Abstract
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are bioactive n-3 long-chain polyunsaturated fatty acids (LCPUFAs) in fish oil that exert immunosuppressive effects. A significant amount of literature shows that n-3 LCPUFAs suppress dendritic cell (DC) function in vitro; however, few studies have determined if the effects are emulated at the animal level. In this study, we first focused on the functional consequences of 5% (weight/weight) fish oil on splenic CD11c+ DCs. Administration of n-3 LCPUFAs, modelling human pharmacological intake (2% of total kcal from EPA,1·3% from DHA), to C57BL/6 mice for 3 weeks reduced DC surface expression of CD80 by 14% and tumour necrosis factor-α secretion by 29% upon lipopolysaccharide stimulation relative to a control diet. The n-3 LCPUFAs also significantly decreased CD11c+ surface expression and phagocytosis by 12% compared with the control diet. Antigen presentation studies revealed a 22% decrease in CD69 surface expression on transgenic CD4+ T lymphocytes activated by DCs from mice fed fish oil. We then determined if the functional changes were mechanistically associated with changes in lipid microdomain clustering or plasma membrane microviscosity with n-3 LCPUFAs, as reported for B and T lymphocytes. Fish oil administration to mice did not influence cholera-toxin induced lipid microdomain clustering or microviscosity, even though EPA and DHA levels were significantly elevated relative to the control diet. Overall, our data show that n-3 LCPUFAs exert immunosuppressive effects on DCs, validating in vitro studies. The results also show that DC microdomain clustering and microviscosity were not changed by the n-3 LCPUFA intervention used in this study.
Keywords: Dendritic cells, diet, fish oil
Introduction
The n-3 long-chain polyunsaturated fatty acids (LCPUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are bioactive molecules found in fish oil, which can suppress some symptoms associated with acute and chronic inflammation.1–4 Given that a variety of disease states are characterized by inflammation, n-3 LCPUFAs may have clinical applications for supplementing or even replacing current pharmacological treatments.5 To effectively develop n-3 LCPUFAs for clinical use, it is essential to determine the cell-specific effects of these fatty acids.
Currently, we know that n-3 LCPUFAs regulate T lymphocyte function in animal models.6–11 For example, murine ex vivo CD4+ T-lymphocyte activation into a classically defined T helper type 1 (Th1) phenotype by hybridomas or CD3/CD28 antibodies is robustly suppressed by n-3 LCPUFAs, as measured by cytokine secretion and proliferation.12,13 Recently, we demonstrated that n-3 LCPUFAs in fish oil suppressed ex vivo murine B-lymphocyte stimulation of cognate naive CD4+ T cells.14 We also showed that n-3 LCPUFAs were not globally immunosuppressive, as murine B-lymphocyte activation was enhanced at several doses in response to the T-cell-independent antigen lipopolysaccharide (LPS).14,15 Our results demonstrated that n-3 LCPUFAs could exhibit differential effects within a single cell type depending on the antigen and the functional end-point.14,15 Less is known about the effects of fish oil on other cell types. In this study, we addressed the effects of n-3 LCPUFAs on dendritic cells (DCs), a cell type not well studied at the animal level with fish oil.
Dendritic cells are the most potent antigen-presenting cells and are responsible for activating T lymphocytes.16 In addition, DCs are scavenger cells, constantly surveying tissues for the presence of a pathogen. Compared to T lymphocytes, DCs are directly activated upon pathogen recognition. However, data addressing how pharmacologically relevant doses of fish oil regulate DC function are limited. The majority of studies with n-3 LCPUFAs and DCs have relied on bone-marrow-derived cells, which require an in vitro model system for experimentation with a lengthy culture period for maturation.17–20 Generally, these studies have revealed that EPA and DHA administration robustly suppresses DC maturation and T-cell stimulation. In this study, we focused on splenic CD11c+ DCs, which circumvented the need for long-term cell culture.
