Abstract
We have previously demonstrated that the anti-inflammatory prostaglandin 15-deoxy-Δ 12,14-prostaglandin J2 (15dPGJ2) delays inflammation-induced preterm labour in the mouse and improves pup survival through the inhibition of nuclear factor-κB (NF-κB) by a mechanism yet to be elucidated. 15dPGJ2 is an agonist of the second prostaglandin D2 receptor, chemoattractant receptor homologous to the T helper 2 cell (CRTH2). In human T helper cells CRTH2 agonists induce the production of the anti-inflammatory interleukins IL-10 and IL-4. We hypothesized that CRTH2 is involved in the protective effect of 15dPGJ2 in inflammation-induced preterm labour in the murine model. We therefore studied the effects of a specific small molecule CRTH2 agonist on preterm labour and pup survival. An intrauterine injection of lipopolysaccharide (LPS) was administered to CD1 mice at embryonic day 16, ± CRTH2 agonist/vehicle controls. Mice were killed at 4.5 hr to assess fetal wellbeing and to harvest myometrium and pup brain for analysis of NF-κB, and T helper type 1/2 interleukins. To examine the effects of the CRTH2 agonist on LPS-induced preterm labour, mice were allowed to labour spontaneously. Direct effects of the CRTH2 agonist on uterine contractility were examined ex vivo on contracting myometrial strips. The CRTH2 agonist increased fetal survival from 20 to 100% in LPS-treated mice, and inhibited circular muscle contractility ex vivo. However, it augmented LPS-induced labour and significantly increased myometrial NF-κB, IL-1β, KC-GRO, interferon-γ and tumour necrosis factor-α. This suggests that the action of 15dPGJ2 is not via CRTH2 and therefore small molecule CRTH2 agonists are not likely to be beneficial for the prevention of inflammation-induced preterm labour.
Keywords: 15-deoxy-Δ12,14-prostaglandin J2; chemoattractant receptor homologous to the T helper 2 cell; lipopolysaccharide; murine preterm labour; nuclear factor-κB
Introduction
Preterm labour is one of the most challenging complications of human pregnancy. Its incidence in the western world remains between 6 and 15% depending on the geography and demographics of the population.1 It is a heterogeneous condition,2 with the only firm causal link being that of infection.3 Despite the increased awareness of the association between infection and inflammation and preterm labour,4 there have been limited advances in the treatment and prevention of preterm labour. Currently, there is a drive to develop anti-inflammatory therapies to not only delay preterm labour, but to prevent the long-term neurological damage thought to be a result of the impact of pro-inflammatory factors on fetal inflammatory response syndrome.
The transcription factor nuclear factor-κB (NF-κB), which is classically associated with inflammation, is central to regulating the biochemical pathways involved in both term labour and preterm labour.5 The oxytocin receptor and cyclo-oxygenase-2 (COX-2) genes contain NF-κB response elements in their promoter regions.6,7 The oxytocin receptor mediates oxytocin-induced myometrial contractions through activation of phospholipase C and downstream calcium release from intracellular stores.8 The COX-2 enzyme is the rate-limiting step for prostaglandin synthesis, which is responsible for uterine contractions and cervical dilatation. NF-κB is also involved in the transcriptional regulation of matrix metalloproteinases, including matrix metalloproteinase-9, which are required for remodelling of the extracellular matrix,9 leading to cervical ripening and fetal membrane rupture. A positive feed-forward loop also exists from activation of NF-κB by the pro-inflammatory cytokines and subsequently their transcriptional activation, including tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β).5,10,11 Hence, premature activation of NF-κB by pro-inflammatory cytokines, or activation of the Toll-like receptors as a result of infection and inflammation, can lead to the amplification of the pro-inflammatory response and the transcription of the labour-associated genes via NF-κB activation, resulting in preterm labour.
Inhibition of NF-κB is an attractive therapeutic target because apart from inhibiting labour-associated genes involved in uterine contractility, cervical ripening and fetal membrane rupture, it would also target pro-inflammatory cytokine production, which may contribute to the neurological damage seen independently of the effect of prematurity. We have previously shown that 15-deoxy-Δ 12,14-prostaglandin J2 (15dPGJ2), an anti-inflammatory cyclopentenone prostaglandin, inhibits NF-κB activity and COX-2 in vitro in both human cultured myocytes and amniocytes.12 In a murine model of inflammation-induced preterm labour, 15dPGJ2 delays preterm labour from 20 hr post lipopolysaccharide (LPS) injection to 30 hr post LPS plus 15dPGJ2 injection. More importantly 15dPGJ2 improved pup survival from 30% with LPS, to 95% with co-injection of LPS and 15dPGJ2.13 The mechanism by which 15dPGJ2 inhibits NF-κB is not entirely understood. The 15dPGJ2 has more than one ligand, including peroxisome proliferator-activated receptor-γ14 and the second prostaglandin D2 (PGD2) receptor chemoattractant receptor homologous to the T helper 2 cell (CRTH2).15 We have shown that 15dPGJ2 does not inhibit NF-κB via the peroxisome proliferator-activated receptor-γ.12 Whether CRTH2 plays a role in the mechanism of NF-κB and COX-2 inhibition by 15dPGJ2 is currently unknown.
CRTH2 is a G protein-coupled receptor linked to the Gαi/o subunit.16 It is the classical receptor of the T helper type 2 (Th2) cell,17 and has also been identified on eosinophils18 and basophils.19 CRTH2 mRNA has been detected in non-pregnant human uterine tissue,20 placenta and choriodecidua.21 Prostaglandin D2 stimulates the production of the Th2 cytokines IL-4, IL-5, IL-13 and IL-10 in cultured Th2 cells in vitro.22 Interleukin-4 is a classic Th2 cytokine that is able to inhibit the Th1 response directly, with IL-10 inhibiting the production of inflammatory mediators indirectly.23 Interleukin-10 has also been shown in the mouse to protect the fetus by reducing fetal loss as a result of pro-inflammatory cytokines.24 The function of CRTH2 in non-immune cells remains unclear.
We sought to determine if a small molecule CRTH2 agonist was able to mimic the effects of 15dPGJ2 by exerting anti-inflammatory effects and subsequently delaying preterm labour and providing neuroprotection for the fetus and increased pup survival. The effect of CRTH2 agonists on murine uterine contractility was examined ex vivo using a myograph.
Materials and methods
Reagents
The small molecule agonist CRTH2, referred to from now on as Pyl A, was synthesized commercially by Oxygen Healthcare, (Cambridge, UK) and is chemically identical to the L-888 607 compound from the Merck Frosst Centre for Therapeutic Research (Quebec, QC, Canada).25 The compound has an indole core with an acetic acid side chain and a phenyl sulphide group, which is para-substituted by a chlorine atom. Based on the pharmacokinetics of 5 mg/kg described by Gervais et al.,25 250 μg of Pyl A was used for intrauterine injection. The CRTH2 antagonist GSKCRTH2X was obtained from Glaxo Smith Kline, (London, UK) and 15dPGJ2 from Cayman Chemicals (Ann Arbor, MI). Escherichia coli LPS serotype 0111:B4 (Sigma, St Louis, MO) was used in the murine model of inflammation-induced preterm labour.
Ethics statement
Human blood from non-pregnant women of childbearing age was collected in accordance with the South East London Ethics Committee approval Ref: 10/H0805/54, and in accordance with Imperial College NHS Healthcare Trust Research and Development department where recruitment took place. All blood was collected with written informed consent. Animal studies were performed under UK Home Office Licence 70/6906 and in accordance with the UK Animals (Scientific Procedures) Act of 1986, and the Imperial College Ethics Review Board.
Flow cytometry of granulocytes for detection of CR3 (CD11b) expression
A protocol based on previous studies on CR3 (CD11b) expression was followed.15 Four millilitres of human blood was collected in sodium citrate vacutainers and the granulocyte fraction was isolated by incubating 1 : 1 blood : 4·5% Dextran (Fluka Analytical, Sigma, Gillingham, UK) in PBS for 45 min at 4°. The leucocyte fraction was centrifuged at 500 g for 10 min, and the pellet was resuspended in PBS containing CaCl2 (0·9 mm) and MgCl2 (0·5 mm) and counted. Cells were then pre-incubated at 37°, followed by treatment with the CRTH2 agonists Pyl A or 15dPGJ2 for 15 min. The reaction was terminated by the addition of 1 ml ice-cold FACSFlow. In experiments with the CRTH2 antagonist, pre-incubation with GSKCRTH2X was performed for 10 min at 37°. The cells were then centrifuged at 400 g for 5 min at 4° and resuspended in PBS with 2% fetal calf serum for labelling with phycoerythrin-conjugated anti-CD11b and allophycocyanin-conjugated anti-CD49d for 10 min at 4° in the dark. The red cells were then lysed by the addition of Optilyse-C for 10 min in the dark at room temperature. Cells were then washed and resuspended in PBS and 1% fetal bovine serum for analysis. Eosinophils were identified as CD49d positive and by high side and forward scatter. Flow cytometry settings were as follows: Forward scatter E0 Voltage, 1·00 Amp gain Lin, and Side scatter of 329 Voltage, 1·00 Amp gain Lin.
