Abstract
The IMiDs® immunomodulatory compounds lenalidomide and pomalidomide are agents with anti-inflammatory, immunomodulatory and anti-cancer activity. An excellent success rate has been shown for multiple myeloma in phase I/II clinical trials leading to Food and Drug Administration approval of lenalidomide. One mechanism by which these drugs could enhance anti-tumour immunity may be through enhanced dendritic cell (DC) function. Thalidomide, a compound structurally related to lenalidomide and pomalidomide, is known to enhance DC function, and we have investigated whether its analogues, pomalidomide and lenalidomide, also have functional effects on DCs. We used mouse bone marrow-derived DCs treated with 5 or 10 μm pomalidomide, or lenalidomide from day 1 of culture. Treatment with IMiD® immunomodulatory compounds increased expression of Class I (H2-Kb), CD86, and pomalidomide also increased Class II (I-Ab) expression in bone marrow-derived DCs, as measured by flow cytometry. Fluorescent bead uptake was increased by up to 45% when DCs were treated with 5 or 10 μm pomalidomide or lenalidomide compared with non-treated DCs. Antigen presentation assays using DCs primed with ovalbumin, and syngeneic T cells from transgenic OTI and OTII mice (containing MHC restricted, ovalbumin-specific, T cells) showed that both pomalidomide and lenalidomide effectively increased CD8+ T-cell cross-priming (by up to 47%) and that pomalidomide alone was effective in increasing CD4+ T-cell priming (by 30%). Our observations suggest that pomalidomide and lenalidomide enhance tumour antigen uptake by DCs with an increased efficacy of antigen presentation, indicating a possible use of these drugs in DC vaccine therapies.
Keywords: CD8, dendritic cell, lenalidomide, pomalidomide, presentation
Introduction
Dendritic cells (DCs) are the principle population of antigen presenting cells for the initiation of T-cell-mediated immunity. They are derived from haematopoietic bone marrow progenitor cells and, like granulocytes and macrophages, express the myeloid marker CD33 and are induced to differentiate in response to granulocyte–macrophage colony-stimulating factor (GM-CSF).1 Dendritic cells circulate throughout the periphery and remain in an immature form (iDC) until exposure to antigen occurs. The mature DC then travels to the lymph node where T helper type 1/type 2-mediated responses are initiated by the presentation of antigen (through MHC Class I and Class II complexes) and co-stimulation markers to naive T cells.2
Dendritic cells have been shown to be important adjuvants in the use of cancer vaccines3 and interest in DC vaccines has grown recently with the Food and Drug Administration's approval of sipuleucel-T (Provenge; Dendreon, Seattle, WA),4 a therapeutic vaccine consisting of autologous DCs that have been loaded with a prostatic acid phosphatase (PAP)–GM-CSF fusion protein ex vivo.5 The PAP, a tumour-associated antigen, is over-expressed in prostate cancers and its fusion with GM-CSF promotes PAP presentation and DC maturation in tandem. Tumour-secreted vascular endothelial growth factor has been shown to inhibit DC maturation6 by the disruption of nuclear factor-κB signalling.7 These partially mature DCs, termed myeloid suppressor cells, have been observed in patient populations and correlate with poor patient survival.8 Generation of myeloid suppressor cells by exposure to antigen in the absence of co-stimulation leads to the expansion of regulatory T (Treg) cells and T-cell anergy.9 The immune suppressive potential of myeloid suppressor cells can be reversed by differentiation into DCs using various chemotherapeutic agents such as phosphodiesterase-5 inhibitors10 and sunitinib.11 An attractive drug candidate for use as an adjuvant with a therapeutic DC vaccine would not only improve the functional response of DCs by improved maturation and antigen presentation; but also suppress the function of myeloid suppressor cells and Treg cells.
The IMID® immunomodulatory drugs lenalidomide (Revlimid®) and pomalidomide (CC-4047) are thalidomide analogues that were developed as tumour necrosis factor-α (TNF-α) inhibitors. Lenalidomide is Food and Drug Administration approved for multiple myeloma in combination with dexamethasone and in del 5q myelodysplastic syndrome based on seminal trials completed in 2007. A current Phase I/II study is testing the combination of antigen-loaded DCs with lenalidomide in patients with multiple myeloma (ClinicalTrial.gov #NCT00698776). These compounds affect tumours directly through the modulation of a variety of signalling pathways such as vascular endothelial growth factor, AKT,12 Notch/Wnt,13 P53 and P38.14
The IMiDs® immunomodulatory compounds primarily function as modulators of immune function and a recent publication demonstrates that lenalidomide increases natural killer cell mediated antibody-dependant cellular cytotoxicity by modulation of Fc-γ receptor signalling in natural killer cells.15 We have previously shown that the IMiDs immunomodulatory compounds suppress Treg cell function,16 and thalidomide and lenalidomide have been identified as co-stimulators of CD8+ effector activity, independent of DC function.17 In the course of this study, we have investigated the effects of lenalidomide and pomalidomide on DC-dependent T-cell activation. We particularly focus on the role of the IMiDs immunomodulatory compounds in the uptake and presentation of antigen by DCs.
