inversely associated with plasma viral load (significantly so for blood: ρ = −0·56, P = 0·02, Fig. 4c top panel; but only a trend towards this at the cervix: ρ = −0·41; P = 0·12, data not shown). Similarly, the frequency of Gag-specific polyfunctional CD8+ T-cell responses in blood was positively associated with blood CD4 counts (Fig. 4c bottom panel; ρ = 0·50; P = 0·04). We found no significant relationship between cervical polyfunctional T-cell responses (or any subset of individual T-cell responses) and cervical viral load (ρ = −0·42; P = 0·1; data not shown). Polyfunctional CD4+ T-cell responses, on the other hand, in both compartments were not similarly impacted by HIV clinical status.
As plasma viral load and HIV shedding were associated, we evaluated whether women shedding HIV in their genital secretions had altered proportions or frequencies of polyfunctional T-cell responses at the cervix compared with women not shedding at the mucosa. We observed no significant differences in magnitude or proportions of one, two, three and four functional responses within the cervical CD4+ and CD8+ T-cell compartment between shedders and non-shedders (data not shown).
Discussion
There is substantial evidence that blood HIV-specific CD8+ T cells play an important role in controlling HIV replication in blood,30–34 but their frequency and the complexity of their effector function at the cervix is not well understood. This study investigated the relationship between HIV clinical status and cervical mucosal T-cell functionality in HIV-infected women. Evaluation of HIV-specific immune responses, particularly at the genital tract, will provide new information for the better understanding of virus–host interactions during HIV infection. This study used multiparameter flow cytometry to simultaneously assess whether HIV-specific T-cell response quality is an important factor in HIV shedding in the female genital tract. The magnitude of responses largely correlated between the two compartments, but interestingly the total magnitude of CD8 T-cell responses was higher in cervical samples and blood. This difference may reflect a higher frequency of effector memory cells in the cervix relative to blood as previously shown directly ex vivo16 or following polyclonal expansion.24
This study detected only a small proportion of CD8+ T cells with two or more simultaneous functions (∼ 1%) at the cervix and the frequency of these polyfunctional responses was not significantly different from those detected in blood (< 0·5%). Monofunctional T cells mediated most of the Gag-specific T-cell responses in both compartments. Previously, Betts et al.6 showed that polyfunctional HIV-specific CD8 T cells are rarely found in the blood of individuals with progressive HIV infection. However, several studies of functional capacity and the magnitude of HIV-specific T cells in other mucosal sites have confirmed the existence of polyfunctional HIV-1 specific T cells at mucosal sites.13–15,35 Brenchley et al.35 simultaneously measured expression of IFN-γ, TNF-α and IL-2 in 26 therapy naive individuals and showed that HIV-specific CD8+ T cells in lungs tended towards a more polyfunctional phenotype (∼ 5%) compared with blood CD8 T cells (< 1%). Moreover, HIV-specific CD4+ T cells from lungs were present at significantly higher frequencies and manifested a significantly more polyfunctional response (∼ 15%) compared with HIV-specific CD4+ T cells from blood (< 1%). The majority of responses present in both lungs and blood were monofunctional (∼ 80%). At the rectal mucosa, Critchfield et al.13 evaluated five distinct effector functions (CD107a, IFN-γ, MIP-1β, IL-2 and TNF-α) of HIV Gag-specific CD8+ T cells in rectal mucosa and blood and found that rectal CD8+ responses are detected at higher frequencies than those in blood (5·9% versus 3·8%). Their data showed that blood and mucosal compartments are dominated by monofunctional T cells, however, the polyfunctionality of T-cell responses increased in individuals who were controlling HIV better in both blood and cervix. Confirming our previous report, comparison between expanded blood and cervical T-cell responses to Gag showed that the magnitude of responses largely correlated significantly between compartments.