The first goal of this study was to test the hypothesis that n-3 LCPUFAs would suppress DC function, as predicted by in vitro studies.17–20 We investigated three major components of DC function: DC activation upon LPS stimulation, phagocytosis of Escherichia coli (E. coli) bioparticles and stimulation of naive CD4+ T cells. Each assay revealed that an element of DC function was suppressed. The second goal was to determine if the immunosuppressive effects of fish oil were mechanistically associated with a reorganization in plasma membrane lipid microdomain spatial distribution and membrane microviscosity (i.e. order) as shown for T and B lymphocytes by our laboratory and others.14,21–24 We addressed this objective by using quantitative imaging to assay for select changes in membrane microdomain organization and membrane order. Unlike studies in lymphocytes, we show that DC GM1 lipid microdomain clustering and membrane microviscosity were not changed with n-3 LCPUFA intervention.
Materials and methods
Mice
Male C57BL/6 mice (Charles River) were fed a purified control diet or a fish oil diet (Harlan-Teklad; Harlan Laboratories, Indianapolis, IN) for 3 weeks, as described previously.14 Both diets contained identical ingredients with the exception of the fatty acid source (soybean oil versus fish oil). The fish oil diet contained approximately 2% of the total kcal from EPA and 1·3% from DHA, which translates to approximately 4 g fish oil of high purity consumed by a human on a daily basis.25 This pharmacological dose is currently used clinically (e.g. Lovaza) for the treatment of elevated triglycerides and in clinical trials.26,27 Mice were killed by CO2 inhalation followed by cervical dislocation. All experiments with mice received previous approval from the Institutional Animal Care and Use Committee at East Carolina University.
Cell isolation
CD11c+ DCs were purified from splenocytes with a positive selection microbead kit (Miltenyi Biotec, Auburn, CA). Briefly, isolated spleens were incubated in collagenase D solution (1·4 mg/ml) for 30 min at 37° in a 5% CO2 incubator. Spleens were then homogenized and red blood cells were lysed. Dendritic cells were labelled with CD11c+ microbeads following the manufacturer's recommended protocol and > 90% DC purity was obtained by sequentially separating cells through two LS columns.28–30 CD4+ T cells (> 85% purity) were purified from B6.Cg-Tg(TcraTcrb)425Cbn/J mice (OT-II) (Jackson Laboratory, Bar Harbor, ME) via negative selection following the manufacturer's suggested protocol with minor modifications (Miltenyi Biotec). The splenocytes were incubated on a rotator in the CD4+ biotin cocktail for 30 min at 4°. The anti-biotin incubation step was also completed on a rotator and separations through two LS columns were required to achieve maximum purity. OT-II transgenic mice were chosen for this assay because of an increased ratio of CD4+ T cells to CD8+ T cells and the ability of the T-cell receptor to specifically recognize chicken ovalbumin (OVA 323–339; Genscript, Piscataway, NJ) presented by H-2 IAb molecules.
LPS stimulation
Dendritic cells were stimulated with 2 μg/ml LPS and incubated for 24 hr in RPMI-1640 1 × media (Mediatech, Manassas, VA) supplemented with 5% heat-inactivated defined fetal bovine serum (Hyclone, Logan, UT), 2 mm l-glutamine, and 1% penicillin/streptomycin at 37° in a 5% CO2 incubator. The concentration of LPS used to stimulate splenic DCs was optimized to ensure high expression of co-stimulatory molecules. The supernatants were collected and the secreted cytokine profiles were measured using an ELISA MAX kit (BioLegend, San Diego, CA) following the manufacturer's suggested protocol. Activation of DCs was determined by measuring the surface expression of activation markers using fluorescently labelled anti-CD80 16-10A1 (Bio X Cell, West Lebanon, NH), anti-MHC class II M5/114.15 (Bio X Cell), and anti-CD11c (Miltenyi Biotec) via a BD LSR II flow cytometer (BD Biosciences, San Jose, CA). MHC class II and CD80 antibodies were conjugated to fluorophores using an antibody conjugation kit (GE Healthcare, Piscataway, NJ).