Materials and methods
Animals
Female C57BL/6 mice were bred and maintained by the Biological Research Facility at St George's, University of London (London, UK). OT-I and OT-II T-cell receptor transgenic mice were purchased from Charles River (Margate, UK) and maintained by the Biological Research Facility at St George's, University of London. Animals were used at 8–10 weeks of age and all procedures conducted had obtained ethics approval and were carried out in accordance with the regulations as described in the Animals (Scientific Procedures) Act 1986, UK (Project licence number: 70/6573).
Reagents
Lenalidomide and pomalidomide were obtained from the Celgene Corporation (Summit, NJ), dissolved in DMSO at 10 mm, and maintained as stock solutions for in vitro experiments at −20° for no longer than 1 month.
Recombinant murine GM-CSF (Peprotech, London, UK) was made up as a stock solution at 10 μg/ml in PBS and used immediately.
The DC medium contained Iscove's modified Dulbecco's medium (Invitrogen, Paisley, UK), 10% fetal calf serum (Invitrogen), 1% weight/volume penicillin streptomycin (P/S) and 50 μm 2-mercaptoethanol (Sigma, Dorset, UK).
Lyophilized ovalbumin (Sigma) was reconstituted in PBS at 45 mg/ml to be used immediately.
Dendritic cell culture
Bone marrow from the femurs and tibias of wild-type (WT) C57BL/6 female mice was suspended in DC medium as a single cell suspension. The cell suspension was forced through a 70-μm filter, and resuspended at 1 × 106 cells/ml in DC media. Both GM-CSF and interleukin-4 (IL-4) were added to a final concentration of 5 ng/ml.
Five-millilitre aliquots of the cell suspension were added to T25 culture flasks supplemented with drugs (lenalidomide and pomalidomide at 5 or 10 μm), and incubated for 3 days at 37° in a humidified atmosphere containing 5% CO2. Lenalidomide and pomalidomide were used at concentrations of 5 and 10 μm based on previous pharmacokinetic studies, where plasma concentrations of up to 10 μm were reached upon dosing with 50 mg/kg – the typical in vivo dose used for murine investigations.
Non-adherent cells were removed after 3 days, then fresh GM-CSF and drugs were added to each flask and incubated for a further 2 days. Loosely adherent DCs were then recovered from culture flasks.
Isolation of ovalbumin T-cell receptor-transgenic splenocytes
OT-I (containing MHC class I-restricted, ovalbumin-specific, CD8+ T cells) and OT-II (containing MHC class II-restricted, ovalbumin-specific, CD4+ T cells) splenocytes were isolated from the spleens of OT-I and OT-II mice (C57BL/6 background), respectively, and, after red blood cell lysis, were maintained in RPMI-1640 medium (RPMI; Sigma Ltd, Poole, UK) supplemented with 10% (v/v) fetal bovine serum, 2 mm l-glutamine and 1 × penicillin/streptomycin (basal medium).
Splenocytes were pooled and suspended in freezing medium (45% basal medium, 45% fetal calf serum, 10% DMSO) and aliquots were stored in liquid nitrogen.
Bead uptake assay
Dendritic cells were prepared as described above and 1 × 106 cells were resuspended in 1 ml DC medium. Cells were incubated with 7 μl fluorescent beads (Fluopheres; Invitrogen) for 3 hr at either 4° or 37°. Cells were then placed on ice, and washed three times with cold PBS before fixation and analysis by flow cytometry.
Immunophenotyping by flow cytometry
Lenalidomide-treated and pomalidomide-treated DCs were resuspended in FACS buffer [1% mouse serum (DAKO, Cambridge, UK) 15 mm NaN3 in PBS] and stained with fluorophore-conjugated monoclonal antibodies for H2-Kb (MHC ClassI), I-Ab (MHC ClassII), CD80, CD86, CD11c, CD40, FAS and FAS ligand (Becton Dickinson, Oxford, UK). Samples were washed with FACS buffer, data were acquired using a FACSCalibur flow cytometer and analysis was performed using Cellquest Pro software (BD) and FCS Express (De Novo software, Los Angeles, CA).
Cytokine detection assays
Supernatants from lenalidomide-treated and pomalidomide-treated DCs were collected and cellular debris was removed by centrifugation. Concentration of the soluble cytokines IL-12-P70, TNF-α, interferon-γ (IFN-γ), macrophage inflammatory protein 1α (MIP-1α), IL-6 and IL-10 were measured by BD Bioscience's Mouse Inflammation CBA kit as per the manufacturer's directions.
Peptide presentation assay
Dendritic cells were cultured as described above. Cells were treated with pomalidomide (5 and 10 μm), lenalidomide (5 and 10 μm) or DMSO (0·1%). After 5 days, loosely adherent DCs were recovered from culture flasks and suspended in DC medium at 5 × 104 cells/ml.
Twelve-well plates were seeded with 1 × 105 cells from each treatment, an ovalbumin stock solution was added to each well (100 μg/ml final concentration) and plates were incubated for 24 hr at 37° in 5% CO2. After 24 hr, lipopolysaccharide was added to the DCs at a final vconcentration of 1 μg/ml to mature the DCs for the presentation assay.
Cells were harvested, washed three times and suspended in RPMI-1640 medium at 1 × 105 cells/ml. Then, 5 × 103 cells were seeded onto 96-well plates (in triplicate).
