Abstract
Human metapneumovirus (hMPV) is the second most common cause of acute lower respiratory tract infections in children, causing a significant public health burden worldwide. Given that hMPV can repeatedly infect the host without major antigenic changes, it has been suggested that hMPV may have evolved molecular mechanisms to impair host adaptive immunity and, more specifically, T-cell memory. Recent studies have shown that hMPV can interfere with superantigen-induced T-cell activation by infecting conventional dendritic cells (DCs). Here, we show that hMPV infects mouse DCs in a restricted manner and induces moderate maturation. Nonetheless, hMPV-infected DCs are rendered inefficient at activating naive antigen-specific CD4+ T cells (OT-II), which not only display reduced proliferation, but also show a marked reduction in surface activation markers and interleukin-2 secretion. Decreased T-cell activation was not mediated by interference with DC–T-cell immunological synapse formation as recently described for the human respiratory syncytial virus (hRSV), but rather by soluble factors secreted by hMPV-infected DCs. These data suggest that although hMPV infection is restricted within DCs, it is sufficient to interfere with their capacity to activate naive T cells. Altogether, by interfering with DC function and productive priming of antigen-inexperienced T cells, hMPV could impair the generation of long-term immunity.
Keywords: conventional dendritic cells, immunological synapse, naive T cells, respiratory viruses, T-cell priming, type I interferons
Introduction
Respiratory viral infections are a major public health burden, especially in the young and the elderly.1,2 Human metapneumovirus (hMPV) is a respiratory virus belonging to the Pneumovirinae subfamily and the Metapneumovirus genus, which has been suggested to be the cause of a significant proportion of respiratory illnesses in the paediatric and adult populations, producing considerable morbidity.1,3–5 Despite modest viral antigenic variability and the presence of anti-viral antibodies, re-infections are recurrent in all age groups.6,7 These findings suggest that hMPV may have evolved molecular mechanisms to evade host immunity and prevent immune clearance.8–12
Dendritic cells (DCs) are professional antigen-presenting cells with the unique capacity to activate naive T cells, which later will exert an anti-viral immune response.13–15 Priming of T cells requires DCs to efficiently capture and present viral proteins as antigenic peptide–MHC complexes and to provide co-stimulatory signals needed for full T-cell activation. These stimulating ligands are provided to T cells through the assembly of an immunological synapse (IS) between DCs and T cells.15,16 Because DCs are essential for the priming and initiation of anti-viral T-cell immunity, interfering with their function can be advantageous for pathogenic viruses.17,18 Here we show that hMPV infects mouse DCs and induces the secretion of interleukin-6 (IL-6), interferon-α (IFN-α) and IFN-β but not IL-12 and tumour necrosis factor-α (TNF-α). Although hMPV-infected DCs significantly up-regulated class II MHC and displayed a mild up-regulation of co-stimulatory molecules on their surface, they failed to efficiently activate antigen-specific naive T cells. Impairment of T-cell activation was not a result of inhibition of IS assembly as we previously described for the human respiratory syncytial virus (hRSV),19 but rather to the action of soluble factors secreted by hMPV-infected DCs. Altogether, hMPV may impair the initiation of T-cell immunity by inducing the secretion of suppressor molecules by DCs.
Materials and methods
Mice
C57BL/6J and BALB/cJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The OT-II transgenic mouse strain encoding a specific T-cell receptor for I-Ab/OVA323–339 was originally obtained from Dr R. Steinman (The Rockefeller University, New York, NY).20 All mice were maintained at the pathogen-free facility of the Pontificia Universidad Católica de Chile (Santiago, Chile) and handled according to institutional guidelines.
Virus preparation
LLC-MK2 cells (American Type Culture Collection #CCL-7, Rockville, MD) were used to propagate and titrate hMPV. Three serogroup A strains were used in this study: a clinical isolate named CZ0107 (obtained from the Laboratorio de Infectología y Virología of the Hospital Clínico de la Pontificia Universidad Católica de Chile), the reference strain NL/1/00 and a recombinant NL/1/00 strain expressing the green fluorescent protein (GFP).21 Virus stock solutions were handled as previously described.22 Non-infectious supernatants from uninfected LLC-MK2 cells were used as mock controls in most experiments. Ultraviolet-inactivated virus (UV-hMPV) was prepared as previously described.19 Opsonized-hMPV (hMPV-IC) was prepared by incubating hMPV for 45 min at 4° with a heat-inactivated anti-hMPV rabbit polyclonal serum generated in our laboratory. Titration of viral stocks, UV-hMPV, IC-hMPV and DC supernatants was performed as described elsewhere over LLC-MK2 cells (ref. 22 and see Supplementary material, Data S1). Where indicated, hMPV-inoculated cells were analysed by flow cytometry to determine the presence of hMPV nucleoprotein (see below) or virally encoded GFP.
Detection of DC infection by flow cytometry and fluorescence microscopy
The DCs were differentiated from C57BL/6J bone marrow-derived precursors as previously described.23 At day 5 of culture, DC preparations (∼ 85% CD11c+ and I-Ab+ cells) were inoculated for 1 hr at 37° with mock, UV-hMPV, IC-hMPV or hMPV at different multiplicities of infection (MOI; see figure legends). Twenty-four hours after inoculation, DCs were stained with anti-CD11c-allophycocyanin/phycoerythrin (APC/PE) (clone HL3; BD Biosciences, Mountainview, CA), washed, fixed with PBS–2% paraformaldehyde, permeabilized with PBS–0·5% Saponin–1% BSA and stained with an anti-hMPV nucleoprotein monoclonal antibody (clone XD10.C7). A highly cross-adsorbed Alexa Fluor-555 conjugated goat anti-mouse IgG antibody was used as the secondary antibody (#A21424; Invitrogen, Carlsbad, CA). Data were acquired in a FACSCanto II flow cytometer (BD Biosciences) and analysed using the FCS express 4 software (De Novo Software, Los Angeles, CA). To determine the percentage of hMPV-infected cells, statistics markers were set in such a way that non-inoculated cells represent ≤ 1% of positive cells. To assess DC infection with hMPV by fluorescence microscopy, cells were seeded over 12-mm diameter coverslips and fixed 24 hr post-inoculation (PBS–1% paraformaldehyde). Then, cells were permeabilized and stained using anti-N/F hMPV FITC-conjugated monoclonal antibodies (EMD Millipore Corporation, Billerica, MA).