Phagocytosis assay
Cells (5 × 105) were transferred to FACS tubes and washed once in Hanks' balanced salt solution at 4°. Dendritic cells were treated with fluorescein-conjugated E. coli Bioparticles (Invitrogen, Grand Island, NY) for 2 hr at a 1 : 9 to 1 : 11 DC to E. coli bioparticle ratio at 37° and 4° as the control. Following the 2-hr incubation period, DCs were placed at 4° to terminate phagocytosis. The DCs were washed once with 1 × PBS and treated with 1 mg/ml trypan blue (Invitrogen) for 2 min at 4° to quench extracellular fluorescence.31 Dendritic cells were then washed twice with 1 × PBS and phagocytosis was determined by mean fluorescence intensity via flow cytometry. The number of bioparticles per experiment was held constant; however, there was some variation in the number of bioparticles between experiments. As a consequence, the data required normalizing to account for the variation between experiments.
Antigen presentation
Dendritic cells were combined with T cells at a 1 : 3 ratio (1 × 105 DCs to 3 × 105 CD4+ T cells) in a 96-well plate. Dendritic cells were treated with 10−5 m OVA 323–339 (Genscript) as previously described.14 The concentration of OVA was optimized for maximum CD4+ T-cell activation. Cells were incubated for 24 hr in RPMI-1640 1 × medium. The supernatants were collected for interleukin-2 (IL-2) and interferon-γ (IFN-γ) analysis via a Multi-Analyte ELISArray kit (SABiosciences, Valencia, CA) according to manufacturer's protocol. The reported absorbance values for IL-2 and IFN-γ correspond respectively to ∼ 420 and ∼ 60 pg/ml. CD4+ T-cell activation was determined by gating on CD4+ cells and measuring the surface expression of CD69 and CD25. Cells were labelled with phycoerythrin-conjugated anti-CD4 (Miltenyi Biotech), FITC-conjugated anti-CD69 (BD Biosciences), and allophycocyanin-conjugated anti-CD25 (BD Biosciences) and assayed via flow cytometry on a BD LSR II (BD Biosciences). SYTOX Blue (Invitrogen) was used in all flow cytometry experiments to differentiate between live and dead cells. Control experiments were conducted with DCs and T cells alone to ensure that CD4+ T-cell activation was due to interactions between the two cell types.
Lipid microdomain staining and image analysis
Dendritic cells were labelled with cholera-toxin subunit B-FITC (Invitrogen) for GM1 molecules and then cross-linked with anti-cholera-toxin to induce clustering. The cells were fixed in 4% paraformaldehyde. Imaging of lipid microdomains was conducted with a Zeiss LSM510 confocal microscope and analysed as previously described.21
Microviscosity studies and analysis
DCs were incubated with 4 μm di-4-ANEPPDHQ (Invitrogen) for 30 min at 4° followed by fixation for 1 hr in 4% paraformaldehyde on ice. DCs were washed three times in 1 × PBS and loaded into vitrotubes for imaging. Images were acquired on an Olympus Fluoview FV1000 with excitation at 488 nm with an argon laser. Instrument settings entailed an SM560 beam splitter equipped with two bandwidth filters between 535–565 nm in channel 1 and 565–675 nm in channel 2. Generalized polarization values were calculated by measuring the fluorescence intensity in each channel as previously reported.14,32
Fatty acid analysis
Total fatty acids were extracted using the Folch method.33 The rationale for isolating total fatty acids, as opposed to plasma membrane fatty acids, was that very large quantities of DCs (> 1 × 108) were required, which was not feasible given the low abundance of DCs in the spleen. Fatty acids were separated on a capillary gas chromatograph (Shimadzu Scientific Instruments, Columbia, MD) with a Restek RT-2560 column as previously described.15 Peaks were identified by their retention times relative to standards (Restek, Bellefonte, PA) and are expressed as per cent of total peak area for a given treatment.
Statistical analysis
Data are presented as mean ± SEM. All data are from several independent experiments. Statistical significance was established using a two-tailed unpaired Student's t-test with the exception of the phagocytosis assay. Mean fluorescence intensity normalization required a paired Student's t-test. P-values < 0·05 were considered significant.