Frozen aliquots of OT-I and OT-II splenocytes were thawed and suspended in RPMI-1640 medium at 1 × 106 cells/ml. Antigen presentation assays were performed by seeding 1 × 105 splenocytes onto wells containing syngeneic DCs (WT) and plates were incubated for 5 days at 37° in 5% CO2, whereupon T-cell proliferation was measured by [H3]thymidine incorporation (0·5 μCi per well) for 16 hr.
Interferon-γ and perforin staining of CD4 and CD8 lymphocytes in DC–splenocyte co-cultures
To determine whether the CD4 and CD8 lymphocytes were activated to a greater extent by lenalidomide-treated or pomalidomide-treated DCs than with the control DCs, we used 24-well plates co-cultured with the splenocytes and DCs pre-treated with DMSO or with lenalidomide or pomalidomide at 5 or 10 μm. After 5 days of co-culture, the cells were treated for 4 hr with brefeldin A, PMA and ionomycin with working concentrations of 10 μg/ml, 50 ng/ml and 1 μg/ml, respectively. An aliquot of the cells was then taken from the wells and washed twice in FACs buffer and resuspended in a volume of 100 μl in FACs tubes. The cells were incubated for 30 min at 4° with fluorophore-conjugated antibodies to anti-CD8 and anti-CD4. After washing twice with FACs buffer, cells were fixed with 2% paraformaldehyde for 20 min at 4° and then washed once with FACs buffer and once with permeabilization buffer (BD Biosciences, Oxford, UK). Pellets were then resuspended in 100 μl permeabilization buffer and incubated with fluorophore-conjugated anti-IFN-γ and anti-perforin for 20 min at room temperature in the dark.
To measure the viability of the cells within the co-cultures after Brefeldin and PMA/ionomycin incubation, a separate aliquot of cells was also taken from each well and tested for viability using an annexin/propidium iodide flow cytometric staining assay (BD Biosciences). Briefly cells were washed with annexin binding buffer, followed by incubation with FITC annexin and propidium iodide for 15 min. Positive staining was assessed by flow cytometric analysis.
Statistical analysis
Statistical analysis of results was performed using repeated measures analysis of variance (anova) on paired data sets followed by Newman–Keuls post testing when appropriate. All statistics were generated using the Graphpad Prism 4.0 software (San Diego, CA).
Results
IMiDs® immunomodulatory compounds increase endocytotic activity of DCs
An immature DC must first endocytose exogenous antigen before it can be presented to the T cell. To this end, we employed a method of measuring DC endocytic activity in which DCs were treated with lenalidomide or pomalidomide at 5 and 10 μm and pulsed with FITC-labelled latex beads. The FITC fluorescence of DCs was analysed by flow cytometry (Fig. 1a). Bead uptake was measured from the percentage of the (gated) DCs found to be FITC-positive. An increase (of up to 10% of the DC population) in fluorescent DCs was observed when treated with pomalidomide at 5 and 10 μm concentrations (Fig. 1b) or 10 μm lenalidomide (Fig. 1c), indicating a small; but significant increase in endocytic activity (P < 0·05, n = 3).
Figure 1.
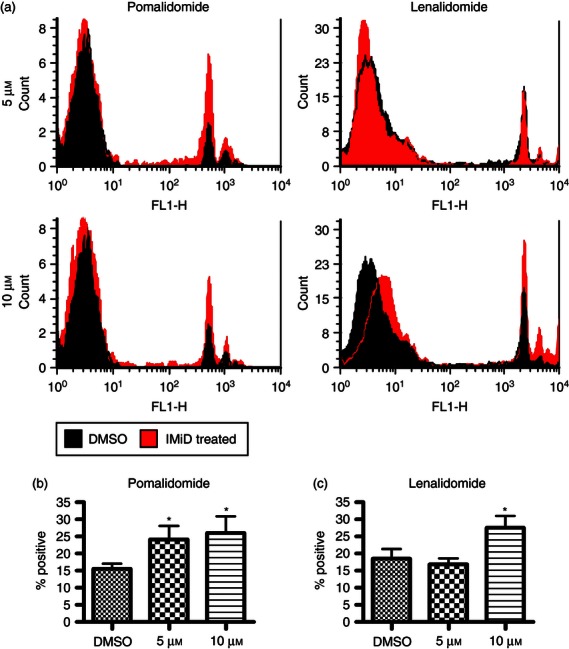
Lenalidomide and pomalidomide increase fluorescent bead uptake. IMiD-treated dendritic cells (DCs) that were treated with fluorescent latex beads endocytosed significantly more beads than untreated DCs (b,c) when analysed by flow cytometry (a). Both 5 and 10 μm pomalidomide can increase uptake of latex beads by DCs by 8% and 10% compared with bead uptake in DMSO-treated DCs [P < 0·05 by repeated measures analysis of variance (anova) and Newman–Keuls, n = 3]. Ten micromoles lenalidomide also increases latex bead uptake by DCs by 10% compared with the DMSO control (P < 0·05 by repeated measures anova and Newman–Keuls, n = 3). NB: The uptake experiments with the lenalidomide (len+ DMSO control have slightly higher basal fluorescence than the experiments comparing pomalidomide with DMSO because of changes in the FL1 channel intensity observed in the FACs calibur. This has no effect on the results as DMSO was used as a control in both sets of experiments. The same gate was used for the DCs in all experiments.