DC–T-cell antigen presentation assays
Twelve hours after inoculation with hMPV, DCs were pulsed with 200 nm ovalbumin323–339 peptide (pOVA) and co-cultured with OT-II CD4+ T cells (1 × 105 or titrated amounts of DCs and 1 × 105 T cells). T cells were previously purified by CD4+ negative selection (> 95% purity; MiltenyiBiotec, Teterow, Germany) from lymph nodes and spleens of OT-II transgenic mice. After 24 hr of co-culture, T-cell activation was determined by supernatant-IL-2 ELISA as described elsewhere19 and by flow cytometry analyses using anti-CD4-APC (clone RM4-5), anti-CD25-FITC (clone 7D4), anti-CD71-PE (clone C2) and CD69-PE (clone H1.2F3) (all from BD Biosciences). To assess T-cell proliferation, purified OT-II CD4+ T cells were stained with 5 μm carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) as previously described.24 Then, T cells were co-cultured with DCs as described below. For T-cell activation assays using DC-supernatants, 2 × 105 purified OT-II CD4+ T cells were added to 96-well plates (Nunc MaxiSorp, eBioscience, San Diego, CA) previously coated with 50 ng/well of each anti-CD3ε and anti-CD28 antibodies (clones 145-2C11 and 37·51, respectively) in the presence of 200 μl DC supernatant plus 100 μl fresh medium. Twenty-four hours after incubation, T-cell activation was measured by ELISA and flow cytometry as described above. Supernatants from DCs inoculated with mock, UV-hMPV and 25 μg/ml poly(I : C) were used as controls. Viability of DCs was determined by trypan blue staining for all experiments (see Supplementary material, Fig. S1b,c). Presentation of pOVA323–339 on the surface of MHC-II molecules (I-Ab) in untreated and hMPV-inoculated DCs was assessed using an FITC-conjugated pOVA323–339 peptide (Genscript, Piscataway, NJ). Briefly, DCs were inoculated for 2 hr either with mock or hMPV. Then, cells were washed twice with PBS and incubated in phenol-red free RPMI medium (10% fetal bovine serum, 10 mm HEPES) with 100 nm, 500 nm, 1 μm or 5 μm of the FITC-labelled pOVA323–339 peptide. Twenty-four hours later, DCs were harvested, washed twice with phenol-red free RPMI medium and stained with anti-CD11c-APC and anti-MHC-II-PE antibodies (BD Biosciences). Finally, DCs were washed twice with phenol-red free RPMI medium and analysed immediately by flow cytometry.
Laser confocal microscopy
Dendritic cells and T cells were labelled with 0·5 μm CMTMR-Orange and 1 μm BODIPY FL C5-Ceramide (both from Molecular Probes, Invitrogen), as previously described.24 The DCs and T cells were seeded into microchambers (Lab-Tek Chamber coverglass; Nalge Nunc, Rochester, NH) previously coated with poly-d-lysine (Sigma-Aldrich). Fluorescence measurements were performed on a FluoView FV1000 Confocal Microscope (Olympus, Melville, NY). To evaluate immunological synapse assembly between DCs and T cells, at least 50 randomly selected fields (minimum of 400 conjugates) were visually scored (double blinded) to determine the percentage of T cells with Golgi apparatus polarized towards DCs in DC–T-cell conjugates.
IFN-α/β ELISA and IFN-blocking assays
Determination of IFN-α and IFN-β levels in the supernatants was performed by ELISA according to the manufacturer's recommendations (PBL interferon source, Piscataway, NJ). An antibody cocktail containing a final concentration of 450 neutralizing units per ml (NU/ml) of each, anti-IFN-α and anti-IFN-β neutralizing polyclonal antibodies (Cell Sciences, Canton, MA) and 20 μg/ml of an IFN-α/β receptor-1 blocking monoclonal antibody (clone MAR1-5A3) was added to T cells for 15 min at room temperature before mixing with DCs. After 36 hr, supernatants and cells were harvested and T-cell activation was determined as described above.
Statistical analyses
All statistical analyses were performed using the graphpad prism software, version 5 (GraphPad Software, Inc, San Diego, CA) and are detailed in each figure legend.
Results
hMPV infects mouse DCs
We evaluated whether immature mouse DCs are permissive to hMPV infection by inoculating DCs with variable amounts of infectious (MOI 1–20) and UV-inactivated hMPV (UV-hMPV). After inoculation, DCs expressing the fusion and nucleoprotein of hMPV could be easily identified by fluorescence microscopy among other non-infected DCs (Fig. 1a). Consistent with this observation, flow cytometry analyses of hMPV-inoculated DCs showed infection rates that ranged from 6% for MOI 1 to 30% for MOI 20 (either for the reference strain NL/1/00, herein hMPVNL/1/00 or the clinical isolate CZ0107, herein hMPVCZ0107) (Fig. 1b). In contrast, mock (non-infectious supernatant) -inoculated and UV-hMPV-inoculated DCs showed no significant antibody staining for the hMPV N protein (Fig. 1a,b). Quantitative PCR analyses were consistent with the findings described above (see Supplementary material, Fig. S1a and Data S1). The hMPV-inoculated DCs were positive for viral nucleoprotein RNA, whereas only negligible amounts of hMPV RNA were amplified from DCs challenged with UV-inactivated hMPV or none for control cells (Fig. S1a). Importantly, viability of DCs did not significantly vary with higher MOIs or longer infection times in these experiments (Fig. S1b,c).
Figure 1.
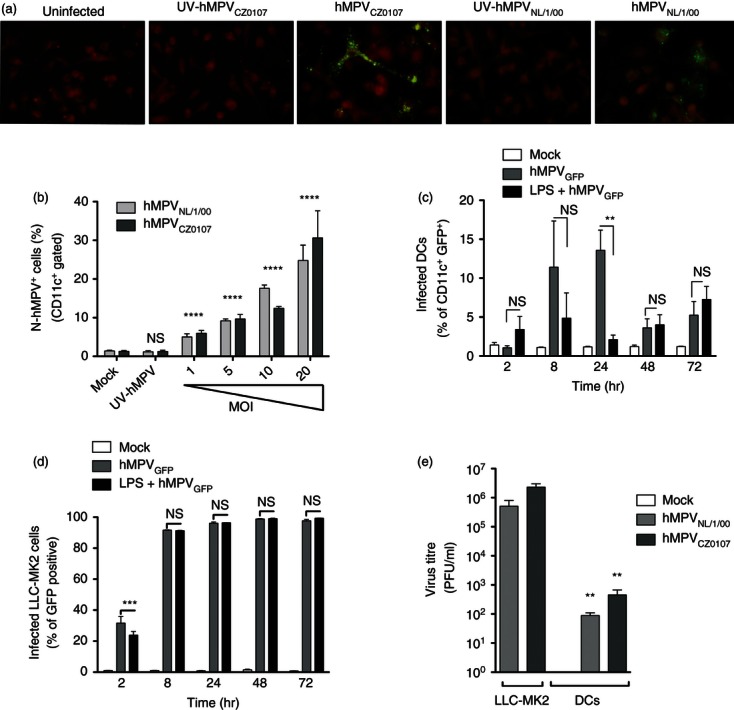
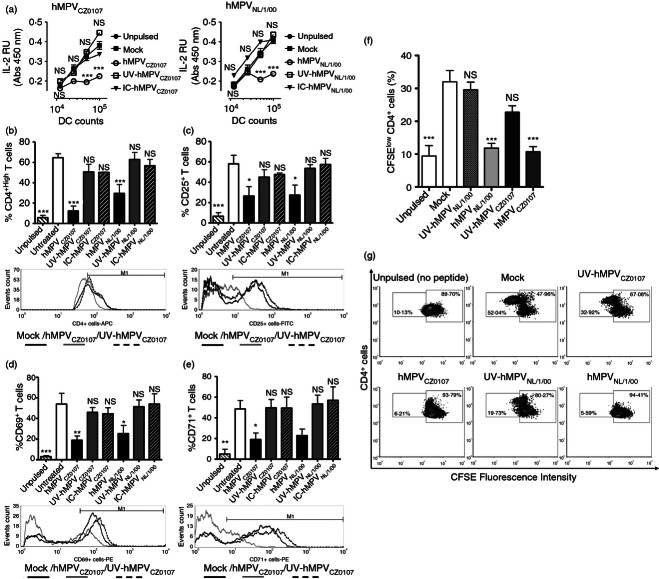