Murine model of infection-induced preterm labour
CD1 outbred virgin female and stud male mice (Charles River, Margate, UK) were purchased at 6–8 weeks of age. All mice were housed in open cages at 21 ± 1°, on a 12 : 12 light : dark cycle regimen, with ad libitum access to standard chow and water. Timed mating was performed, with the presence of a copulatory plug being classed as E0 (day 0) of gestation. A mini-laparotomy was performed on embryonic day 16 (E16) of gestation correlating with human gestation of between 33 and 34 weeks. Morphine analgesia (2·5 mg/kg) was administered subcutaneously 20 min before surgery. Both uterine horns were exteriorized and the number of live fetuses per horn was determined. Twenty micrograms (25 μl total volume) Escherichia coli LPS serotype 0111:B4 (Sigma) or sterile PBS was injected into the upper right uterine horn between the first and second sacs taking care not to enter the amniotic cavity. Two-hundred and fifty micrograms of Pyl A or vehicle control was then injected between the second and third sacs. Treatment groups consisted of (i) vehicle, (ii) LPS, (iii) LPS and Pyl A and (iv) Pyl A alone.
Animals were allowed to recover before fetal wellbeing assessment and tissue collection (myometrium and pup brain) at 4·5 hr post injection. A qualitative assessment of fetal viability was made in accordance with Pinto-Machado.26 Fetuses were deemed viable if they were pink and moved spontaneously or in response to stimulus. In subsequent experiments dams were allowed to deliver spontaneously. Continuous monitoring was achieved via a remote infrared CCTV system. A dose—response for the LPS was first performed to obtain the lowest dose at which preterm delivery was consistently obtained. For tissue harvesting, mice were anaesthetized and killed by cervical dislocation. A laparotomy was performed immediately and pups were killed by decapitation in accordance with the project licence. Before processing tissue, uteri were incised in the longitudinal direction and pups were expelled. Right and left horns of the uterus were snap frozen separately with placentas and vasculature removed. Myometrium from the frozen left uterine horns were used for analysis. Pup brains were also extracted and snap frozen. Tissue was stored at −80° until processing.
Protein extraction
Tissue was ground with a pestle and mortar in liquid nitrogen and homogenized in whole cell lysis buffer (150 mm NaCl, 20 mm Tris–HCl pH 7·5, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, with phosphatase Inhibitor (Sigma) and protease inhibitor (Roche, Burgess Hill, UK). The homogenate was incubated on ice for 5 min and centrifuged for 20 min at 16 200 g at 4°. The supernatant was stored at −80° until use. Protein quantification was performed using the Bio-Rad assay, measuring absorbance at 655 nm (Bio-Rad, Hemel Hemstead, UK).
SDS–PAGE and Western blotting
Approximately 15 μg of extracted protein per sample was resolved by SDS–PAGE and subsequently transferred onto PVDF membranes (GE Healthcare, Little Chalfont, UK) at 100 constant V at 4°. Following transfer, the membrane was then blocked in 5% (weight/volume) milk in Tris-buffered saline with tween (TBST×1) for 1 hr. The membrane was then probed with phospho-p65 (Ser 536) (Cell Signalling, Danvers, MA) primary antibody (1 : 1000 in TBS) overnight at 4° or COX-2 (Santa Cruz, Dallas, TX) primary antibody (1 : 2000 in 1% milk in TBS) for 2·5 hr at room temperature, followed by secondary antibody (1 : 2000 in 1% milk/TBS) for 1 hr at room temperature. Chemiluminescence detection was then carried out with ECL Plus (GE Healthcare). The membranes were developed using a high-performance chemiluminescence film (GE Healthcare). Blots were scanned and densitometry was performed with ImageJ (v1.44p).
Detection of CRTH2 and interleukin mRNA
Total RNA was isolated from tissue with Trizol© according to the manufacturer's instructions. Tissue was washed in PBS and homogenized using the power homogenizer in 1 ml Trizol© per 100 mg of tissue. 1 µg RNA was incubated with 1 μl DNase and 1 μl DNase buffer made up to 10 μl volume with diethylpyrocarbonate-treated water for 15 min at room temperature for removal of contaminating DNA. Eight microlitres of the DNAse-treated mix was incubated with 1 μl 10 mm dNTP and 1 μl oligo-dT(12–18) (0·5 µg/ml) for 5 min at 65°. To this mix, 2 μl 10X RT buffer, 4 μl 25 mm MgCl2, 2 μl 0·1 mm dithiothreitol, 1 μl RNAse Out and 1 μl Superscript III was added. (In the reverse transcriptase controls no Superscript III was added.) The mix was incubated at 42° for 10 min and the reaction was terminated at 70° for 15 min. Then 0·5 μl RNAse H was added and the mix was incubated at 37° for 20 min. Samples were stored at −20° until further use. PCR was used to amplify the cDNA. Paired oligonucleotide primers for amplification of the genes of interest were designed to produce amplicons where the intron/exon boundary was crossed wherever possible. Non-template reverse transcriptase controls were used. Table 1 provides the primers for CRTH2, L-19, COX-2 and the cytokines IL-4, IL-10, interferon-γ (IFN-γ) and TNF-α.
Table 1.
Primer sequences used for amplification of CRTH2, cyclo-oxygenase 2 (COX-2), L-19 and the cytokines: interleukin-4 (IL-4), IL-10, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)
| Forward 5′–3′ | Reverse 5′–3′ | bp | |
|---|---|---|---|
| CRTH2 | CGTAGCCGTGAGCCTGCGACTG | GGCGATTGCGGAGCCCACCACT | 344 |
| COX-2 | AGCCAGGCAGCAAATCCTTGCTGTT | TCAAATCCTGTGCTCATACATTCC | 71 |
| IFN-γ | AGCGGCTGACTGAACTCAGATTGTAG | CGGGTGTAGTCACAGTTTTCAGCTGTATAGG | 252 |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | 175 |
| IL-4 | CGAAGAACACCACAGAGAGTGAGCT | GACTCATTCATGGTGCAGCTTATCG | 237 |
| IL-10 | ACCTGGTAGAAGTGATGCCCCAGGCA | CTATGCAGTTGATGAAGATGTCAAA | 181 |
| L-19 | GAAAAAGAAGGTCTGGTTGGA | TGATCTGCTGACGGGAGTTG | 72 |
Multi-spot enzyme-linked immunosorbent assay
The mesoscale discovery multi-spot ultrasensitive mouse Th1/Th2 9-plex assay was used as per the manufacturer's protocol for the detection of the following cytokines: IL-12, IFN-γ, TNF-α, IL-1β, KC/GRO, IL-4, IL-5, IL-10 and IL-2. Cytokines were quantified against an eight-point calibration curve from 0 to 2500 pg/ml, constructed from serially diluted standards provided by the kit. The 96-well multi-spot plate was blocked in 1% BSA in PBS for 1 hr before the addition of 40 μg of murine myometrium or 100 μg of pup brain protein lysate and incubated for 2 hr at room temperature. The multi-spot ELISA plate was read using a Sanger 2400 imager. The quantities of cytokines were determined against the standard curve and transferred into an excel spreadsheet for further analysis.
Myometrial contractility
Mice were killed by cervical dislocation at E15–16 of gestation; the uterus was harvested, kept in PBS on ice and was used within 5 hr of harvesting. The uterus was dissected either in the longitudinal or horizontal direction to expel the fetuses and the placentas. Vasculature and decidua were removed macroscopically, and 5 × 10 mm strips were mounted on the DMT myograph (DMT, Aarhus, Denmark) in the orientation dependent on the muscle type being examined; longitudinal direction for longitudinal muscle and horizontally for the circular muscle orientation. Strips were stretched to 3 g of tension in the organ baths containing 4 ml Krebs solution (glucose 2·0 g/l, magnesium sulphate 0·141 g/l, potassium phosphate 0·16 g/l, potassium chloride 0·35 g/l, sodium chloride 6·9 g/l, calcium chloride dehydrate 0·373 g/l, sodium bicarbonate 2·1 g/l, pH 7·4) and was gassed with 95% O2 and 5% CO2. Tissue was allowed to equilibrate for 30 min. Cumulative dose responses were performed after 30 min of spontaneous contractions were recorded to serve as baseline contractility. At the end of the experiment 10−7 m oxytocin was added to demonstrate strip viability. Concentrations from 0·1 to 100 μm were added every 20 min at the time of organ bath wash out. Contractility was analysed using the Powerlab software V 5.5.6 (ADI instruments, Oxford, UK) using the peak parameters extension. Data were transferred from the datapad of the Powerlab software onto an excel spreadsheet for analysis. Response to treatment was measured by normalizing to baseline spontaneous contractility and divided by the relevant time-point for the vehicle control.