IMiDs®immunomodulatory compounds modulate phenotypic markers on DCs
Dendritic cells initiate T-cell activation by the presentation of MHC bound antigen and co-stimulation markers (including CD80 and CD86) to the naive T-cell. Having investigated the role of antigen endocytosis, we then examined the modulation of MHC and co-stimulation marker expression on DCs that had been treated with lenalidomide and pomalidomide. The DCs were treated with 10 μm lenalidomide or pomalidomide and the expression of the phenotypic markers MHC Class I (H-2Kb), MHC Class II (I-Ab), CD80, CD86, CD11c, CD40, FAS and FAS ligand were measured by flow cytometry. Expression of H-2Kb (Fig. 2a) and CD86 (Fig. 2c) was significantly increased by up to 73% and 53%, respectively, with treatment (P < 0·05, n = 4). Pomalidomide alone up-regulated I-Ab expression on DCs by 72% (P < 0·05, n = 4; Fig. 2b). No significant changes were observed in CD80, CD11c, CD40, FAS and FAS ligand expression (see Supplementary material, Fig. S1).
Figure 2.
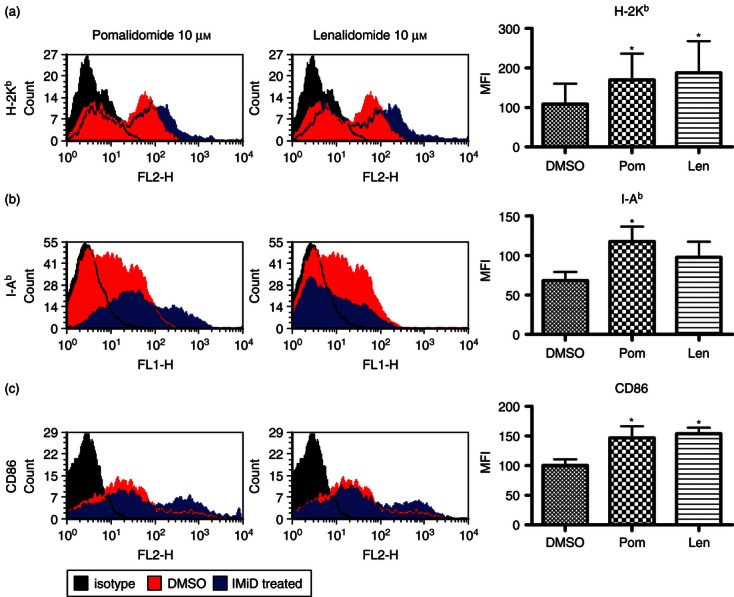
Lenalidomide and pomalidomide alter phenotypic marker expression on dendritic cells (DCs). Expression is measured as mean fluorescence index (MFI) which represents the intensity of fluorescence observed. Flow cytometric analysis of IMiD-treated DCs showed that expression of MHC Class I (H-2Kb) was increased by up to 73% by IMiD treatment [P < 0·05 by repeated measures analysis of variance (anova) and Newman–Keuls, n = 4] (a) and CD86 was increased by up to 53% by IMiD treatment (P < 0·05 by repeated measures anova and Newman–Keuls, n = 4) (c). Pomalidomide, but not lenalidomide, increased expression of MHC Class II (I-Ab) by 72% (b) compared with untreated DCs (P < 0·05 by repeated measures anova and Newman–Keuls, n = 4).
Lenalidomide treatment up-regulates MIP-1α and TNF-α expression in DCs
Dendritic cells were treated with 10 μm lenalidomide or pomalidomide and supernatants were collected after 5 days. Expression of IL-12 p70, TNF-α, IFN-γ, MIP-1α, IL-6 and IL-10 was assayed by cytometric bead array. Lenalidomide-treated DCs produced 945% more MIP-1α (P < 0·01, n = 4; Fig. 3a) and 180% more TNF-α (P < 0·05, n = 4; Fig. 3b) than either untreated or pomalidomide-treated DCs. Expression of all other cytokines remained unchanged (see plementary material, Fig. S2).
Figure 3.
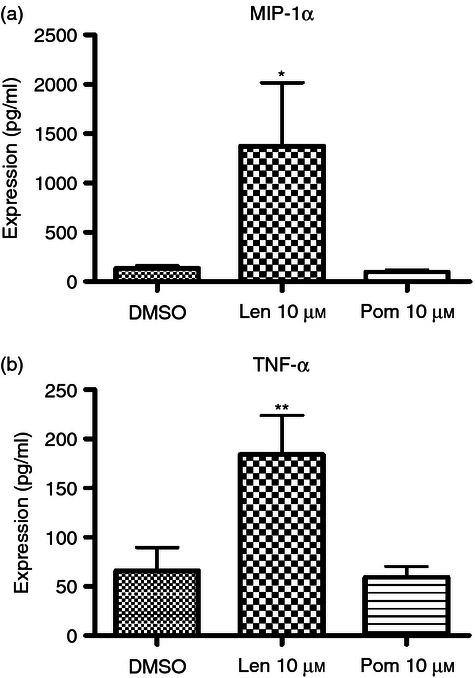
Lenalidomide effects dendritic cell (DC) cytokine expression. Supernatants were collected from IMiD-treated DCs and expression of inflammatory cytokines was assayed by cytometric bead array. Lenalidomide-treated DCs produced 945% more macrophage inflammatory protein-1α (MIP-1α) (P < 0·01, n = 4) (a) and 180% more tumour necrosis factor-α (TNF-α) (P < 0·05 by repeated measures analysis of variance and Newman–Keuls, n = 4) (b) than either untreated or pomalidomide-treated DCs.