Dendritic cells (DCs) are infected by human metapneumovirus (hMPV). (a) Fluorescence micrographs (100 × magnification) of DCs 24 hr post-inoculation with mock, viable or UV-inactivated hMPV CZ0107 and NL/1/00 strains (Green-Fluorescence: anti-hMPV Nucleoprotein/Fusion protein monoclonal antibodies, Red: Evan's Blue counterstain). One representative experiment out of four independent assays is shown. (b) Flow cytometry detection of hMPV infection in DCs (gate on CD11c+ cells) inoculated either with mock, UV-inactivated [multiplicity of infection (MOI 20)] or increasing amounts of hMPV (MOIs 1– 20). (c) Flow cytometry analysis of DCs inoculated with the green fluorescent protein (GFP) -expressing hMPVNL/1/00 strain (MOI 10), at different time-points post-infection (2–72 hr). Gated CD11c+ cells were assessed for virus-derived GFP expression. Mature (treated with 5 μg/ml LPS) and immature (untreated) DCs were inoculated with mock or the GFP-expressing hMPVNL/1/00 strain at MOI 10. Bars represent flow cytometry data as the percentage of CD11c+ cells expressing GFP. Maturation of lipopolysaccharide (LPS) -treated DCs was confirmed by flow cytometry by measuring MHC class-II, CD40, CD80 and CD86, which were up-regulated as compared to untreated cells (data not shown). (d) Flow cytometry analysis of LLC-MK2 cells inoculated with the GFP-expressing hMPVNL/1/00 strain (MOI 10), at different time-points post-infection (2–72 hr). Untreated or LPS-treated cells were assessed for virus-derived GFP expression. (e) Titration of hMPV in the supernatants of DCs inoculated either with mock or hMPV (strains CZ0107 and NL/1/00). Supernatants were harvested 24 hr post-inoculation and applied over LLC-MK2 monolayers. Supernatants from hMPV infected-LLC-MK2 were used as positive controls for virus-detection. Bars in (b–e) are means of at least three independent experiments ± SEM. One-way analysis of variance and the Bonferroni post-test were used for (b), (d) and (e). Two-way analysis of variance and the Bonferroni post-test were used to compare each time-point against its paired mock in (c). ****P < 0·0001; ***P < 0·001; **P < 0·01; *P < 0·05; n.s.: non-significant.
DC infection with hMPV is restricted
To determine whether DCs are fully permissive to hMPV infection, we assessed the kinetics of infection for DCs with this virus and compared it with that of LLC-MK2 cells. LLC-MK2 cells and mouse DCs were infected either with a recombinant hMPV strain expressing the green fluorescent protein (NL/1/00-GFP) or the two other hMPV strains described above (hMPVNL/1/00 and hMPVCZ0107). Infection of cells was analysed by flow cytometry at different time-points. As shown in Fig. 1(c,d), compared with LLC-MK2 fluorescence derived from the expression of GFP encoded within hMPV was poorly detectable at early times post-infection in DCs. However, starting 8 hr post-inoculation GFP-fluorescence within both cell types was significantly up-regulated and peaked at 24 hr post-inoculation. Unlike LLC-MK2 cells, which displayed sustained green fluorescence throughout the assay, GFP-fluorescence significantly decreased within DCs at 48 and 72 hr post-inoculation (Fig. 1c,d). These results suggest that infection of mouse DCs by hMPV is transient, implying a limited number of virus replication cycles in these cells and probably reduced virion release. As expected, similar results were obtained for the hMPV clinical isolate and the non-fluorescent parental reference strain NL/1/00 when infection was determined using antibodies against the virus (Fig. S1d). Because infection of DCs with viruses may depend on the maturation state of these cells,25,26 hMPV-infection was assessed in immature and lipopolysaccharide-treated DCs using the GFP-expressing virus. As shown in Fig. 1c, immature DCs were infected more efficiently with hMPV than mature DCs. Similar observations were made on DCs derived from another mouse genetic background (Fig. S1e). LLC-MK2 infection was unaffected by lipopolysaccharide treatment (Fig. 1d). These data suggest that replication of the hMPV isolates studied here was transient and inefficient in murine DCs. To further assess this notion, titration of supernatants from hMPV-inoculated DCs was performed over LLC-MK2 cell monolayers to determine whether they contained newly synthesized infective viral particles. Consistent with the results described above, we recovered up to 10 000-fold less virus from the supernatants of hMPV-inoculated DCs as compared with supernatants from LLC-MK2-infected cells (Fig. 1e).
Maturation and cytokine release by hMPV-challenged DCs
To evaluate the effect of hMPV on the capacity of DCs to secrete cytokines, secretion of IL-6, IL-10, IL-12p70, TNF-α and transforming growth factor-β1 (TGF-β1) was measured in the supernatants of hMPV-inoculated cells. When produced by DCs, these cytokines can modulate CD4+ T-cell activation, differentiation and effector functions. DCs inoculated either with the reference or the clinical hMPV strains at various MOIs secreted significant amounts of IL-6 compared with mock-inoculated DCs (Fig. 2a). However, no significant production of IL-10, IL-12p70, TNF-α or TGF-β1 could be detected, even with high viral titers (i.e. MOI equal to 10 or 20) (see Supplementary material, Fig. S2a–d). The Toll-like receptor agonists, poly(I : C) and lipopolysaccharide were used as positive controls for DC cytokine secretion. Dendritic cells inoculated with UV-inactivated hMPV also produced high levels of IL-6 (Fig. 2a).
Figure 2.
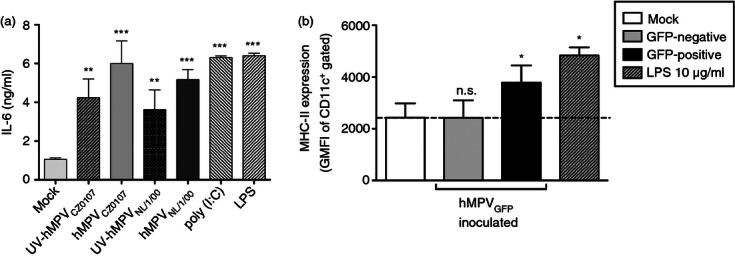
Secretion of cytokines and up-regulation of maturation markers after human metapneumovirus (hMPV) inoculation. (a) Supernatants from hMPV-inoculated and control dendritic cells (DCs) were assessed by ELISA for the presence of interleukin-6 (IL-6). (b) Expression of MHC class II (I-Ab) was measured by flow cytometry at 24 hr post-inoculation (geometric mean fluorescence intensity; GMFI). The hMPV-infected [CD11c+/green fluorescent protein (GFP)+] and non-infected (CD11c+/GFP−) DC populations (within the hMPVGFP-inoculated DC population) were analysed separately and compared with mock- and lipopolysaccharide (LPS) -inoculated DCs. Bars are means of at least four independent experiments ± SEM. Statistical analysis was performed by one-way analysis of variance and the Bonferroni post-test comparing all groups against mock-inoculated DCs. ***P < 0·001; **P < 0·01; *P < 0·05; n.s.: non-significant.