Results
CD80 surface expression and tumour necrosis factor-α production from LPS-stimulated DCs is lowered by n-3 LCPUFAs
To determine the effects of n-3 LCPUFA-enriched fish oil on DC activation, splenic DCs were purified and stimulated with the T-cell-independent antigen LPS for 24 hr. The surface expression of co-stimulatory CD80 and MHC class II molecules was measured (Fig. 1a). In both the unstimulated (−LPS) and stimulated (+LPS) states, MHC class II surface expression did not change with n-3 LCPUFA relative to the control (Fig. 1a). In the absence of LPS stimulation, n-3 LCPUFA lowered CD80 surface expression by 28% (Fig. 1b); in addition, LPS stimulation showed a decrease in CD80 surface expression by 14% (Fig. 1b) with fish oil. The levels of tumour necrosis factor-α (TNF-α), Interleukin-10 (IL-10), IL-6, IL-12p40 and transforming growth factor-β (TGF-β) were also measured (Fig. 1c). Quantification of cytokine secretion revealed that TNF-α production was decreased by 29% with n-3 LCPUFA (Fig. 1c). IL-10, IL-12p40, IL-6 and TGF-β secretion were unaffected by n-3 LCPUFA (Fig. 1c).
Figure 1.
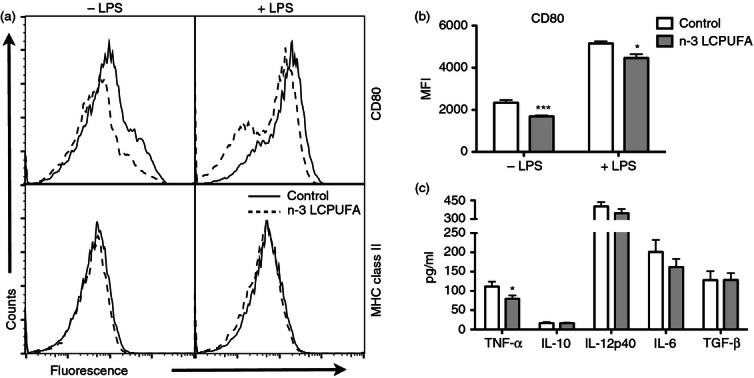
CD80 surface expression and tumour necrosis factor-α (TNF-α) secretion from lipopolysaccharide (LPS) -stimulated dendritic cells (DCs) is lowered with n-3 long-chain polyunsaturated fatty acids (LCPUFAs). DCs were isolated from C57BL/6 mice consuming a control or n-3 LCPUFA diet and stimulated for 24 hr with LPS. (a) Sample flow cytometry histograms of CD80 and MHC class II. (b) The mean fluorescence intensity (MFI) of CD80 in the absence and presence of LPS stimulation. (c) TNF-α, interleukin-10 (IL-10), IL-12p40, IL-6 and transforming growth-factor-β (TGF-β) cytokine secretion was measured after 24 hr of LPS stimulation. Data are from 6 to 10 independent experiments. Asterisks indicate significance from control: *P < 0·05, ***P < 0·001.
CD11c+ surface expression and uptake of E. coli bioparticles by DCs is suppressed with n-3 LCPUFAs
Immediately following DC purification, we measured a 12% decrease in the surface expression of CD11c, a key marker involved in phagocytosis, with the n-3 LCPUFA diet (Fig. 2a).34,35 Therefore, we assessed if phagocytosis was reduced by quantifying DC uptake of fluorescently labelled opsonized E. coli bioparticles at 37° and 4° (Fig. 2b). The 4° control was required to ensure that measured fluorescence was a result of phagocytosed bioparticles as opposed to E. coli bioparticles bound to the cell surface. We discovered that the percentage of DCs engulfing E. coli bioparticles did not change (data not shown); however, the amount of E. coli bioparticles taken up was lowered by 12% (Fig. 2c). The data in Fig. 2(c) were normalized to account for slight variations in the number of bioparticles used between experiments. Raw mean fluorescence intensity values ranged from ∼ 3200 to 4200 but showed a consistent decrease with fish oil in each experiment.