IMiD® immunomodulatory compound-treated DCs are more efficient initiators of MHC Class I restricted T-cell activation
Having observed elevated MHC Class I and MHC Class II expression on IMiD immunomodulatory compound-treated DCs (as well as the co-stimulation marker CD86), we sought to investigate whether these observations would correlate with increased antigen presentation to naive T cells. Ovalbumin-specific CD8+ and CD4+ T cells from transgenic mice (OT-I and OT-II) are commonly used in model systems of antigen presentation18 and cross-presentation19 where T-cell priming is initiated by MHC-restricted presentation of exogenously derived ovalbumin on antigen-presenting cells (such as bone marrow-derived DCs). In this study, DCs were treated with lenalidomide and pomalidomide at 5 and 10 μm concentrations then used in an antigen presentation assay with splenocytes extracted from OT-I or OT-II transgenic mice. Proliferation of MHC class I-restricted (ovalbumin-specific) CD8+ T cells (OT-I) or MHC class II-restricted (ovalbumin-specific) CD4+ T cells (OT-II) was measured by [H3]thymidine incorporation. CD8+ T-cell cross-priming was increased by 35% (P < 0·05 by repeated measures anova and Newman–Keuls, n = 7) and 47% (P < 0·001 by repeated measures anova and Newman–Keuls, n = 7) in antigen presentation assays containing 5 and 10 μm lenalidomide-pre-treated DCs, respectively, and by 43% (P < 0·05 by repeated measures anova and Newman–Keuls, n = 7) in antigen presentation assays containing 10 μm pomalidomide-pre-treated DCs (Fig. 4a). CD4+ T-cell priming was increased by 10 μm pomalidomide-pre-treated DCs by 30% (P < 0·05 by repeated measures anova and Newman–Keuls, n = 7; Fig. 4b). Lenalidomide-pre-treated DCs had no effect on CD4 T-cell proliferation. The mean percentage proliferation of DCs or T cells alone was, on average, 10% of the mean DMSO control, as were assays containing DCs that had not been loaded with ovalbumin (see plementary material, Fig. S3). Hence, DCs alone, and T cells not exposed to ovalbumin-primed DCs have little or no proliferative capacity.
Figure 4.
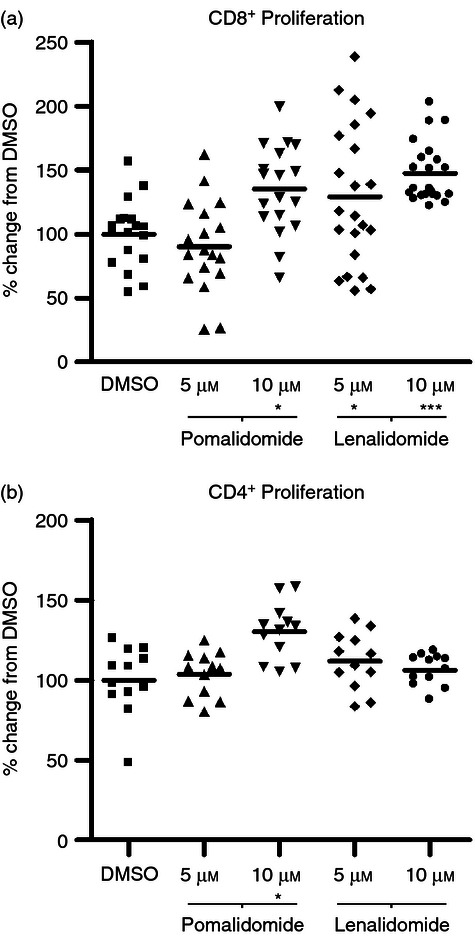
Lenalidomide and pomalidomide increase dendritic cell (DC) dependent T-cell expansion. IMiD-treated DC were loaded with ovalbumin, used in antigen presentation assays with OT-I or OT-II splenocytes and proliferation was measured by [H3]thymidine incorporation. A significantly higher CD8+ T-cell cross-priming was seen (35% and 47% higher than in antigen presentation assays containing 5 and 10 μm of lenalidomide-pre-treated DCs, respectively) compared with the DMSO control [P < 0·05 by repeated measures analysis of variance (anova) and Newman–Keuls, n = 7] and 43% higher in antigen presentation assays containing 10 μm pomalidomide-pre-treated DCs (a) (P < 0·001 by repeated measures anova and Newman–Keuls, n = 7). CD4+ T-cell priming was increased by 30% in 10 μm pomalidomide-pre-treated DCs (P < 0·05 by repeated measures anova and Newman–Keuls, n = 7) (b). Lenalidomide-pre-treated DCs had no effect on CD4 T-cell proliferation.