To further evaluate the effect of hMPV infection on DC maturation, we measured the expression of molecules on the surface of DCs that promote or inhibit T-cell activation. The DCs were challenged with the recombinant GFP-expressing NL/1/00 hMPV strain and the expression of MHC class II (I-Ab), CD40, CD80 (B7-1), CD86 (B7-2), PD-L1 (CD274, B7-H1), PD-L2 (CD273, B7-DC) and the adhesion molecules CD48 and intercellular adhesion molecule 1 (ICAM-1) were measured by flow cytometry in GFP+ and GFP− DCs (Fig. S2e–l). Of all molecules assessed, only MHC class II showed significant up-regulation in hMPV-infected (GFP+) DCs (Fig. 2b), whereas no significant variation was observed for most of the other molecules assessed, such as the co-stimulatory receptors CD80 and CD86, when compared with mock-inoculated cells and GFP-negative cells (Fig. S2). These results are in agreement with recent reports showing modest-to-null DC maturation in response to hMPV infection.10,27 Although we observed poor DC maturation in response to hMPV, the phenotype acquired by these cells could potentially lead to T-cell activation because of increased MHC class II expression and mild up-regulation of co-stimulatory molecules.
hMPV infection dampens T-cell activation by DCs
To assess the effect of hMPV on the capacity of DCs to activate naive CD4+ T cells, antigen-specific stimulation assays were performed using control and hMPV-inoculated DCs in the presence of an exogenously added antigenic peptide (pOVA323–339) that is recognized by OT-II CD4+ T cells. The use of this peptide reduces possible variations in antigen presentation to T cells that could result from the differential uptake and processing of whole ovalbumin protein between virus-inoculated and control DCs.
First, we measured IL-2 secretion by OT-II T cells stimulated with increasing numbers of control or hMPV-inoculated DCs loaded with pOVA. Twenty-four hours after incubation, a significant reduction in IL-2 release was observed for T cells stimulated with DCs infected either with hMPVNL/1/00 or hMPVCZ0107, as compared with T cells stimulated either with untreated, mock-inoculated or UV-hMPV-inoculated DCs (Fig. 3a). Equivalent data were obtained at various MOIs (see Supplementary material, Fig. S3a). Importantly, decreased T-cell activation by hMPV-inoculated DCs was not due to reduced antigen presentation, as hMPV-infected DCs expressed increased levels of MHC-II compared with non-infected DCs (Fig. 2b) and bound equivalent amounts of pOVA-FITC on their surface (Fig. S3b). Furthermore, hMPV was also capable of inhibiting activation of CD4+ T cells stimulated with alloantigens in mixed lymphocyte reactions (Fig. S3c). As an additional control, T cells were stimulated by DCs inoculated with hMPV opsonized with neutralizing antibodies (IC-hMPV). As shown in Fig. 3(a), antibody neutralization restored the T-cell priming capacity of DCs, suggesting that hMPV needs to infect DCs to subvert their T-cell priming capacity. Consistent with this notion, DCs challenged with UV-inactivated hMPV retained their capacity to activate T cells (Fig. 3a).
Figure 3.
Human metapneumovirus (hMPV)-inoculated dendritic cells (DCs) display a reduced capacity to activate naive CD4+ T cells. Increasing amounts of either uninfected, mock-inoculated, UV-inactivated-, or viable hMPV-inoculated DCs were used to stimulate antigen-specific T cells in the presence of exogenously added antigenic peptide (pOVA323–339). T cells were assessed for (a) interleukin-2 (IL-2) secretion in the supernatant by ELISA (left panel: hMPV clinical isolate CZ0107, right panel: hMPV reference strain NL/1/00) and surface expression of (b) CD4, (c) CD25, (d), CD69 and (e) CD71, 24 hr after co-culture (gating on CD4+ T cells). Representative histograms are shown for each marker assessed. (f) The percentage of proliferating T cells after co-culture with antigen-pulsed DCs was determined using a CFSE dilution assay. (g) Flow cytometry density plots depict proliferating subsets of CD4+ T cells at 24 hr of co-culture (low-CFSE fluorescent populations). Data are means ± SEM of at least three independent experiments. Negative (non-antigenic peptide/unpulsed) control is not shown in (a). Positive (Type IV concanavalin A) controls are not shown in (a–g). One-way analysis of variance and the Bonferroni post-test were used to compare all groups against T cells co-cultured with mock-inoculated DCs. ***P < 0·001; **P < 0·01; *P < 0·05; n.s.: non-significant.
Further, we determined T-cell activation in response to hMPV-inoculated DCs by measuring the surface expression of activation markers, such as CD4 (pMHC co-receptor), CD25 (IL-2 receptor/IL-2Rα), CD71 (transferrin receptor) and CD69.28 Consistent with the IL-2 results, T cells co-cultured with hMPVNL/1/00-inoculated and hMPVCZ0107-inoculated DCs showed reduced expression of all these activation markers (CD4, CD25, CD69, CD71, Fig. 3b–e) when compared with T cells stimulated either with untreated, mock-, UV-hMPV- or opsonized (IC)-hMPV-inoculated DCs. Equivalent data were obtained at various MOIs (Fig. S3d) and DC : T-cell ratios (Fig. S3e).
Because T cells can undergo proliferation after antigen stimulation, despite reduced expression of activation markers,29 this parameter was measured in T cells stimulated with antigen-pulsed hMPV-inoculated DCs using a CFSE dilution assay. Consistent with the T-cell activation results described above, T-cell proliferation was significantly reduced 24 hr after co-culture using DCs that were inoculated with both hMPV strains (Fig. 3f,g). However, the opposite was observed for T cells stimulated with uninfected, mock-inoculated or UV-hMPV-inoculated DCs (Fig. 3f,g).
To evaluate whether decreased T-cell activation by hMPV-infected DCs was the result of cell death during the experiments, caspase 3 activation was measured for DCs and T cells before and after co-cultures (see Supplementary material, Data S1). No significant apoptosis was induced after hMPV inoculation of DCs, either alone or in the presence of T cells (see Supplementary material, Fig. S4a,b, respectively). Similarly, no significant apoptosis was observed for T cells challenged with hMPV alone or co-cultured with hMPV-inoculated DCs (Fig. S4c). These results suggest that the lack of T-cell activation observed by hMPV-inoculated DCs was not the result of virus-induced cell death, but rather an effect of the virus over the immunogenic capacity of DCs.