Statistical analysis
Experimental groups consisted of at least three replicates unless otherwise stated. Statistical analysis was performed with Graph-Pad Prism v5 (GraphPad Software, San Diego, CA). One-way analysis of variance or analysis of variance of repeated measures was conducted, with either Dunnett's or Bonferroni's multiple comparisons tests. Samples with P < 0·05 were considered to be statistically significant.
Results
CRTH2 mRNA
CRTH2 mRNA was detected in murine myometrium by RT-PCR, using L-19 as a housekeeping gene. No significant difference in CRTH2 expression was seen between the treatment groups (Fig. 1). Amplification of CRTH2 was seen by cycle 33 and L-19 by cycle 19.
Figure 1.
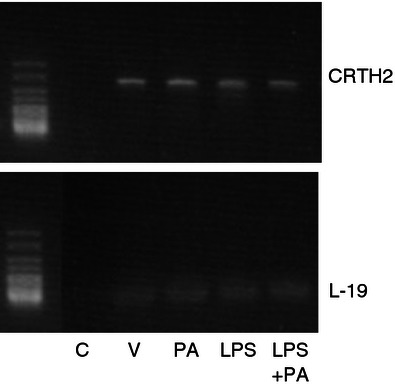
Murine myometrial CRTH2 mRNA. The mRNA was isolated from murine uterus and converted to cDNA (n = 3 per treatment group). RT-PCR was used to amplify CRTH2 showing a product size of 344 bp. No difference in CRTH2 expression was seen between treatment groups. CRTH2 mRNA expression was comparable between mice. C = Non-template control, V = vehicle, PA = Pyl A, LPS = lipopolysaccharide.
Pyl A up-regulates CR3 (CD11b) via CRTH2
The CRTH2 agonists PGD2 and 15dPGJ2 increase the expression of CR3 (CD11b) on eosinophils and basophils via CRTH2.15,27 Before experiments with the CRTH2 agonist Pyl A, activity at the CRTH2 receptor was confirmed by demonstrating up-regulation of CR3 (CD11b) in human eosinophils. We used flow cytometry to detect CR3 (CD11b) expression on eosinophils, identified by high intensity CD49d expression and forward and side scatter characteristics (Fig. 2). Up-regulation of CR3 (CD11b) expression with Pyl A treatment was demonstrated by an increase in mean fluorescence intensity of CD11b-PE (P < 0·01). This effect was attenuated with previous incubation of cells with the CRTH2 antagonist GSKCRTH2X (Fig. 2a,b). The effect of Pyl A was identical to the effect of 15dPGJ2 in causing increased expression of CR3 (Fig. 2c).
Figure 2.
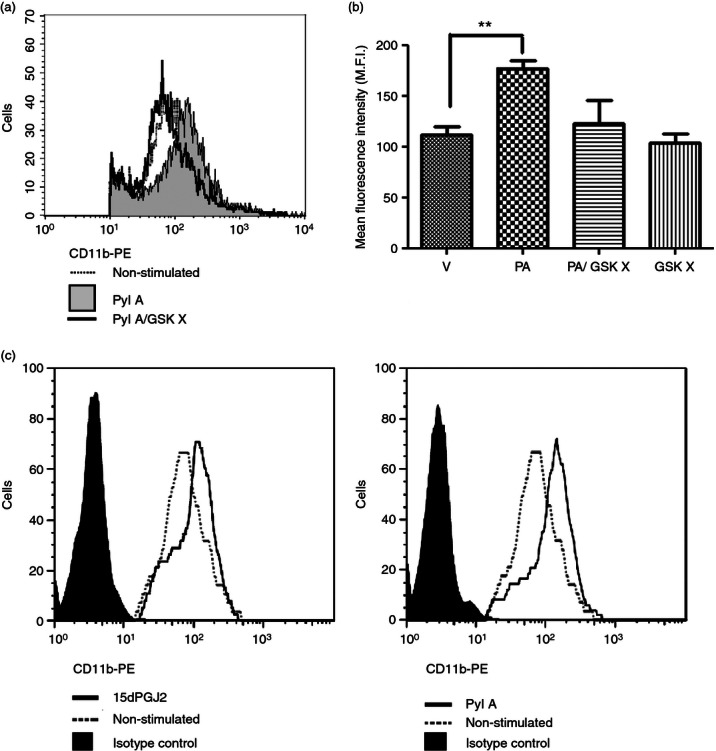
The effect of Pyl A and GSKCRTH2X on CR3 (CD11b) expression on eosinophils. Pyl A (32 μm) was used to increase CR3 (CD11b) expression on eosinophils. Pre-treatment with the CRTH2 antagonist GSKCRTH2X (100 μm) was used to confirm this effect was via CRTH2. Eosinophils were identified by labelling with anti-CD49d and on the basis of forward and side scatter. A representative histogram reveals a clear shift to the right with Pyl A treatment indicative of an increase in CR3 (CD11b) expression. This effect was attenuated with CRTH2 antagonist pre-treatment (a). A summary of CR3 (CD11b) expressed in eosinophils with each treatment is shown in the graph (n = 3) (b). The effect of Pyl A on CR3 expression is identical to that of 15dPGJ2. A representative histogram is shown for the effect of both 15dPGJ2 and Pyl A on CR3 expression (c). V = vehicle, PA = Pyl A, GSK X = GSKCRTH2X. For statistical analysis, analysis of variance of repeated measures with Dunnett's post hoc test was used; **P < 0.01.
Pyl A induces preterm labour in the mouse
We sought to determine if the CRTH2 agonist Pyl A had the same tocolytic and feto-protective effect as 15dPGJ2 in delaying preterm labour in LPS-treated mice. A dose–response effect was demonstrated with LPS (serotype 0111:B4) since varying potencies can be seen between serotypes and within batches.28 Administration of 20 μg LPS led to reliable preterm delivery with the least variation between mice (Fig. 3a). No surviving pups at the time of delivery were seen with concentrations above 10 μg (Fig. 3b). Subsequent experiments were performed with 20 μg LPS. Twenty micrograms of LPS with vehicle or 250 μg Pyl A was given by intrauterine injection under general anaesthetic and mice were allowed to labour spontaneously. Vehicle control mice delivered 64·5 hr post injection and LPS-treated mice delivered 7·7 hr post injection (P < 0·001) (Fig. 4a). Co-injection of LPS and Pyl A augmented delivery to 5·8 hr (mean) post injection (Fig. 4a). This effect was more pronounced with a higher dose of Pyl A (500 μg) and lower dose of LPS (10 μg), shortening delivery time from 14·7 to 8·7 hr post injection (P < 0·01) (Fig. 4b). Although at 250 μg Pyl A alone did not induce labour, at 500 μg labour was induced at 44·8 hr post injection from 64·6 hr in the vehicle control group. None of the vehicle control-treated mice delivered preterm.
Figure 3.
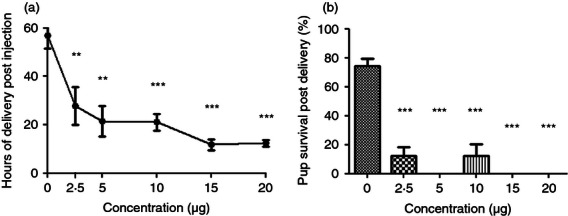
Dose–response of lipopolysaccharide (LPS) induced preterm labour. CD1 mice received an intrauterine injection of vehicle or LPS at E16 and were allowed to deliver, (n = 3–10 per treatment group). No vehicle control mice delivered preterm and LPS induced preterm labour in a dose–response effect, (a). No surviving pups were seen at an LPS dose of >10 μg (b). For statistical analysis, one-way analysis of variance with Dunnett's post hoc test comparing all groups to the vehicle control was used; **P < 0.01, ***P < 0.001.
Figure 4.
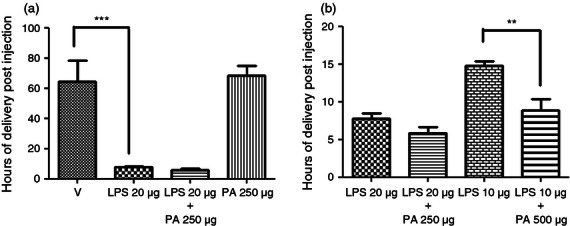
The effect of Pyl A on lipopolysaccharide (LPS) -induced preterm labour. CD1 mice received an intrauterine injection of vehicle, LPS or Pyl A at E16 and were allowed to deliver, (n = 4 per treatment group). No vehicle control mice delivered preterm, and at 250 μg Pyl A alone did not induce preterm labour (a). LPS induced preterm labour in a dose–response manner, and this effect was augmented with the addition of Pyl A (b). V = vehicle, PA = Pyl A. For statistical analysis, one-way analysis of variance with Bonferroni's multiple comparison's test was used; **P < 0.01, ***P < 0.001.