Studies investigating the relationship between the magnitude or breadth of HIV-specific cytotoxic T lymphocytes in blood and HIV clinical status have reported conflicting findings.30–32,36,37 Few studies have evaluated the relationship between the frequency or functionality of HIV-specific T-cell responses at mucosal surfaces and HIV clinical status. A significant inverse relationship was observed between plasma viral load and CD8+ T cells expressing CD107a in fresh blood, expanded blood and expanded cervical lines. This indicates that the ability of CD8+ T cells from both the cervix and in blood to degranulate was highest in women with lowest plasma viral load. It is likely that polyfunctional responses systemically as well as at mucosal surfaces are a consequence of an intact immune system in individuals with low viral replication, rather than the cause of low virus replication. The finding that cervical and systemic CD107a responses are similarly effected by clinical status is not surprising given our finding that (i) blood CD107a responses are significantly associated with cervical CD107a responses; (ii) plasma viral load is strongly associated with genital tract HIV shedding (either as the result of passive transudate from plasma across mucosal surfaces or through active homing of HIV-infected target cells to the mucosa in individuals with high systemic viral loads), and (iii) CD4 counts between compartments are also linked.
Gumbi et al.16 showed that IFN-γ+ T-cell responses to HIV Gag were also not associated with protection from HIV genital shedding. Similarly, we show here by measuring four functions of cervical Gag-specific CD8+ T cells that these cells are not able to protect against genital HIV shedding. It could be argued that the measurement of four functions will not necessarily be more biologically relevant than a single function, but recent findings have demonstrated the added value of this approach. Critchfield et al.13 reported that the frequency of rectal mucosal Gag-specific CD8+ T cells capable of three or more effector functions was significantly associated with blood CD4 count and inversely related to plasma viral load suggesting that these responses may play an important role in mucosal immune surveillance, as suggested by their relative enrichment among persons who control HIV in the absence of therapy. Alternatively, worse clinical disease status may be associated with compromised HIV-specific polyfunctional responses. Accumulation of polyfunctional HIV-specific cellular responses have been associated with restoration of mucosal CD4+ T cells, despite persistent mucosal CD4+ T-cell proviral reservoirs and immune activation in individuals on long-term HAART.14 However, data from the present study suggest that systemic viral loads were the major determinant for improved functionality in blood and at the genital tract.
In summary, we demonstrate that HIV Gag-specific T-cell responses detectable in the female genital tract were largely monofunctional with only a small proportion of HIV-infected women having polyfunctional responses. Polyfunctional cells were largely present in women with high CD4 T-cell counts in blood and low plasma viral loads. As a result of the differing microenvironments in blood and at the cervix, it is important to understand the differences in immune pressures imposed on HIV in these compartments. This study adds to our understanding of localized cellular responses against HIV in the genital tract of chronically infected women. The observation that CD107a responses correlated between compartments and was associated with the HIV disease status of the women perhaps argues against differences in immune pressure. Perhaps an important question for future studies will be to determine the degree of trafficking of specific cellular populations between blood and the genital mucosa.
Acknowledgments
We thank the women from the Nyanga Day Hospital who kindly participated in the study and Mrs Janine Jones for collecting all the specimens. We gratefully acknowledge the provision of recombinant human IL-2 by the National Institutes of Health AIDS Research and Reference Reagent Program.
Disclosure
This work was funded by the Wellcome Trust and the International AIDS Vaccine Initiative (IAVI). AB, WB and JP received training in the USA as part of the Columbia University-Southern African Fogarty AITRP Program. JP and WB are Wellcome Trust Intermediate Fellows in Infectious Diseases/Public Health and Tropical Medicine.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Impact of polyclonal expansion on the cumulative and individual net frequencies of functional responses to HIV Gag measured in fresh versus expanded blood T-cell lines.