Because DCs can support viral infection of bystander cells, such as previously observed for HIV-1-engulfing DCs, which later can infect T cells in trans,30 we evaluated whether T cells become infected after co-culture with hMPV-inoculated DCs. Both CD4+ T cells obtained from DC–T-cell co-cultures and purified T cells that were directly inoculated with hMPV-GFP were negative for hMPV-encoded proteins and GFP-fluorescence (Fig. S4d,e, respectively). These data suggest that the decreased activation observed for T cells co-cultured with hMPV-infected DCs is not a result of T-cell infection.
hMPV does not interfere with DC–T-cell synapse assembly
We have previously described that hRSV, a virus closely related to hMPV, has the ability to prevent IS assembly between DCs and T cells after infecting DCs.31 To evaluate whether hMPV uses a similar strategy to keep DCs from priming T cells, we measured DC–T-cell IS assembly with hMPV-inoculated DCs using equivalent experimental conditions as previously described for hRSV.31 Because T cells polarize their Golgi apparatus towards DCs during IS formation, we assessed this process as a readout for IS assembly.31–33 As shown in Fig. 4, hMPV failed to suppress the capacity of DCs to induce T-cell polarization in response to antigenic stimulation when compared with mock or UV-hMPV-inoculated DCs (Fig. 4a,b). These results indicate that hMPV-infected DCs retain their capacity to trigger the polarization of antigen-specific T cells. Therefore, the mechanism of inhibition of T-cell activation by hMPV-infected DCs does not involve impairment of IS assembly and so differs from hRSV.
Figure 4.
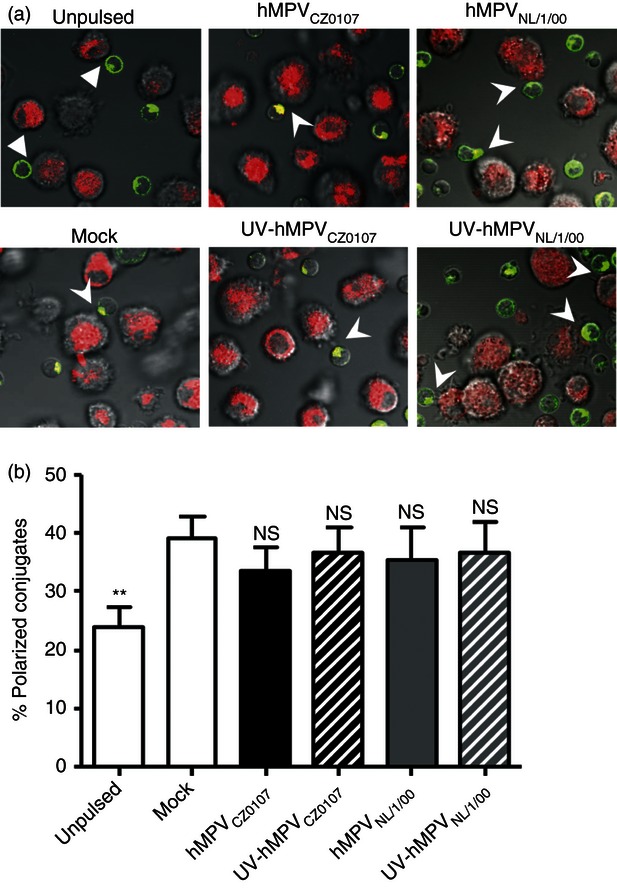
Human metapneumovirus (hMPV) does not prevent T-cell polarization towards dendritic cells (DCs) during immunological synapse (IS) formation. DC–T-cell IS assembly was assessed for uninfected, hMPV- and UV-hMPV-inoculated DCs (red fluorescence) co-cultured with antigen-specific T cells (green fluorescence). Golgi apparatus polarization within T cells was measured as a read-out of synapse formation by laser confocal microscopy. (a) A representative micrograph is shown per treatment. Arrowheads show polarization of the Golgi apparatus (green fluorescence) at the interface formed between T cells and contacting DCs (red fluorescence). (b) IS assembly was quantified by determining the percentage of T cells with their Golgi apparatus polarized towards DCs within the total number of DC–T-cell conjugates. Data are means ± SEM of at least 400 visualized DC–T-cell conjugates captured in three independent experiments. One-way analysis of variance and the Bonferroni post-test were used to compare groups. **P < 0·01; n.s.: non-significant.
Reduced T-cell activation is mediated by a soluble factor secreted by hMPV-infected DCs
To assess whether soluble factors secreted by hMPV-inoculated DCs interfere with T-cell activation, purified CD4+ T cells were stimulated with plate-bound anti-CD3ε plus anti-CD28 antibodies in the presence of supernatants obtained from control and hMPV-infected DCs at different MOIs. Remarkably, supernatants derived from DCs inoculated with hMPVNL/1/00 and hMPVCZ0107, but not their UV-inactivated counterparts, suppressed both the secretion of IL-2 and the up-regulation of activation markers in T cells (Fig. 5a,b). These findings suggest that hMPV-inoculated DCs are likely to secrete soluble molecules that down-modulate T-cell activation. To further assess whether supernatants derived from other hMPV-infected cells, different from DCs also down-modulate T-cell activation, we cultured purified CD4+ T cells over plate-bound anti-CD3ε plus anti-CD28 in the presence of supernatants obtained from LLC-MK2-hMPV-infected cells. In contrast to the T-cell inhibition results observed above with supernatants derived from hMPV-inoculated DCs, supernatants from LLC-MK2 that contained high titers of hMPV did not inhibit T-cell activation (Fig. S4f). These results suggest that hMPV induced the secretion of T-cell inhibitory factors by infected DCs and that hMPV virions were unable to directly suppress T-cell activation.
Figure 5.