Pyl A prevents LPS-induced intrauterine death
We then determined if the CRTH2 agonist Pyl A maintained the same feto-protective effect as 15dPGJ2 by examining fetal wellbeing at 4·5 hr post intrauterine injection of LPS with vehicle or Pyl A. Mice were anaesthetized and underwent a caesarean section. Fetuses were assessed for viability by assessment of colour and movement with or without mechanical stimulus. A significant improvement in fetal viability was observed when LPS-treated mice were co-injected with Pyl A compared with LPS and vehicle control. There was a clear difference in the appearance between both groups, in that the LPS-treated mice were clearly dead with no respiratory effort, whereas the LPS/Pyl A-treated mice were pink, moved spontaneously or with stimulus, and had respiratory effort. Fetal survival was increased from 20% in LPS-treated mice to 100% in LPS/Pyl A-treated mice, (P < 0·0001) (Fig. 5a). However, following spontaneous labour no pups were viable in the LPS-treated and LPS/Pyl A-treated groups (Fig. 5b).
Figure 5.
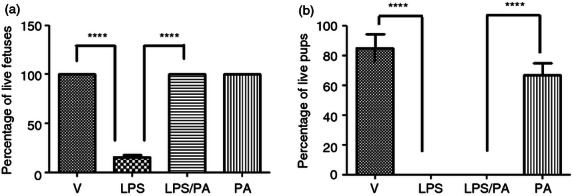
Effect of Pyl A on intrauterine fetal viability at 4.5 hr post intrauterine injection. Dams were killed at 4.5 hr post intrauterine injection of 20 μg of lipopolysaccharide (LPS), 250 μg of Pyl A and pup viability was assessed (n = 3 dams). An average of 11–14 pups per dam was seen in each treatment condition. Pyl A significantly increased viability at 4.5 hr post injection from 20% survival to 100% (a). In a subsequent experiment mice were allowed to deliver spontaneously. No pups in the LPS-treated or LPS/Pyl A-treated groups survived premature delivery (b). V = vehicle, PA = Pyl A. For statistical analysis, one-way analysis of variance with Bonferroni's multiple comparison test was used; ****P < 0.0001.
The effect of Pyl A on the inflammatory cascade in the myometrium and fetal brain
To explore the mechanisms behind Pyl A-augmented LPS-induced preterm labour, key mediators of inflammation in the myometrium were investigated. Myometrium and pup brain were harvested at 4·5 hr post intrauterine injection and Western blotting was used to detect whole cell phospho-p65 and COX-2. Administration of LPS did not lead to an increase in NF-κB in the myometrium; however, an increase was seen with co-administration of LPS and Pyl A (P < 0·05) (Fig. 6a). A reduction was seen in NF-κB in pup brain with LPS compared with vehicle control, with no increase with co-administration with Pyl A (Fig. 6b). No significant difference in COX-2 protein expression was seen between treatment groups in the myometrium or pup brain at this time-point (Fig. 6c,d). However, the messenger RNA of COX-2 was increased in the myometrium of dams treated with Pyl A and LPS compared with other treatment groups (Fig. 6e).
Figure 6.
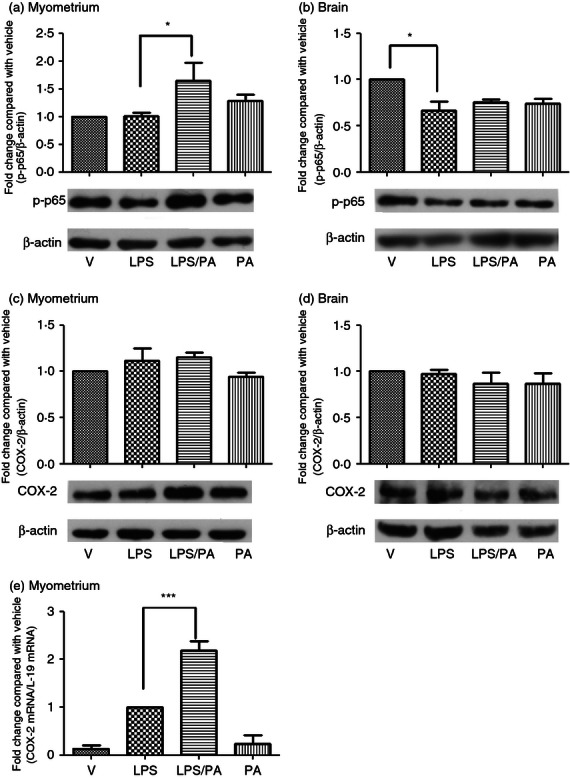
The effect of lipopolysaccharide (LPS) and Pyl A on myometrial and pup brain nuclear factor-κB (NF-κB) and cyclo-oxygenase 2 (COX 2). Dams were killed at 4.5 hr post intrauterine injection and tissue was harvested for protein analysis of phospho-p65 and COX-2 (n = 3). Co-injection of LPS and Pyl A increased myometrial phospho p65 (a), with no effect on pup brain phospho-p65. Phospho-p65 was decreased in brains of pups from dams treated with LPS alone (b). Representative phospho-p65 immunoblots are shown for each treatment group with B actin used as a loading control. No significant changes in COX-2 protein expression were seen in any treatment group in both myometrium and pup brain (c,d). However, an increase in messenger RNA of COX-2 was seen in LPS-treated and LPS/Pyl A-treated mice (e). For statistical analysis, one-way analysis of variance with Bonferroni's multiple comparison test was used; *P < 0.05, ***P < 0.001.
The effect of Pyl A on inflammatory cytokines
We next sought to determine whether activation of NF-κB resulted in downstream activation of pro-inflammatory cytokines. As the CRTH2 agonist PGD2 induces the production of the Th2 cytokines IL-10 and IL-4 in human T cells,22 we anticipated that Pyl A would lead to an increase in these anti-inflammatory cytokines and an inhibition of the pro-inflammatory cytokines. However, consistent with NF-κB activation, Pyl A and LPS led to an increase in the pro-inflammatory cytokines (Fig. 7). Messenger RNA of the Th1 cytokines IFN-γ and TNF-α was significantly increased at 4·5 hr post injection (P < 0·05 and P < 0·01, respectively); however, the increase in protein expression did not reach statistical significance. Protein expression levels of other pro-inflammatory cytokines were significantly elevated including IL-1β, KC/GRO (the murine chemokine equivalent of human IL-829), and IL-12 (P < 0·05).
Figure 7.
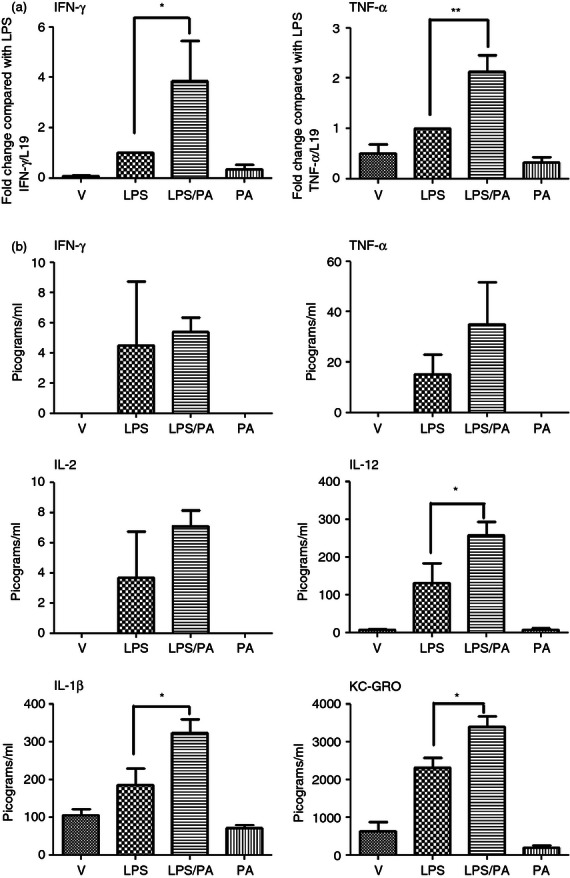
Effect of lipopolysaccharide (LPS) and Pyl A on the pro-inflammatory cytokines in the myometrium. Dams were killed at 4.5 hr post intrauterine injection of 20 µg LPS/250 µg Pyl A and mRNA and protein was extracted from the myometrium and analysed by PCR or ELISA (n = 3). With co-injection of LPS and Pyl A, interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) mRNA were significantly increased (a) as well as protein levels of interleukin-12 (IL-12), IL-1β and KC-GRO (b). V = vehicle, PA = Pyl A. For statistical analysis, one-way analysis of variance with Bonferroni's multiple comparison's test was used; *P < 0.05, **P < 0.01.