Figure 2.
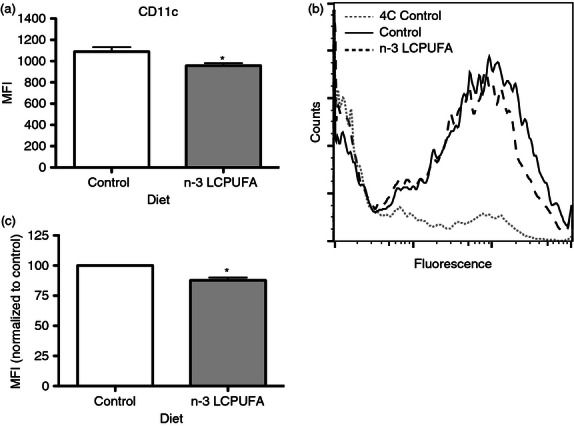
Dendritic cell (DC) CD11c+ surface expression and phagocytosis are suppressed by n-3 long-chain polyunsaturated fatty acids (LCPUFAs). (a)The surface expression of CD11c on DCs isolated from C57BL/6 mice consuming a control or n-3 LCPUFA diet. (b) Sample flow cytometry histograms of fluorescence uptake of Escherichia coli bioparticles. (c) The amount of E. coli bioparticles taken up as indicated by the mean fluorescence intensity (MFI), which was was normalized due to a slight variation in the number of bioparticles administered to the cells between experiments. Data are from six to eight independent experiments in (a) and four for (b) and (c). Asterisk indicates significance from control: *P < 0·05.
CD69 surface expression on the surface of CD4+ T cells was lowered with n-3 LCPUFAs upon DC stimulation
Dendritic cells are the most potent antigen-presenting cells, which activate naive T cells. Therefore, we determined if n-3 LCPUFAs suppressed the ability of DCs to present antigen to CD4+ T cells. We first optimized activation of naive T cells as a function of peptide dose and discovered that 10−5 m peptide provided the most robust response (data not shown). We measured the surface expression of CD69 and CD25 on the surface of activated CD4+ T cells after 24 hr of stimulation (Fig. 3a). The percentage of CD69+ T cells remained unchanged (data not shown); however, CD69 surface expression was decreased by 22% with n-3 LCPUFAs relative to the control diet (Fig. 3b). No change was observed in CD25 surface expression (Fig. 3b). Secretion of the cytokines IL-2 and IFN-γ for the control and experimental diets were equivalent (Fig. 3c). We also conducted select experiments at 48 hr of T-cell stimulation and measured no differences in the Th2 cytokines IL-4 and IL-5 in response to n-3 LCPUFAs (data not shown).
Figure 3.
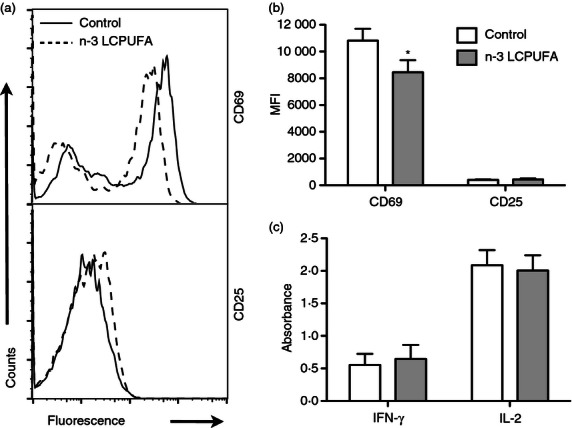
CD69 expression on CD4+ T cells is lowered upon stimulation with dendritic cells (DCs) isolated from mice fed n-3 long-chain polyunsaturated fatty acids (LCPUFAs). The DCs were isolated from C57BL/6 mice consuming a control or n-3 LCPUFA diet and incubated with naive CD4+ T cells isolated from transgenic mice for 24 hr. T-cell activation was (a) measured by CD69 and CD25 surface expression and (b) quantified in terms of mean fluorescence intensities (MFI). (c) Interleukin-2 (IL-2) and interferon-γ (IFN-γ) secretion were measured with ELISAs. Data are from seven independent experiments. Asterisk indicates significance from control: *P < 0·05.