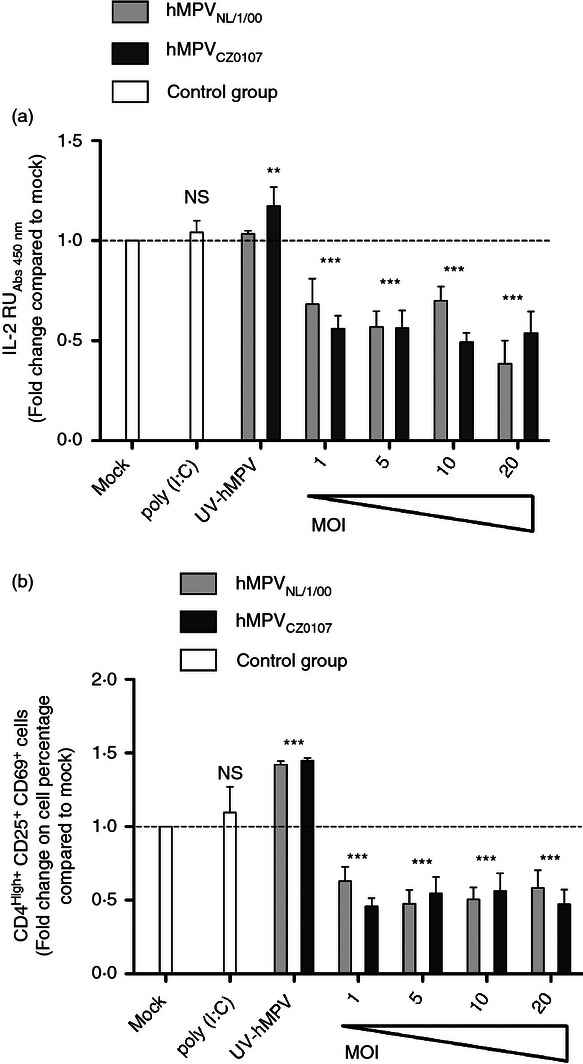
Reduced T-cell activation by human metapneumovirus (hMPV) -inoculated dendritic cells (DCs) is mediated by soluble factors. (a) Interleukin-2 (IL-2) secretion (ELISA) by T cells incubated in the presence of supernatants from hMPV-inoculated DCs and plate-bound anti-CD3ε plus anti-CD28 antibodies (50 ng/well each). Increasing multiplicities of infection (MOIs) were used (range 1–20) to assess the effects of virus doses over the inhibitory capacity of DC supernatants from both hMPVCZ0107- and hMPVNL/1/00-inoculated DCs. Supernatants from DCs treated with 25 μg/ml of poly (I : C) or inoculated with mock or UV-hMPV (MOI 20) were used as controls (white bars, right side of each graph). Data are means ± SEM of at least three independent experiments. (b) FACS analyses assessing the expression of CD25 and CD69 in CD4+ T cells used for the experiments shown in (a). Data represent means ± SEM of at least four independent experiments performed in duplicate. One-way analysis of variance and the Bonferroni post-test were used to compare all groups against T cells treated with supernatants from mock-inoculated DC. ***P < 0·001; **P < 0·01; n.s.: non-significant.
Type-I IFNs do not mediate suppression of naive T-cell activation by hMPV-inoculated DCs
Type-I IFNs are a group of cytokines secreted by a wide range of cell types in response to virus infection, which can modulate DC and T-cell functions.34–37 As shown in Fig. 6, DCs secrete large amounts of IFN-α and IFN-β in response to hMPV inoculation. On the contrary, no secretion of either IFN-α or IFN-β was observed for mock- or UV-inactivated hMPV-inoculated DCs, suggesting that viral replication within DCs is required to induce the secretion of these cytokines (Fig. 6a,b).
Figure 6.
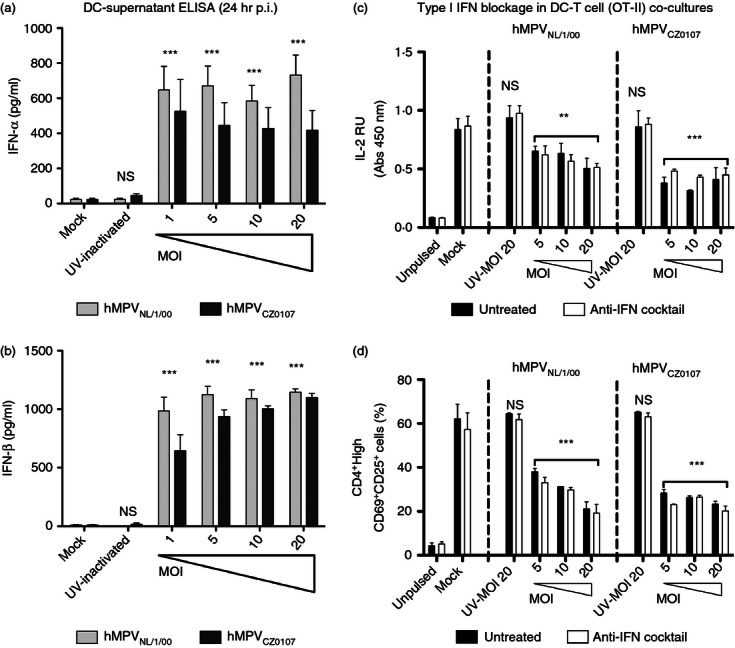
Neutralization of type I interferons (IFNs) does not restore T-cell activation, (a) IFN-α and (b) IFN-β secretion by dendritic cells (DCs) was measured by ELISA in supernatants collected 24 hr post-inoculation (hr p.i.) with either mock or different multiplicities of infection (MOIs; varying from 1 to 20) of the human metapneumovirus (hMPV) NL/1/00 and CZ0107 strains. Bars represent means of at least three independent experiments performed in duplicate ± SEM. (c, d) To assess potential inhibitory roles of IFN-α/β in the supernatants, a cocktail of antibodies were used to block the type I IFN receptor as well as soluble IFN-α/β during DC–T-cell co-cultures. Blockade of the IFN-α/β receptor was corroborated by an IFN-α/β ELISA that showed an increase in soluble IFNs in the supernatants of DC–T-cell co-cultures (data not shown). (c) Interleukin-2 (IL-2) ELISA of co-culture supernatants recovered at 36 hr of culture. (d) Flow cytometry analyses were performed over cells harvested from (c). The percentage of CD4High+/CD69+/CD25+ activated T cells observed for each condition is shown. Black bars: untreated co-cultures, white bars: co-cultures treated with the antibody cocktail. Bars represent means of at least three (a, b) and four (c, d) independent experiments ± SEM. For IFN-α/β ELISA statistical analyses were performed by one-way analysis of variance and the Bonferroni post-test. For IFN-blockage assays, statistical analyses were performed by two-way analysis of variance and the Bonferroni post-test compared with mock both, for the effects of either virus infection or IFN-blockade over T-cell activation. ***P < 0·001; **P < 0·01; n.s.: non-significant.
To assess the contribution of IFN-α and IFN-β over T-cell suppression in our DC–T-cell co-cultures, IFN-α and IFN-β, as well as their common IFN-α/β receptor 1 were neutralized with a cocktail of blocking antibodies. No significant T-cell activation could be recovered in these type-I IFN blockade assays, either by measuring IL-2 secretion or by evaluating the expression of activation markers on the surface of T cells (Fig. 6c,d). Further, neutralization of IFN-α/β in the supernatants of hMPV-infected DCs failed to restore the activation of T cells stimulated by anti-CD3ε plus anti-CD28-coated plates (data not shown).
Discussion
Priming of virus-specific CD4+ T cells is a critical process for mounting an antiviral immune response. Because of its importance, several viruses have evolved molecular mechanisms to impair T-cell activation and gain advantage over the host immune response to disseminate within infected tissues.33,38 Given that DCs play a major role in naive T-cell priming, several pathogens have evolved molecular mechanisms to impair the function of DCs.10,31,39 A recent study using antigen-specific mouse T cells showed that conventional DCs isolated from hMPV-infected lungs fail to induce the proliferation of pOVA-specific DO11.10 T cells.10 However, DC infection with hMPV was not shown in this study.10 Here, we observed that hMPV infects mouse DCs in a restricted and abortive manner, which was characterized by limited expression of virus-encoded proteins, as well as limited production of infectious particles. Despite this fact, DC infection with hMPV rendered these cells inefficient at priming antigen-specific naive T cells, which were not infected by this virus under our experimental conditions. Importantly, DC infection with hMPV was necessary for inhibiting T-cell activation, because DCs inoculated either with UV-inactivated or opsonized hMPV failed to suppress T-cell activation.