The effect of Pyl A on the anti-inflammatory cytokines
The mechanism of increased in utero fetal survival seen with Pyl A was explored by analysing the mRNA and protein expression of Th2 anti-inflammatory cytokines in the myometrium and pup brains. There was no difference in IL-4 mRNA between treatment groups, and protein concentrations were below the detection level of the assay. There was a slight increase in the production of IL-5, and an increase in both mRNA and protein expression of IL-10, which did not achieve statistical significance (Fig. 8). These interleukins were not detectable in fetal brain samples (data not shown).
Figure 8.
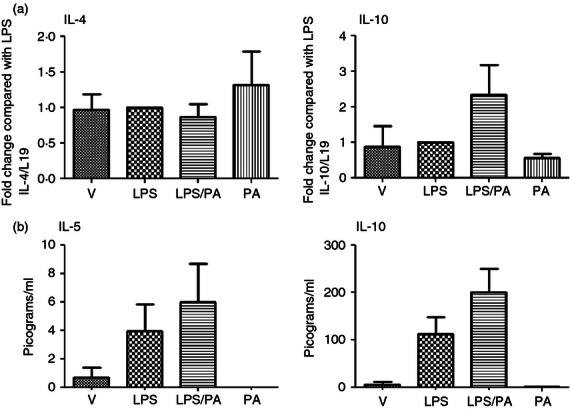
Effect of lipopolysaccharide (LPS) and Pyl A on the anti-inflammatory cytokines in the myometrium. Dams were killed at 4.5 hr post intrauterine injection of 20 μg LPS/250 μg Pyl A and mRNA and protein was extracted from the myometrium and analysed by PCR or ELISA. No change in interleukin-4 (IL-4) mRNA expression was seen, and a non-significant increase in IL-10 was seen with co-administration of Pyl A and LPS (a). Although there was an increase in protein levels of IL-10 and IL-5 with co-injection of LPS and Pyl A, this did not reach statistical significance (b). V = Vehicle, PA = Pyl A.
The effect of Pyl A on uterine contractility
To determine if Pyl A had a direct effect on uterine contractility, uteri were harvested from mice on E15–16, dissected and mounted on the myograph in the circular orientation. Pyl A inhibited myometrial contractility from a concentration of 10 μm (P < 0·01), with complete inhibition seen with 100 μm (P < 0·001) (Fig. 9a,b). The effect of Pyl A on longitudinal muscle was also examined by mounting the strips along the longitudinal orientation. Contractility was not maintained in the longitudinal orientation for the whole duration of the experiment in control strips to robustly examine the effect of Pyl A on longitudinal muscle contractility. Despite this, the clear inhibition seen in the circular muscle was not evident in the longitudinal strips (data not shown). The inhibition of contractility in circular muscle was probably not CRTH2-mediated because other agonists, 15dPGJ2 and 13,14-dihydro-15-keto-prostaglandin D2 (DK-PGD2), did not have the same effect (Fig. 9c–f).
Figure 9.
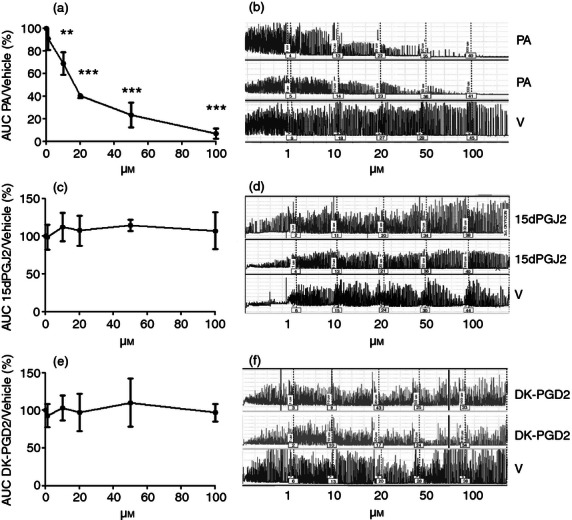
The effect of CRTH2 agonists on circular muscle contractility. CD1 mice were killed on E15–16 and myometrial tissue was harvested to mount on the myograph in the circular orientation (n = 3). A cumulative dose–response of CRTH2 agonists or vehicle was performed and the average area under the curve was determined. An inhibition in myometrial contractility was seen with Pyl A, but no effect with 15dPGJ2 or DK-PGD2. Graphs showing the effects of the CRTH2 agonists are shown (a,c,e), with representative traces of myometrial contractility (b,d,f). V = vehicle, PA = Pyl A. For statistical analysis, analysis of variance of repeated measures was used with Dunnett's multiple comparison test; **P < 0.01, ***P < 0.001.
Discussion
The search for preventative therapies for both preterm birth and related neurological injury has largely focused upon anti-inflammatory strategies. It is generally accepted that parturition is a pro-inflammatory event, with preterm labour being associated with an exaggerated inflammatory response and infection. When women present in preterm labour, it is likely that inflammation precedes any clinical symptoms. We have previously reported that the anti-inflammatory cyclopentenone prostaglandin and CRTH2 agonist 15dPGJ2 delays inflammation-induced preterm labour in the mouse and increases pup survival.13 In this study we have examined the potential for acute administration of a small molecule CRTH2 agonist to improve both maternal and fetal outcomes in LPS-induced murine preterm labour.
The CRTH2 agonist Pyl A was manufactured based on the L-888 607 compound from the Merck Frosst Centre for Therapeutic Research in which radioligand binding assays were used to demonstrate the affinity of the compound to the CRTH2 receptor.25 The CRTH2 agonist activity of Pyl A was confirmed with a gold standard experiment based on the work of Cossette, Monneret and Nagata, in which the CRTH2 agonists PGD2, DK-PGD2, indomethacin and 15dPGJ2 cause up-regulation of CR3 (CD11b) in granulocytes.15,27,30–32 Pyl A caused a significant increase in the expression of CR3 (CD11b) in human eosinophils, which could be attenuated by pre-incubation with the CRTH2 antagonist GSKCRTH2X (Fig. 2), further confirming activity at the CRTH2 receptor. CR3 (CD11b) up-regulation via CRTH2 is believed to aid cell adhesion to the vascular wall for migration of cells from the blood into tissue at sites of inflammation.33 The murine CRTH2 gene was first cloned and characterized by Abe et al.34 and shares 77% homology with the human CRTH2 receptor gene. The pharmacologies of the human and mouse CRTH2 receptors are virtually identical, and the receptors share 90% homology within the transmembrane domains.35 The CRTH2 agonists PGD2, DK-PGD2, 15dPGJ2 and indomethacin all show activity to the mouse CRTH2 receptor.36–39 15dPGJ2 binds to the mouse CRTH2 receptor with an affinity several orders of magnitude greater than that seen for peroxisome proliferator-activated receptor-γ.39,40 We detected CRTH2 mRNA in the mouse myometrium using the primers used by Abe et al.,34 (Fig. 1). There was no difference in mRNA expression between vehicle and Pyl A-treated or LPS-treated mice and LPS/Pyl A-treated mice. However, the degree of expression seen at the mRNA level suggests that CRTH2 is expressed in the myometrium. Determining if expression is seen on both myocytes and infiltrating leucocytes or leucocytes alone has not been possible because of the lack of available specific antibodies to murine CRTH2. Human studies have demonstrated mRNA expression in the myometrium, but flow cytometry confirms the absence of the expressed protein in cultured myocytes.41 CRTH2 positive leucocytes are also detected in the endometrium and are likely to be recruited to decidua via PGD2.42,43
We have previously reported that the CRTH2 agonist 15dPGJ2 delays LPS-induced preterm labour in the mouse, which is thought to be via NF-κB inhibition in the myometrium.13 15dPGJ2 also inhibits NF-κB in human cultured amniocytes and myocytes;12 however, the mechanism by which NF-κB inhibition is achieved is unclear. It was therefore hypothesized that Pyl A could reproduce the effects of 15dPGJ2 of delaying preterm labour by diminishing the pro-inflammatory effect of LPS via NF-κB inhibition. However, co-injection of LPS-treated mice with Pyl A was found to exacerbate time to preterm labour in a dose-dependent response (Fig. 4b). To establish the mechanism of Pyl A-augmented labour onset we first examined the effect of Pyl A and LPS on the pro-inflammatory and pro-labour transcription factor NF-κB and its downstream targets. Treatment of animals with Pyl A alone increased NF-κB activity in the myometrium, which was enhanced with co-administration of LPS (Fig. 6a). The inability of Pyl A to inhibit NF-κB implies that CRTH2 is not involved in the mechanism of 15dPGJ2-mediated inhibition. In support of this, we demonstrated that CRTH2 is not required for 15dPGJ2-mediated inhibition of NF-κB in human amniocytes, myocytes and lymphocytes.41
Surprisingly, myometrial COX-2 protein levels remained unchanged 4·5 hr post treatment in all groups. As preterm labour was typically induced following LPS/Pyl A treatment at 5·8 hr (SEM ± 0·7) it was expected that any COX-2 up-regulation in the myometrium should have already been apparent by 4·5 hr post treatment. It is possible that COX-2 was already up-regulated before intrauterine injection in preparation for term labour, which is one limitation of using a model at E16. Progesterone withdrawal in the mouse occurs late E16 and so downstream activation of pro-labour genes is not likely to have been initiated in our model.44 Consistent with this the majority of labour-associated proteins such as PGE2, PGF2α, the oxytocin receptor and Connexin-43 are not significantly up-regulated until E18.45,46 We have shown, however, that COX-2 is suppressed in pregnancy and is up-regulated from E16, which was not increased further in term labour.47 We further explored the possibility that, despite seeing no change at the protein level, COX-2 was still activated by LPS and LPS plus Pyl A. Messenger RNA was indeed increased in LPS-treated mice, and was further increased with co-injection of Pyl A (Fig. 6e). COX-2 requires peroxidases for activation and the endogenous peroxide tone of smooth muscle cells can be mimicked by nitration.48 Previous studies have shown that peroxynitrite increases the activity of COX-2 with no alteration of COX-2 protein expression.49,50 Consistent with our results, Aisemberg et al.51 demonstrated an increase in LPS-induced mRNA COX-2 with no effect at the protein level. It is plausible that this is a result of LPS-induced NO leading to the formation of peroxynitrite, which in turn, activates COX-2 without alteration of protein expression. Alternatively, it is also plausible that the nitrated form of COX-2 is not recognized by the COX-2 antibody.