CD8 T cells activated by IMiD® immunomodulatory compound treated DCs express greater levels of intracellular IFN γ and perforin
Co-cultures of either OTI or OTII splenocytes and DCs pre-treated with either DMSO, or with 5 or 10 μm lenalidomide or pomalidomide were assessed after 5 days for their ability to express intracellular IFN-γ or perforin (in the case of OTI splenocytes only). Intracellular IFN-γ and perforin levels were assayed by intracellular staining with phycoerythrin-conjugated anti-IFN-γ or phycoerythrin-anti-perforin antibodies after Brefeldin A treatment and PMA–ionomycin stimulation. Anti-CD4 or anti-CD8 surface markers were also used for selection of the OTI or OTII population by FACs. Separate aliquots of the co-cultured cells were stained with FITC-annexin and propidium iodide (PI) to confirm the viability of the lymphocytes. Results showed no increase in expression of intracellular IFN-γ in OTII splenocytes when co-cultured with DCs that were pre-treated with the IMiDs (compared with the DMSO control; data not shown). However, a significant increase of intracellular IFN-γ expression from 4% up to 9% occurred in OTI splenocytes when co-cultured with either 5 or 10 μm lenalidomide-treated or pomalidomide-treated DCs (Fig. 5a,c; P < 0·05 by repeated measures anova and Newman–Keuls post test). Perforin levels in OTI cells were also increased from 4% to over 8% when co-cultured with 10 μm lenalidomide-treated or pomalidomide-treated DCs (Fig. 5b,c; P < 0·05 by repeated measures anova and Newman–Keuls post test). The viability of the OTI or OTII lymphocytes incubated with IMiD-pre-treated DCs was not significantly different to that of the lymphocytes incubated with DMSO-pre-treated DCs as assessed by annexin/PI staining of the gated lymphocytes within the co-cultures (1% or less positive annexin and or PI-stained cells; data not shown).
Figure 5.
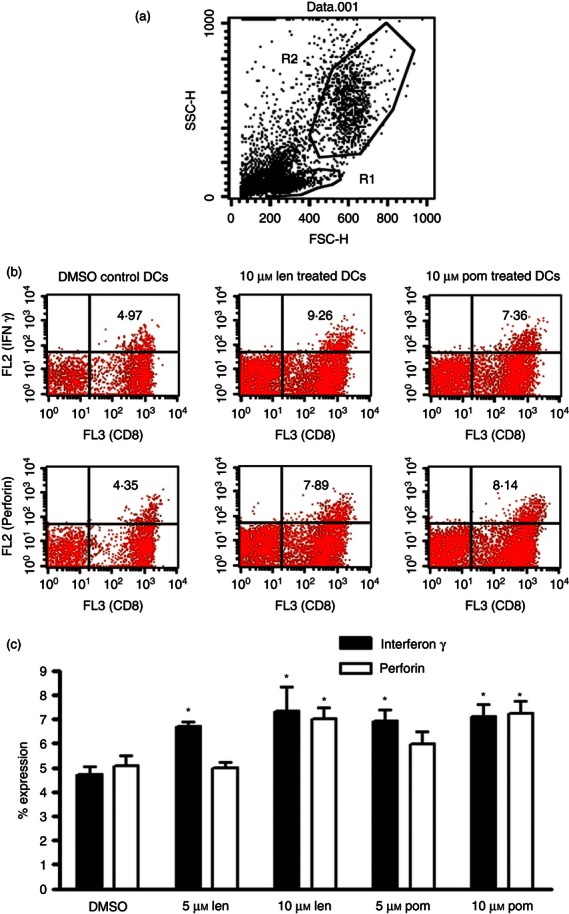
CD8 (OTI) T cells expanded by pomalidomide- and lenalidomide-treated dendritic cells (DCs) are more active than CD8 (OTI) T cells cultured with DMSO-pre-treated DCs. OTI cells co-cultured for 5 days with DCs treated with IMiDs or DMSO control were assessed for intracellular interferon-γ (IFN-γ) and perforin expression by intracellular staining as described under the Materials and methods. Results are expressed in panel (a) to show the separate gating of the lymphocytes (G1) which was then used as the gate to assess staining with peridinin chlorophyll protein-conjugated CD8 (FL3) and either phycoerythrin-conjugated perforin or phycoerythrin-conjugated IFN-γ. The G1 gate contains a population of lymphocytes which are all alive (as assessed by < 1% positive annexin and/or propidium iodide staining of cells within this gate in separate FACs tubes (data not shown). Representative dot plots are shown in panel (b). These show that the CD8-positive T cells within the G1 gate (indicated by the positive FL3 staining) also express IFN-γ and perforin (indicated by FL2 staining of the FL3-positive cells in the upper right quadrant). Panel (c) shows that expression of intracellular IFN-γ and perforin are increased from 4% up to 9% when CD8 (OTI) splenocytes are treated with 10 μm lenalidomide- or pomalidomide-pre-treated DCs [P < 0·05 by repeated measures analysis of variance (anova) and Newman–Keuls, n = 3 experiments]. DCs pre-treated with 5 μm lenalidomide and pomalidomide also significantly increase IFN-γ expression in OTI CD8 T cells (P < 0·05 by repeated measures anova and Newman–Keuls, n = 3 experiments).