Upon inoculation with viable hMPV, DCs secreted IL-6 and type-I IFNs. Remarkably, inoculation of DCs with UV-inactivated hMPV induced IL-6 but not IFN-α or IFN-β. These differences are probably the result of distinct pattern recognition receptors engaged by infectious and inactivated virus in DCs, such as Toll-like receptors, retinoic acid-inducible gene-1-like family receptors and protein kinase R. For instance, some Toll-like receptors may engage virion molecules present both in infectious and inactivated viruses, such as the hMPV matrix protein.40 On the other hand, molecules such as protein kinase R, Toll-like receptor 3, and retinoic acid-inducible gene-1-like family receptors are specialized at recognizing RNA species that mainly occur in replicating viruses.41–43 However, despite the recognition of viable or inactivated virus (UV-treated), we observed that DCs secreted poor amounts of cytokines that support T-cell activation, such as IL-12 and TNF-α. Furthermore, DCs only modestly up-regulated co-stimulatory molecules on their surface after hMPV inoculation. This DC phenotype could explain the reduced naive T-cell activation observed.
Previous reports have shown that hMPV infects human DCs and interferes with their capacity to activate T cells.12,27,44 However, another study showed no inhibition of human T-cell activation when stimulated with hMPV-inoculated monocyte-derived DCs pulsed with a super-antigen9. Because the quality of the TCR ligand and TCR/pMHC binding kinetics can deeply influence the outcome of T-cell activation,24,45 here we used an antigenic peptide that is presented to T cells bound to MHC-II molecules in a physiological context. Furthermore, in this study we extended the characterization of the phenotype of T cells co-cultured with hMPV-inoculated DCs by assessing the expression of soluble molecules secreted by T cells, as well as membrane-bound activation markers on these cells. Because hRSV, a closely related paramyxovirus was recently described by our group to interfere with the capacity of DCs to establish activating immunological synapses with naive T cells,31 we hypothesized that hMPV may use a similar mechanism to dampen T-cell activation. However, our results showed that hMPV-inoculated DCs were capable of assembling an IS with T cells, which induced the polarization of the T-cell secretory machinery to a similar extent to control and UV-inactivated hMPV-inoculated DCs. These results suggest that hRSV and hMPV may have evolved different virulence mechanisms to subvert DC function and the adaptive immune system, which should be further studied.
In view of these results, we decided to assess the contribution of soluble determinants secreted by hMPV-inoculated DCs. Here, we observed that soluble factors secreted by hMPV-inoculated DCs and not LLC-MK2 cells played an important role at inhibiting T-cell activation. These soluble factors were able to inhibit T-cell activation in response to a strong stimulus, such as plate-bound anti-CD3ε plus anti-CD28 antibodies. Because IFN-α/β can significantly modulate T-cell activation, either positively or negatively,34,36,37 we decided to assess their role in suppressing T-cell activation after inoculating DCs with hMPV. We observed that IFN-α/β secreted by hMPV-infected DCs does not seem to play a significant role in the observed inhibition of T-cell activation. Indeed, T-cell activation was unaltered in the presence of an anti-IFN cocktail, suggesting that type I IFNs are not required during naive CD4+ T-cell priming by DCs.
Taken together, our results suggest that soluble factors, different from IL-10, TGF-β1, IFN-α/β and hMPV virions, mediate the inhibition of T-cell activation by hMPV-infected DCs. Further research is required to identify the nature and mechanisms of action of soluble components secreted by hMPV-infected DCs that negatively modulate T-cell activation.
Acknowledgments
We would like to thank Dr Ron Fouchier at the Erasmus Medical Centre, Rotterdam, the Netherlands for kindly providing the hMPV NL/1/00 reference strain and its GFP recombinant derivative. We also thank Dr Pierre Pothier at the University of Dijon for kindly providing us with XD10.C7 monoclonal antibody. We are grateful to Dr Susan M. Bueno and Dr Leandro J. Carreño for critically reading the manuscript. This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico Grants (FONDECYT) 3100090, 1070352; FONDEF D061008; Biomedical Research Consortium CTU06 and the Millennium Institute on Immunology and Immunotherapy (P07/088-F). PAG and PFC are supported by the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT). AMK is a Chaire De La Région Pays De La Loire, Chercheur Étranger D'excellence, France.
Glossary
- hMPV
human metapneumovirus
- DCs
dendritic cells
- IFN-α
interferon-α
- IFN-β
interferon-β
- hRSV
human respiratory syncytial virus
Disclosures
The authors of this work declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Dendritic cell (DC) infection and viability after human metapneumovirus (hMPV) inoculation
Figure S2. Dendritic cell (DC) cytokine secretion and up-regulation of maturation markers after human metapneumovirus (hMPV) inoculation
Figure S3. Human metapneumovirus (hMPV) -inoculated dendritic cells (DCs) display reduced capacity to activate T cells at different multiplicities of infection (MOI) and DC : T-cell ratios. The hMPV does not interfere with the loading of pOVA-FITC on treated DCs
Figure S4. Reduced T-cell activation is not a consequence of programmed cell death of dendritic cells (DCs) or T cells. CD4+ T cells are not affected by viral particles after activation with plate-bound anti-CD3ε plus anti-CD28.