Analysis of pup brain extracts collected from LPS-treated dams revealed a decrease in levels of phosphorylated p65 (ser 536). It is thought that this may reflect protein degradation induced by the pre-terminal state of the live pups (Fig. 6b). A significant increase in in utero fetal viability was achieved with Pyl A treatment (Fig. 5a) but this was not associated with altered NF-κB activity. This also highlights the contrasting effects of Pyl A compared with the 15dPGJ2 because we have previously shown that 15dPGJ2 inhibits NF-κB in the pup brain of dams treated with LPS.13 Co-injection of Pyl A and LPS led to an increase in the production of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-12, IL-1β and IL-8 (Fig. 7b). Pro-inflammatory stimuli such as LPS52 and the cytokines TNF-α53 and IL-154 have been shown to activate NF-κB via the canonical pathway by phosphorylating serines, leading to I kappa B (IκB) degradation and translocation of the NF-κB complex into the nucleus, thereby activating gene expression of pro-inflammatory cytokines, which augments the pro-inflammatory response in a positive feed-forward loop.5 The assessed cytokines were also increased but to a lesser degree in the absence of NF-κB activation with LPS treatment alone (Fig. 7a,b). It is therefore plausible that LPS and Pyl A co-administration activates a strong cytokine response, which then further induces NF-κB activation via a feed-forward mechanism. Lipopolysaccharide induces a strong inflammatory response and leads to the recruitment of leucocytes.55,56 CRTH2 agonists also chemoattract CRTH2-positive leucocytes,19,57 including Th2 cells,19 eosinophils58 and dendritic cells.37 The increase in inflammatory cytokines seen with combined injection of both LPS and the CRTH2 agonist Pyl A may be as a result of the increase in infiltrating leucocytes rather than a direct effect on myocytes. Importantly, CRTH2 is also expressed on Th1 cells in the mouse, unlike the human, which is likely to have contributed to the unexpected pro-inflammatory response seen in the mouse.
Several murine studies with CRTH2 agonists/antagonists and the use of CRTH2 knock-out mice have shown a pro-inflammatory role for the CRTH2 receptor.36,38,59–63 The CRTH2 agonist DK-PGD2 causes eosinophilia in mouse lung63 and intra-peritoneal administration of DK-PGD2 causes a two-fold induction of monocyte chemoattractant protein-1 and a 25-fold induction of macrophage inflammatory protein-2.36 Furthermore, in a murine study of FITC-induced inflammation of the skin (a model of contact hypersensitivity), a CRTH2 antagonist was found to significantly reduce the production of the pro-inflammatory cytokines TNF-α, IL-1β and the chemokines macrophage inflammatory protein-2 and GRO-α.64 However, no distinction between the Th1 or Th2 type cytokines being modulated was made. Similarly reduced levels of lung IFN-γ, IL-4 and IL-5 have been observed in a mouse model of airway inflammation upon administration of a CRTH2 antagonist.62
Our finding of increased fetal viability with Pyl A in LPS-treated mice was surprising in view of the shortened time interval from injection to delivery. Although following spontaneous labour there were no surviving pups in the LPS and LPS/Pyl A treatment groups (Fig. 5b). We attribute this to the pups delivering preterm, based on unpublished data showing non-viability at E16 even in the absence of inflammation-induced preterm labour. The CRTH2 agonists indomethacin and PGD2 lead to an increase in the production of the Th2 cytokines in human T cells in vitro,22,30 which in turn can antagonize the Th1 response. In vitro stimulation of Th2 cells by PGD2 requires much higher concentrations to stimulate IL-10 production compared with IL-4, IL-5 and IL-13.1,22 We therefore examined the effect of Pyl A on the Th2-type anti-inflammatory cytokines in the myometrium (Fig. 8). Although no changes in levels of IL-4 were detected, an increase (non-significant) in IL-5 was observed (Fig. 8). Moreover, a non-significant increase in IL-10 mRNA and protein with LPS and Pyl A treatment was detected consistent with improved protection against LPS-induced fetal loss in mice65 as well as the reduced rate of naturally occurring fetal loss in IL-10-deficient mice.24
Although Pyl A led to a small increase in the pro-labour transcription factor NF-κB and the pro-inflammatory cytokines, we did not see an increase in COX-2 protein expression. We therefore examined the direct effect of Pyl A on myometrial contractility ex vivo. Contrary to the expected uterotonic effect, Pyl A administration resulted in complete inhibition of circular muscle contractility (Fig. 9), but had no effect on longitudinal muscle. There is limited knowledge on the functional role of the individual muscle layers of the mouse uterus, the inner circular and outer longitudinal muscle, in pregnancy and parturition. In the myometrium of other species such as the pig and rat, it has been suggested that the function of the longitudinal muscle is to move luminal contents by contraction66 and that tonic contraction of the circular muscle may be required for spacing and retention of embryos/fetuses.67 Circular muscle cells have a higher spontaneous electrical activity than longitudinal muscle cells during rat pregnancy,68 and weak high-frequency contractions in the circular muscle layer prevent movement of fetuses towards the cervix during pregnancy,69 supporting its potential role in the maintenance of pregnancy. If circular muscle contraction is necessary for retention of uterine contents, this would explain how inhibition of circular muscle contraction by Pyl A leads to preterm expulsion of the fetuses, as seen in this study. Consistent with this, relaxation of uterine tone is also believed to be important during human labour.70 It is proposed that relaxation of the lower segment of the uterus, in conjunction with contractions of the fundal region, is required for the passage of the fetus through the birth canal.
Alternatively, relaxation of circular muscle may not be important in murine labour. Many rodent studies suggest that by term, the function of circular muscle becomes more similar to the longitudinal layer, and that contractility of both the circular and longitudinal muscle is required for labour.71–74 It is possible that despite the inhibitory effect on contractions seen with Pyl A ex vivo, that the overwhelming in vivo inflammatory effect was enough to overcome the tocolytic effect resulting in preterm labour. It is also plausible that the in vivo uterotonic effect of Pyl A occurs through indirect stimulation of immune cells and activation of the myometrium via an inflammatory response, rather than a direct action of Pyl A on myocytes. This is comparable to the indirect effect of LPS-induced labour, because addition of LPS to myometrial strips ex vivo does not lead to increased myometrial contractility.
The observed inhibition in myometrial contractility seen with Pyl A is likely to be through a CRTH2-independent mechanism as the other CRTH2 agonists 15dPGJ2 and DK-PGD2 did not show the same effect. At higher concentrations, Pyl A is able to bind to other prostanoid receptors with the rank of order of affinity as follows: CRTH2> TP> EP3> DP> EP4> EP2> FP> IP> EP1.25 Since the TP/IP/EP3/EP1 receptors are considered to be excitatory and the EP2/EP4 and DP1 receptors relaxatory, we hypothesize that Pyl A may be having off-target effects on one of the latter mentioned receptors. The effect of DP1 agonists on murine contractility has been investigated previously by several groups. We have shown that the EP2 agonist, but not EP4 agonist, inhibits human myometrial contractility.75 Stimulation of the EP2 and EP4 receptors leads to cAMP production via the G protein Gs leading to smooth muscle relaxation.76 Hence the effect seen in our study is potentially a result of non-specific binding with the EP2 receptor.