References
- 1.Jin X, Bauer DE, Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–8. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–10. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goulder PJ, Phillips RE, Colbert RA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–7. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 5.Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, Bangham CR, Phillips RE. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci U S A. 1997;94:1890–5. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204:1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darrah PA, Patel DT, De Luca PM, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 9.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–63. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boaz MJ, Waters A, Murad S, Easterbrook PJ, Vyakarnam A. Presence of HIV-1 gag-specific IFN-γ+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J Immunol. 2002;169:6376–85. doi: 10.4049/jimmunol.169.11.6376. [DOI] [PubMed] [Google Scholar]
- 11.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenchley JM, Knox KS, Asher AI, et al. High frequencies of polyfunctional HIV-specific T cells are associated with preservation of mucosal CD4 T cells in bronchoalveolar lavage. Mucosal Immunol. 2008;1:49–58. doi: 10.1038/mi.2007.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchfield JW, Lemongello D, Walker DH, Garcia JC, Asmuth DM, Pollard RB, Shacklett BL. Multifunctional human immunodeficiency virus (HIV) gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J Virol. 2007;81:5460–71. doi: 10.1128/JVI.02535-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–88. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 15.Ferre AL, Hunt PW, Critchfield JW, et al. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood. 2009;113:3978–89. doi: 10.1182/blood-2008-10-182709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gumbi PP, Nkwanyana NN, Bere A, et al. J Virol. 2008;82:8529–36. doi: 10.1128/JVI.00183-08. Impact of mucosal inflammation on cervical human immunodeficiency virus (HIV-1)-specific CD8 T-cell responses in the female genital tract during chronic HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaul R, Plummer FA, Kimani J, et al. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–11. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 18.Shacklett BL, Beadle TJ, Pacheco PA, et al. Characterization of HIV-1-specific cytotoxic T lymphocytes expressing the mucosal lymphocyte integrin CD103 in rectal and duodenal lymphoid tissue of HIV-1-infected subjects. Virology. 2000;270:317–2. doi: 10.1006/viro.2000.0299. [DOI] [PubMed] [Google Scholar]
- 19.Ibarrondo FJ, Anton PA, Fuerst M, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–97. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shacklett BL, Yang O, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 21.Nkwanyana NN, Gumbi PP, Roberts L, et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128:e746–57. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebenberg LJ, Gamieldien H, Mkhize NN, Jaumdally SZ, Gumbi PP, Denny L, Passmore JA. Stability and transport of cervical cytobrushes for isolation of mononuclear cells from the female genital tract. J Immunol Methods. 2011;367:47–55. doi: 10.1016/j.jim.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bere A, Denny L, Hanekom W, Burgers WA, Passmore JA. Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women. J Immunol Methods. 2010;354:68–79. doi: 10.1016/j.jim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bere A, Denny L, Burgers WA, Passmore JA. Polyclonal expansion of cervical cytobrush-derived T cells to investigate HIV-specific responses in the female genital tract. Immunology. 2010;130:23–3. doi: 10.1111/j.1365-2567.2009.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal SM, Ball TB, Kimani J, Kiama P, Thottingal P, Embree JE, Fowke KR, Plummer FA. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–3. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 26.Passmore JA, Milner M, Denny L, et al. Comparison of cervical and blood T-cell responses to human papillomavirus-16 in women with human papillomavirus-associated cervical intraepithelial neoplasia. Immunology. 2006;119:507–14. doi: 10.1111/j.1365-2567.2006.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trickett AE, Kwan YL, Cameron B, Dwyer JM. Ex vivo expansion of functional T lymphocytes from HIV-infected individuals. J Immunol Methods. 2002;262:71–83. doi: 10.1016/s0022-1759(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 28.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate data sets. Cytometry. 2011;79A:167–74. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Columb MO, Sagadai S. Multiple comparisons. Curr Anaesth Crit Car. 2006;17:233–6. [Google Scholar]
- 30.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–92. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, Casazza JP, Koup RA. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol Lett. 2001;79:117–25. doi: 10.1016/s0165-2478(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 32.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaufmann DE, Bailey PM, Sidney J, et al. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol. 2004;78:4463–77. doi: 10.1128/JVI.78.9.4463-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramduth D, Chetty P, Mngquandaniso NC, et al. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J Infect Dis. 2005;192:1588–96. doi: 10.1086/496894. [DOI] [PubMed] [Google Scholar]
- 35.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–9. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 36.Dalod M, Dupuis M, Deschemin JC, et al. Weak anti-HIV CD8+ T-cell effector activity in HIV primary infection. J Clin Invest. 1999;104:1431–9. doi: 10.1172/JCI7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogg GS, Dong T, Hansasuta P, et al. Four novel cytotoxic T-lymphocyte epitopes in the highly conserved major homology region of HIV-1 gag, restricted through B*4402, B*1801, A*2601, B*70 (B*1509) AIDS. 1998;12:1561–3. doi: 10.1097/00002030-199812000-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.