Discussion
The advent of two Food and Drug Administration-approved DC vaccines has highlighted DCs as an important component of the cancer therapeutic arsenal.20,21 We now demonstrate that lenalidomide and pomalidomide can directly enhance the maturation and cross-priming function of dendritic cells in vitro, and therefore these drugs could be a useful adjuvant to any DC therapeutic programme. It has been shown previously that lenalidomide can increase the cross-priming function of DCs in a nude mouse model of myeloma22 through activation of natural killer cells and can increase CD8+ T cells co-cultured with DCs,17 but a direct in vitro effect of pomalidomide and lenalidomide has not yet been demonstrated on DCs.
We first studied whether lenalidomide or pomalidomide increased uptake of fluorescent latex beads into DCs. The uptake of antigenic material is a crucial step in DC function, and some chemotherapeutic agents such as paclitaxel and rapamycin can inhibit the uptake of antigen by DCs.23 Both lenalidomide and pomalidomide increased uptake of FITC dextran beads by DCs by up to twofold at 10 μm. The mechanism by which the drugs increase uptake is not clear, however, increased antigen uptake is known to require an activation of the phosphoinositide 3-kinase pathway,24 which can be induced by lenalidomide and pomalidomide in T cells and B cells.25,26
We then assessed whether common markers of maturation were affected by lenalidomide or pomalidomide. Although no changes occurred to FAS, FAS ligand or CD80, there were significant increases in CD86 and class I expression induced by both lenalidomide and pomalidomide, and pomalidomide also increased class II expression on the DCs. Lenalidomide has previously been shown to increase CD86 antigen expression in B cells of patients with chronic lymphocytic leukaemia.27 Although matured DC are not efficient at taking up antigen in the human, antigen uptake by DC is possible in the matured state28 and so the augmentation of DC maturation by lenalidomide or pomalidomide does not contradict the ability of the drugs to increase antigen uptake in these cells.
We next determined the effects of lenalidomide and pomalidomide on cross-presentation of antigen to T cells, measuring the proliferation of the T cells in response to drug-treated DCs. In our cross-presentation assays, pomalidomide- and lenalidomide-treated DCs were more efficient at inducing OT-I antigen-specific proliferation of CD8 T cells than non-treated DCs. This is most likely due to the increased expression of class I antigens on the DCs as a result of the lenalidomide and pomalidomide pre-treatment, and due to the up-regulation of CD86, which also induces a sustained co-stimulatory effect on CD8+ T cells, compared with CD80.29 Both lenalidomide-treated and pomalidomide-treated DCs increased OT-I antigen-specific (CD8+) T-cell proliferation by up to 50% more than the control DMSO treatment. This increase was not the result of any effects on T cells as DCs were pre-incubated with the drugs and then washed thoroughly before exposure to the T cells. Pomalidomide-treated DCs, in contrast to lenalidomide, also increased proliferation of OT-II antigen-specific CD4+ T cells by up to 50% at 10 μm. This may be a result of the ability of pomalidomide to increase class II expression on the DCs, which was not observed with lenalidomide treatment.
To examine the functional effects of lenalidomide and pomalidomide on DCs we also examined their effects on the secretion of common cytokines from the cells. Although no effects were seen on IL-12 p70, IFN-γ, IL-6 or IL-10, significant increases were seen in TNF-α and in MIP-1α by lenalidomide, but not pomalidomide, treatment. In addition to its chemokinetic activity, MIP-1α is known to potently enhance CD8 stimulation by DCs30 and also increases production of TNF-α by DCs. As lenalidomide is known to decrease TNF-α secretion in LPS-stimulated monocytes, it is not clear whether the drug directly up-regulates TNF-α in the DCs or whether this is through an effect on MIP-1α. However, since pomalidomide treatment of DCs induces an antigen-specific increase in the proliferation of CD8 and CD4 T cells without increasing DC secretion of any of the cytokines tested, it is possible that the effects of both drugs are due to an increased CD86 co-stimulatory effect on the DCs. This needs to be explored in further mechanistic studies.
Both pomalidomide and lenalidomide treatment of DCs also results in an increase in the cytotoxic activity (as measured by perforin) and the intracellular interferon-γ expressed by OTI splenocytes. OTII splenocytes are not similarly activated by lenalidomide or pomalidomide with regard to their IFN-γ secretion, but it is deduced that a greater activity of OTII cells will result in the presence of pomalidomide-treated DCs as the OTII cells proliferate more in the presence of these DCs.
In conclusion, pomalidomide and lenalidomide pre-treatment of DCs actively initiates class I restricted T-cell activation with an additional class II restricted activation by pomalidomide and this mechanism is independent of any effects of the drugs on T cells or other effector cell types. These initial studies demonstrate the potential of lenalidomide and pomalidomide as adjuvants for use in DC vaccine therapies.
Acknowledgments
We would like to acknowledge the support of the MRC Centre for Transplantation and the NIHR Biomedical Research centre at Kings College London UK and the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosures
This study has been funded in part by a grant given to A.G Dalgleish by the Celgene corporation.
Authors contributions
J.Y.H, C.G. and A.G.D. designed the research; J.Y.H. performed the experiments; Celgene supplied thalidomide, lenalidomide and pomalidomide; J.Y.H and B.M. analysed data; B.M. and J.Y.H. prepared figures; and J.Y.H, P.D. and C.G. wrote the paper. All authors reviewed and approved the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Effects of lenalidomide and pomalidomide on phenotypic marker expression of dendritic cells.