Conclusion
This study presents evidence that the CRTH2 agonist Pyl A augments a pro-inflammatory response in LPS-induced preterm labour in the mouse. Pyl A shortened the time interval from intrauterine injection to preterm delivery via increased NF-κB activity and increased production of pro-inflammatory cytokines. We also demonstrated a non-CRTH2-mediated inhibition of circular myometrial contractility ex vivo, which was likely to contribute to rapid expulsion of the fetus. Despite increased fetal viability seen with Pyl A in LPS-treated dams, an overwhelming pro-inflammatory response was seen with the CRTH2 agonist in the mouse. This may be secondary to a functional CRTH2 receptor on murine Th1 cells, unlike in humans. We conclude that 15dPGJ2-mediated inhibition of NF-κB is not mediated via CRTH2. The CRTH2 agonist seems to augment inflammation-induced preterm birth, so CRTH2 is unlikely to be a suitable therapeutic target for the prevention of preterm labour and neonatal morbidity.
Acknowledgments
This study was funded by a Wellbeing of Women research training fellowship, grant 148 (to LS). PRB is funded by the Imperial College NIHR Biomedical Research Centre.
Disclosures
The authors have no financial disclosures or competing interests.
References
- 1.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002;360:1489–97. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 4.Sykes L, MacIntyre D, Teoh T, Bennett P. Targeting immune activation in the prevention of preterm labour. Eur Obstet Gynecol. 2011;6:100–6. [Google Scholar]
- 5.Lindstrom TM, Bennett PR. The role of nuclear factor κB in human labour. Reproduction. 2005;130:569–81. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 6.Terzidou V, Lee Y, Lindstrom T, Johnson M, Thornton S, Bennett PR. Regulation of the human oxytocin receptor by nuclear factor-κB and CCAAT/enhancer-binding protein-β. J Clin Endocrinol Metab. 2006;91:2317–26. doi: 10.1210/jc.2005-2649. [DOI] [PubMed] [Google Scholar]
- 7.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-κB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod. 2001;7:581–6. doi: 10.1093/molehr/7.6.581. [DOI] [PubMed] [Google Scholar]
- 8.Phaneuf S, Europe-Finner GN, Varney M, MacKenzie IZ, Watson SP, Lopez Bernal A. Oxytocin-stimulated phosphoinositide hydrolysis in human myometrial cells: involvement of pertussis toxin-sensitive and -insensitive G-proteins. J Endocrinol. 1993;136:497–509. doi: 10.1677/joe.0.1360497. [DOI] [PubMed] [Google Scholar]
- 9.Choi SJ, Oh S, Kim JH, Roh CR. Changes of nuclear factor κB (NF-κB), cyclooxygenase-2 (COX-2) and matrix metalloproteinase-9 (MMP-9) in human myometrium before and during term labor. Eur J Obstet Gynecol Reprod Biol. 2007;132:182–8. doi: 10.1016/j.ejogrb.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-κB mediates interleukin-1β-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol. 1999;181:359–66. doi: 10.1016/s0002-9378(99)70562-4. [DOI] [PubMed] [Google Scholar]
- 11.Kniss DA, Rovin B, Fertel RH, Zimmerman PD. Blockade NF-κB activation prohibits TNF-α-induced cyclooxygenase-2 gene expression in ED27 trophoblast-like cells. Placenta. 2001;22:80–9. doi: 10.1053/plac.2000.0591. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom TM, Bennett PR. 15-Deoxy-δ12,14-prostaglandin j2 inhibits interleukin-1β-induced nuclear factor-κB in human amnion and myometrial cells: mechanisms and implications. J Clin Endocrinol Metab. 2005;90:3534–43. doi: 10.1210/jc.2005-0055. [DOI] [PubMed] [Google Scholar]
- 13.Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-δ 12,14-prostaglandin J2 delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 14.Berry EB, Keelan JA, Helliwell RJ, Gilmour RS, Mitchell MD. Nanomolar and micromolar effects of 15-deoxy-δ 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors γ and δ and nuclear factor κB. Mol Pharmacol. 2005;68:169–78. doi: 10.1124/mol.104.009449. [DOI] [PubMed] [Google Scholar]
- 15.Monneret G, Li H, Vasilescu J, Rokach J, Powell WS. 15-Deoxy-δ 12,14-prostaglandins D2 and J2 are potent activators of human eosinophils. J Immunol. 2002;168:3563–9. doi: 10.4049/jimmunol.168.7.3563. [DOI] [PubMed] [Google Scholar]
- 16.Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, O'Neill GP, Gervais FG. Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol. 2002;137:1163–72. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata K, Tanaka K, Ogawa K, et al. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol. 1999;162:1278–86. [PubMed] [Google Scholar]
- 18.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–8. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 19.Hirai H, Tanaka K, Yoshie O, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata K, Hirai H. The second PGD2 receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2003;69:169–77. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- 21.Helliwell RJ, Keelan JA, Marvin KW, et al. Gestational age-dependent up-regulation of prostaglandin D synthase (PGDS) and production of PGDS-derived antiinflammatory prostaglandins in human placenta. J Clin Endocrinol Metab. 2006;91:597–606. doi: 10.1210/jc.2005-1982. [DOI] [PubMed] [Google Scholar]
- 22.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Huunter MG, Pettipher R. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 23.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–50. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 24.Chaouat G, Assal Meliani A, Martal J, Raghupathy R, Elliott JF, Mosmann T, Wegmann TG. IL-10 prevents naturally occurring fetal loss in the CBA x DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-τ. J Immunol. 1995;154:4261–8. [PubMed] [Google Scholar]
- 25.Gervais FG, Morello JP, Beaulieu C, Sawyer N, Benis D, Greig G, Melebranche AD, O'Neill GP. Identification of a potent and selective synthetic agonist at the CRTH2 receptor. Mol Pharmacol. 2005;67:1834–9. doi: 10.1124/mol.104.009068. [DOI] [PubMed] [Google Scholar]
- 26.Pinto-Machado J. Categorization of near-term mouse fetuses according to their viability as a tool for developmental toxicity screening. Biol Neonate. 1990;57:54–9. doi: 10.1159/000243159. [DOI] [PubMed] [Google Scholar]
- 27.Cossette C, Walsh SE, Kim S, Lee GJ, Lawson JA, Bellone S, Rokach J, Powell WS. Agonist and antagonist effects of 15R-prostaglandin (PG) D2 and 11-methylene-PGD2 on human eosinophils and basophils. J Pharmacol Exp Ther. 2007;320:173–9. doi: 10.1124/jpet.106.111062. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Yen H, Chen CH, Jasani N, Soni R, Koscica K, Reznik SE. Prevention of inflammation-associated preterm birth by knockdown of the endothelin-1-matrix metalloproteinase-1 pathway. Mol Med. 2010;16:505–12. doi: 10.2119/molmed.2010.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiratori Y, Hikiba Y, Mawet E, et al. Modulation of KC/gro protein (interleukin-8 related protein in rodents) release from hepatocytes by biologically active mediators. Biochem Biophys Res Commun. 1994;203:1398–403. doi: 10.1006/bbrc.1994.2340. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Hirai H, Takano S, Nakamura M, Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem Biophys Res Commun. 2004;316:1009–14. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura-Uchiyama C, Iikura M, Yamaguchi M, et al. Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2. Clin Exp Allergy. 2004;34:1283–90. doi: 10.1111/j.1365-2222.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- 32.Monneret G, Cossette C, Gravel S, Rokach J, Powell WS. 15R-methyl-prostaglandin D2 is a potent and selective CRTH2/DP2 receptor agonist in human eosinophils. J Pharmacol Exp Ther. 2003;304:349–55. doi: 10.1124/jpet.102.042937. [DOI] [PubMed] [Google Scholar]
- 33.Gervais FG, Sawyer N, Stocco R, et al. Pharmacological characterization of MK-7246, a potent and selective CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells) antagonist. Mol Pharmacol. 2011;79:69–76. doi: 10.1124/mol.110.068585. [DOI] [PubMed] [Google Scholar]
- 34.Abe H, Takeshita T, Nagata K, et al. Molecular cloning, chromosome mapping and characterization of the mouse CRTH2 gene, a putative member of the leukocyte chemoattractant receptor family. Gene. 1999;227:71–7. doi: 10.1016/s0378-1119(98)00599-x. [DOI] [PubMed] [Google Scholar]
- 35.Hata AN, Lybrand TP, Breyer RM. Identification of determinants of ligand binding affinity and selectivity in the prostaglandin D2 receptor CRTH2. J Biol Chem. 2005;280:32442–51. doi: 10.1074/jbc.M502563200. [DOI] [PubMed] [Google Scholar]