Data S1. Virus titration
References
- 1.Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Curr Opin Infect Dis. 2003;16:255–8. doi: 10.1097/00001432-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Williams JV, Martino R, Rabella N, Otegui M, Parody R, Heck JM, Crowe JE., Jr A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–5. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowe JE., Jr Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23:S215–21. doi: 10.1097/01.inf.0000144668.81573.6d. [DOI] [PubMed] [Google Scholar]
- 5.Xepapadaki P, Psarras S, Bossios A, Tsolia M, Gourgiotis D, Liapi-Adamidou G, et al. Human metapneumovirus as a causative agent of acute bronchiolitis in infants. J Clin Virol. 2004;30:267–70. doi: 10.1016/j.jcv.2003.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebihara T, Endo R, Ishiguro N, Nakayama T, Sawada H, Kikuta H. Early reinfection with human metapneumovirus in an infant. J Clin Microbiol. 2004;42:5944–6. doi: 10.1128/JCM.42.12.5944-5946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto M, Sugawara K, Takashita E, Muraki Y, Hongo S, Nishimura H, Matsuzaki Y. Longitudinal course of human metapneumovirus antibody titers and reinfection in healthy adults. J Med Virol. 2010;82:2092–6. doi: 10.1002/jmv.21920. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez R, Tripp RA. The immune response to human metapneumovirus is associated with aberrant immunity and impaired virus clearance in BALB/c mice. J Virol. 2005;79:5971–8. doi: 10.1128/JVI.79.10.5971-5978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Nouen C, Hillyer P, Munir S, Winter CC, McCarty T, Bukreyev A, et al. Effects of human respiratory syncytial virus, metapneumovirus, parainfluenza virus 3 and influenza virus on CD4+ T cell activation by dendritic cells. PLoS ONE. 2010;5:e15017. doi: 10.1371/journal.pone.0015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero-Plata A, Kolli D, Hong C, Casola A, Garofalo RP. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J Immunol. 2009;182:3072–83. doi: 10.4049/jimmunol.0802262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douville RN, Bastien N, Li Y, Pochard P, Simons FE, HayGlass KT. Human metapneumovirus elicits weak IFN-γ memory responses compared with respiratory syncytial virus. J Immunol. 2006;176:5848–55. doi: 10.4049/jimmunol.176.10.5848. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am J Respir Cell Mol Biol. 2006;34:320–9. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tobar JA, Carreno LJ, Bueno SM, Gonzalez PA, Mora JE, Quezada SA, Kalergis AM. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect Immun. 2006;74:6438–48. doi: 10.1128/IAI.00063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobar JA, Gonzalez PA, Kalergis AM. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc γ receptors on dendritic cells. J Immunol. 2004;173:4058–65. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- 15.Grayson MH, Holtzman MJ. Emerging role of dendritic cells in respiratory viral infection. J Mol Med. 2007;85:1057–68. doi: 10.1007/s00109-007-0212-3. [DOI] [PubMed] [Google Scholar]
- 16.Carreno LJ, Gonzalez PA, Bueno SM, Riedel CA, Kalergis AM. Modulation of the dendritic cell-T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy. 2011;3:6–11. doi: 10.2217/imt.11.38. [DOI] [PubMed] [Google Scholar]
- 17.Thoulouze MI, Sol-Foulon N, Blanchet F, Dautry-Varsat A, Schwartz O, Alcover A. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity. 2006;24:547–61. doi: 10.1016/j.immuni.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Raftery MJ, Winau F, Kaufmann SH, Schaible UE, Schonrich G. CD1 antigen presentation by human dendritic cells as a target for herpes simplex virus immune evasion. J Immunol. 2006;177:6207–14. doi: 10.4049/jimmunol.177.9.6207. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14999–5004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–12. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 21.de Graaf M, Herfst S, Schrauwen EJ, van den Hoogen BG, Osterhaus AD, Fouchier RA. An improved plaque reduction virus neutralization assay for human metapneumovirus. J Virol Methods. 2007;143:169–74. doi: 10.1016/j.jviromet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Tollefson SJ, Cox RG, Williams JV. Studies of culture conditions and environmental stability of human metapneumovirus. Virus Res. 2010;151:54–9. doi: 10.1016/j.virusres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carreno LJ, Riquelme EM, Gonzalez PA, Espagnolle N, Riedel CA, Valitutti S, Kalergis AM. T-cell antagonism by short half-life pMHC ligands can be mediated by an efficient trapping of T-cell polarization toward the APC. Proc Natl Acad Sci U S A. 2010;107:210–5. doi: 10.1073/pnas.0911258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Perlman S. Preferential infection of mature dendritic cells by mouse hepatitis virus strain JHM. J Virol. 2006;80:2506–14. doi: 10.1128/JVI.80.5.2506-2514.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavrois M, Neidleman J, Kreisberg JF, Fenard D, Callebaut C, Greene WC. Human immunodeficiency virus fusion to dendritic cells declines as cells mature. J Virol. 2006;80:1992–9. doi: 10.1128/JVI.80.4.1992-1999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le NouenC, Munir S, Losq S, Winter CC, McCarty T, Stephany DA, et al. Infection and maturation of monocyte-derived human dendritic cells by human respiratory syncytial virus, human metapneumovirus, and human parainfluenza virus type 3. Virology. 2009;385:169–82. doi: 10.1016/j.virol.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ridgway W, Fasso M, Fathman CG. Following antigen challenge, T cells up-regulate cell surface expression of CD4 in vitro and in vivo. J Immunol. 1998;161:714–20. [PubMed] [Google Scholar]
- 29.Tsang J, Jiang S, Tanriver Y, Leung E, Lombardi G, Lechler RI. In-vitro generation and characterisation of murine CD4+ CD25+ regulatory T cells with indirect allospecificity. Int Immunopharmacol. 2006;6:1883–8. doi: 10.1016/j.intimp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci U S A. 2006;103:738–43. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez PA, Prado CE, Leiva ED, Carreno LJ, Bueno SM, Riedel CA, Kalergis AM. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci USA. 2008;105:14999–5004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, et al. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–94. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez PA, Carreno LJ, Figueroa CA, Kalergis AM. Modulation of immunological synapse by membrane-bound and soluble ligands. Cytokine Growth Factor Rev. 2007;18:19–31. doi: 10.1016/j.cytogfr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ. Type I interferon (IFN α) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol. 2009;183:2915–20. doi: 10.4049/jimmunol.0801607. [DOI] [PubMed] [Google Scholar]
- 35.Lachgar A, Bizzini B. Involvement of α-interferon in HIV-1 induced immunosuppression A potential target for AIDS prophylaxis and treatment. Biomed Pharmacother. 1994;48:73–7. doi: 10.1016/0753-3322(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 36.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 37.Erickson S, Matikainen S, Thyrell L, Sangfelt O, Julkunen I, Einhorn S, Grander D. Interferon-α inhibits Stat5 DNA-binding in IL-2 stimulated primary T-lymphocytes. Euro J Biochem. 2002;269:29–37. doi: 10.1046/j.0014-2956.2002.02626.x. [DOI] [PubMed] [Google Scholar]
- 38.Bueno SM, Gonzalez PA, Kalergis AM. Use of genetically modified bacteria to modulate adaptive immunity. Curr Gene Ther. 2009;9:171–84. doi: 10.2174/156652309788488587. [DOI] [PubMed] [Google Scholar]
- 39.Cao S, Li Y, Ye Y, Yang X, Chen L, Liu X, Chen H. Japanese encephalitis virus wild strain infection suppresses dendritic cells maturation and function, and causes the expansion of regulatory T cells. Virol J. 2011;8:39. doi: 10.1186/1743-422X-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bagnaud-Baule A, Reynard O, Perret M, Berland JL, Maache M, Peyrefitte C, et al. The human metapneumovirus matrix protein stimulates the inflammatory immune response in vitro. PLoS ONE. 2011;6:e17818. doi: 10.1371/journal.pone.0017818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goutagny N, Jiang Z, Tian J, Parroche P, Schickli J, Monks BG, et al. Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein. J Immunol. 2010;184:1168–79. doi: 10.4049/jimmunol.0902750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao S, Bao X, Liu T, Lai S, Li K, Garofalo RP, Casola A. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J Gen Virol. 2008;89:1978–86. doi: 10.1099/vir.0.2008/000778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–9. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Tan MC, Battini L, Tuyama AC, Macip S, Melendi GA, Horga MA, Gusella GL. Characterization of human metapneumovirus infection of myeloid dendritic cells. Virology. 2007;357:1–9. doi: 10.1016/j.virol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carreno LJ, Gonzalez PA, Kalergis AM. Modulation of T cell function by TCR/pMHC binding kinetics. Immunobiology. 2006;211:47–64. doi: 10.1016/j.imbio.2005.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.