Figure S2. Cytometric bead array analysis of inflammatory cytokines expressed in lenalidomide and pomalidomide treated dendritic cells reveal no significant changes in interferon-γ, interleukin-10, interleukin-12 P70 and interleukin-6 expression (no significance by repeated measures analysis of variance or Newman–Keuls, n = 4).
Figure S3. Antigen-specific T-cell proliferation: data is presented as in Fig. 4, with additional sets of control data.
References
- 1.Young JW, Steinman RM. The hematopoietic development of dendritic cells: a distinct pathway for myeloid differentiation. Stem Cells. 1996;14:376–87. doi: 10.1002/stem.140376. [DOI] [PubMed] [Google Scholar]
- 2.Ilett EJ, Prestwich RJ, Melcher AA. The evolving role of dendritic cells in cancer therapy. Expert Opin Biol Ther. 2010;10:369–79. doi: 10.1517/14712590903559830. [DOI] [PubMed] [Google Scholar]
- 3.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 4.Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P, Kantoff PW. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–4. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 5.Patel PH, Kockler DR. Sipuleucel-T: a vaccine for metastatic, asymptomatic, androgen-independent prostate cancer. Ann Pharmacother. 2008;42:91–8. doi: 10.1345/aph.1K429. [DOI] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 7.Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. [PubMed] [Google Scholar]
- 8.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34+ cells which suppress immune functions within cancers that secrete granulocyte–macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 9.Gandhi R, Anderson DE, Weiner HL. Cutting Edge: immature human dendritic cells express latency-associated peptide and inhibit T cell activation in a TGF-β-dependent manner. J Immunol. 2007;178:4017–21. doi: 10.4049/jimmunol.178.7.4017. [DOI] [PubMed] [Google Scholar]
- 10.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu WM, Henry JY, Meyer B, Bartlett JB, Dalgleish AG, Galustian C. Inhibition of metastatic potential in colorectal carcinoma in vivo and in vitro using immunomodulatory drugs (IMiDs) Br J Cancer. 2009;101:803–12. doi: 10.1038/sj.bjc.6605206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu WM, Laux H, Henry JY, Bolton TB, Dalgleish AG, Galustian C. A microarray study of altered gene expression in colorectal cancer cells after treatment with immunomodulatory drugs: differences in action in vivo and in vitro. Mol Biol Rep. 2010;37:1801–14. doi: 10.1007/s11033-009-9614-3. [DOI] [PubMed] [Google Scholar]
- 14.Henry JY, Lu L, Adams M, Meyer B, Bartlett B, Dalgleish AG, Galustian C. Lenalidomide enhances the anti-prostate cancer activity of docetaxel in vitro and in vivo. Prostate. 2012;72:856–67. doi: 10.1002/pros.21488. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–7. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 16.Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58:1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haslett PA, Hanekom WA, Muller G, Kaplan G. Thalidomide and a thalidomide analogue drug costimulate virus-specific CD8+ T cells in vitro. J Infect Dis. 2003;187:946–55. doi: 10.1086/368126. [DOI] [PubMed] [Google Scholar]
- 18.Galea-Lauri J, Wells JW, Darling D, Harrison P, Farzaneh F. Strategies for antigen choice and priming of dendritic cells influence the polarization and efficacy of antitumor T-cell responses in dendritic cell-based cancer vaccination. Cancer Immunol Immunother. 2004;53:963–77. doi: 10.1007/s00262-004-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J Exp Med. 1997;186:239–45. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahnisch H, Fussel S, Kiessling A, et al. Dendritic cell-based immunotherapy for prostate cancer. Clin Dev Immunol. 2010;2010:517493. doi: 10.1155/2010/517493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill DW. Dendritic cells and T cells in immunotherapy. J Drugs Dermatol. 2010;9:1383–92. [PubMed] [Google Scholar]
- 22.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, Wallace P, Czuczman MS. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 23.John J, Ismail M, Riley C, Askham J, Morgan R, Melcher A, Pandha H. Differential effects of Paclitaxel on dendritic cell function. BMC Immunol. 2010;11:14. doi: 10.1186/1471-2172-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–94. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 25.Lapalombella R, Andritsos L, Liu Q, et al. Lenalidomide treatment promotes CD154 expression on CLL cells and enhances production of antibodies by normal B cells through a PI3-kinase-dependent pathway. Blood. 2010;115:2619–29. doi: 10.1182/blood-2009-09-242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeBlanc R, Hideshima T, Catley LP, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 27.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–9. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- 28.Drutman SB, Trombetta ES. Dendritic cells continue to capture and present antigens after maturation in vivo. J Immunol. 2010;185:2140–6. doi: 10.4049/jimmunol.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas IJ, Petrich de Marquesini LG, Ravanan R, et al. CD86 has sustained costimulatory effects on CD8 T cells. J Immunol. 2007;179:5936–46. doi: 10.4049/jimmunol.179.9.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flesch IE, Stober D, Schirmbeck R, Reimann J. Monocyte inflammatory protein-1α facilitates priming of CD8+ T cell responses to exogenous viral antigen. Int Immunol. 2000;12:1365–70. doi: 10.1093/intimm/12.9.1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