- 36.Higuchi S, Kobayashi M, Yoshikawa Y, Tsuneyama K, Fukami T, Nakajima M, Yokoi T. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicol Lett. 2011;200:139–45. doi: 10.1016/j.toxlet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel AB, Trottein F. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: consequence on the polarization of naive Th cells. J Immunol. 2003;170:4943–52. doi: 10.4049/jimmunol.170.10.4943. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto Y, Otani S, Hirai H, et al. Dual functions of prostaglandin D2 in murine contact hypersensitivity via DP and CRTH2. Am J Pathol. 2011;179:302–14. doi: 10.1016/j.ajpath.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hata AN, Zent R, Breyer MD, Breyer RM. Expression and molecular pharmacology of the mouse CRTH2 receptor. J Pharmacol Exp Ther. 2003;306:463–70. doi: 10.1124/jpet.103.050955. [DOI] [PubMed] [Google Scholar]
- 40.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 41.Sykes L, Lee Y, Khanjani S, MacIntyre DA, Yap XJ, Ponnampalam S, Teoh TG, Bennett PR. Chemoattractant receptor homologous to the T Helper 2 Cell (CRTH2) is not expressed in human amniocytes and myocytes. PLoS ONE. 2012;7:e50734. doi: 10.1371/journal.pone.0050734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michimata T, Ogasawara MS, Tsuda H, et al. Distributions of endometrial NK cells, B cells, T cells, and Th2/Tc2 cells fail to predict pregnancy outcome following recurrent abortion. Am J Reprod Immunol. 2002;47:196–202. doi: 10.1034/j.1600-0897.2002.01048.x. [DOI] [PubMed] [Google Scholar]
- 43.Saito S, Tsuda H, Michimata T. Prostaglandin D2 and reproduction. Am J Reprod Immunol. 2002;47:295–302. doi: 10.1034/j.1600-0897.2002.01113.x. [DOI] [PubMed] [Google Scholar]
- 44.Pointis G, Rao B, Latreille MT, Mignot TM, Cedard L. Progesterone levels in the circulating blood of the ovarian and uterine veins during gestation in the mouse. Biol Reprod. 1981;24:801–5. doi: 10.1095/biolreprod24.4.801. [DOI] [PubMed] [Google Scholar]
- 45.Cella M, Farina MG, Dominguez Rubio AP, Di Girolamo G, Ribeiro ML, Franchi AM. Dual effect of nitric oxide on uterine prostaglandin synthesis in a murine model of preterm labour. Br J Pharmacol. 2010;161:844–55. doi: 10.1111/j.1476-5381.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook JL, Zaragoza DB, Sung DH, Olson DM. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: myometrial activation, stimulation, and preterm labor. Endocrinology. 2000;141:1718–28. doi: 10.1210/endo.141.5.7474. [DOI] [PubMed] [Google Scholar]
- 47.Lee YS, Macintyre DA, Pirianov G, Herbert B, Terzidou V, Johnson MR, Bennett PR. Inhibition of Ap-1 delays LPS-induced preterm labour and improves pup outcome in the murine model. Reprod Sci. 2012;19:0–164. [Google Scholar]
- 48.Bachschmid M, Schildknecht S, Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun. 2005;338:536–42. doi: 10.1016/j.bbrc.2005.08.157. [DOI] [PubMed] [Google Scholar]
- 49.Beharka AA, Wu D, Serafini M, Meydani SN. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radic Biol Med. 2002;32:503–11. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]
- 50.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996;93:15069–74. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aisemberg J, Vercelli C, Billi S, et al. Nitric oxide mediates prostaglandins' deleterious effect on lipopolysaccharide-triggered murine fetal resorption. Proc Natl Acad Sci U S A. 2007;104:7534–9. doi: 10.1073/pnas.0702279104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 53.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I κB-α on serines 32 and 36 controls I κB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–83. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF, Romero R, Sharma S. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstet Gynecol. 2009;200:308–e1. doi: 10.1016/j.ajog.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–90. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 57.Michimata T, Tsuda H, Sakai M, Fujimura M, Nagata K, Makamura M, Saito S. Accumulation of CRTH2-positive T-helper 2 and T-cytotoxic 2 cells at implantation sites of human decidua in a prostaglandin D2-mediated manner. Mol Hum Reprod. 2002;8:181–7. doi: 10.1093/molehr/8.2.181. [DOI] [PubMed] [Google Scholar]
- 58.Gervais FG, Cruz RP, Chateauneuf A, Gale S, Sawyer N, Nantel F, Metters KM, O'Neill GP. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J Allergy Clin Immunol. 2001;108:982–8. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 59.He R, Oyoshi MK, Wang JY, Hodge MR, Jin H, Geha RS. The prostaglandin D receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. J Allergy Clin Immunol. 2010;126:784–90. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oiwa M, Satoh T, Watanabe M, Niwa H, Hirai H, Nakamura M, Yokozeki H. CRTH2-dependent, STAT6-independent induction of cedar pollen dermatitis. Clin Exp Allergy. 2008;38:1357–66. doi: 10.1111/j.1365-2222.2008.03007.x. [DOI] [PubMed] [Google Scholar]
- 61.Boehme SA, Franz-Bacon K, Chen EP, Ly TW, Kawakami Y, Bacon KB. Murine bone marrow-derived mast cells express chemoattractant receptor-homologous molecule expressed on T-helper class 2 cells (CRTh2) Int Immunol. 2009;21:621–32. doi: 10.1093/intimm/dxp031. [DOI] [PubMed] [Google Scholar]
- 62.Lukacs NW, Berlin AA, Franz-Bacon K, et al. CRTH2 antagonism significantly ameliorates airway hyperreactivity and downregulates inflammation-induced genes in a mouse model of airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2008;295:L767–79. doi: 10.1152/ajplung.90351.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, Trottein F, Dombrowicz D. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. J Immunol. 2005;174:3703–8. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 64.Boehme SA, Franz-Bacon K, Chen EP, Sasik R, Sprague LJ, Ly TW, Hardiman G, Bacon KB. A small molecule CRTH2 antagonist inhibits FITC-induced allergic cutaneous inflammation. Int Immunol. 2009;21:81–93. doi: 10.1093/intimm/dxn127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–96. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 66.Cao J, Shayibuzhati M, Tajima T, Kitazawa T, Taneike T. In vitro pharmacological characterization of the prostanoid receptor population in the non-pregnant porcine myometrium. Eur J Pharmacol. 2002;442:115–23. doi: 10.1016/s0014-2999(02)01489-9. [DOI] [PubMed] [Google Scholar]
- 67.Crane LH, Martin L. In vivo myometrial activity during early pregnancy and pseudopregnancy in the rat. Reprod Fertil Dev. 1991;3:233–44. doi: 10.1071/rd9910233. [DOI] [PubMed] [Google Scholar]
- 68.Okabe K, Inoue Y, Kawarabayashi T, Kajiya H, Okamoto F, Soeda H. Physiological significance of hyperpolarization-activated inward currents (Ih) in smooth muscle cells from the circular layers of pregnant rat myometrium. Pflugers Arch. 1999;439:76–85. doi: 10.1007/s004249900140. [DOI] [PubMed] [Google Scholar]
- 69.Nesheim BI. Effect of noradrenaline and isoprenaline on the circular and longitudinal muscle of the oestrogen dominated rabbit uterus. Acta Pharmacol Toxicol (Copenh) 1972;31:296–304. doi: 10.1111/j.1600-0773.1972.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 70.Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–33. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 71.Bengtsson B, Chow EM, Marshall JM. Calcium dependency of pregnant rat myometrium: comparison of circular and longitudinal muscle. Biol Reprod. 1984;30:869–78. doi: 10.1095/biolreprod30.4.869. [DOI] [PubMed] [Google Scholar]
- 72.Tuross N, Mahtani M, Marshall JM. Comparison of effects of oxytocin and prostaglandin F2-α on circular and longitudinal myometrium from the pregnant rat. Biol Reprod. 1987;37:348–55. doi: 10.1095/biolreprod37.2.348. [DOI] [PubMed] [Google Scholar]
- 73.Osa T, Katase T. Physiological comparison of the longitudinal and circular muscles of the pregnant rat uterus. Jpn J Physiol. 1975;25:153–64. doi: 10.2170/jjphysiol.25.153. [DOI] [PubMed] [Google Scholar]
- 74.Kuriyama H, Suzuki H. Changes in electrical properties of rat myometrium during gestation and following hormonal treatments. J Physiol. 1976;260:315–33. doi: 10.1113/jphysiol.1976.sp011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sykes L, Kandola M, Teoh T, Bennett PR. The EP2 agonist Butaprost inhibits human myometrial contractility, but the EP4 agonist compound 1F does not. Reprod Sci. 2011;18:135A. [Google Scholar]
- 76.Palliser HK, Hirst JJ, Ooi GT, Rice GE, Dellios NL, Escalona RM, Parkington HC, Young IR. Prostaglandin E and F receptor expression and myometrial sensitivity at labor onset in the sheep. Biol Reprod. 2005;72:937–43. doi: 10.1095/biolreprod.104.035311. [DOI] [PubMed] [Google Scholar]