Lipid microdomain GM1 clustering and membrane microviscosity on the surface of DCs is not influenced by n-3 LCPUFAs despite increased levels of EPA and DHA
We and others have previously reported that fish oil mechanistically targets B-lymphocyte and T-lymphocyte activation and antigen presentation by disrupting the clustering of lipid microdomains.6–8,14 To investigate if the changes in DC function were dependent on the underlying lipid microdomain organization, we measured changes in GM1 microdomain clustering with n-3 LCPUFA intervention (Fig. 4a). Image analysis revealed there were no differences in the distribution of size of the lipid microdomains between control and n-3 LCPUFA diets (Fig. 4b). In addition, when analysing the percentage of cells exhibiting clustered lipid microdomains, we observed no distinction between control and n-3 LCPUFA (data not shown).
Figure 4.
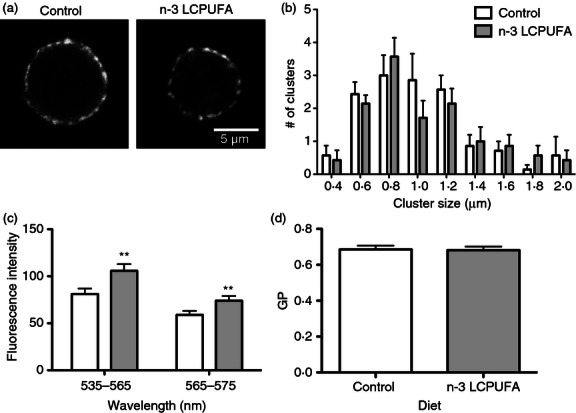
Dendritic cell (DC) GM1 lipid microdomain clustering and membrane microviscosity are not influenced by n-3 long-chain polyunsaturated fatty acids (LCPUFAs). DCs were isolated from C57BL/6 mice consuming either a control or n-3 LCPUFA diet. (a) Confocal images of cholera toxin-induced lipid microdomains and (b) distribution of microdomain size measured in terms of Feret's diameter. (c) Fluorescence intensity of di-4-ANEPPDHQ measured in the 535–565 nm and 565–675 nm channels. (d) Generalized polarization (GP) of di-4-ANEPPDHQ. Data are from six to eight independent experiments. Asterisks indicate significance from control: **P < 0·01.
We next determined if there was a decrease in DC microviscosity (i.e. increase in fluidity) with n-3 LCPUFAs. Imaging studies with the polarity sensitive membrane dye di-4-ANEPPDHQ revealed that there was an increase in di-4-ANEPPDHQ uptake with fish oil intervention as indicated by increased fluorescence intensity in channel 1 (535–565) by 23% and in channel 2 (565–675) by ∼ 21% (Fig. 4c). However, the calculated generalized polarization values showed no change between the control and n-3 LCPUFA diets (Fig. 4d).
Finally, we measured the total fatty acid content of DCs to determine if there was an increase in the amount of EPA and DHA with the fish oil diet (Fig. 5a). Palmitoleic (16 : 1), EPA (20 : 5), docosapentaenoic acid (22 : 5 n-3) and DHA (22 : 6) all increased with an n-3 LCPUFA-enriched diet (Fig. 5a). Interestingly, we did not observe a change in palmitic acid (16 : 0) between control and fish oil, as seen previously with B lymphocytes (Fig. 5a).14 We observed a decrease in oleic acid (18 : 1), linoleic acid (18 : 2) and arachidonic acid (20 : 4) (Fig. 5a) with the n-3 LCPUFA diet relative to the control diet. There was no change in the total levels of saturated and monounsaturated fatty acids (Fig. 5b). A decrease in total n-6 PUFAs concomitant with an increase in total n-3 PUFAs was measured with DCs from the n-3 LCPUFA diet relative to controls, lowering the n-6/n-3 ratio (Fig. 5b).
Figure 5.
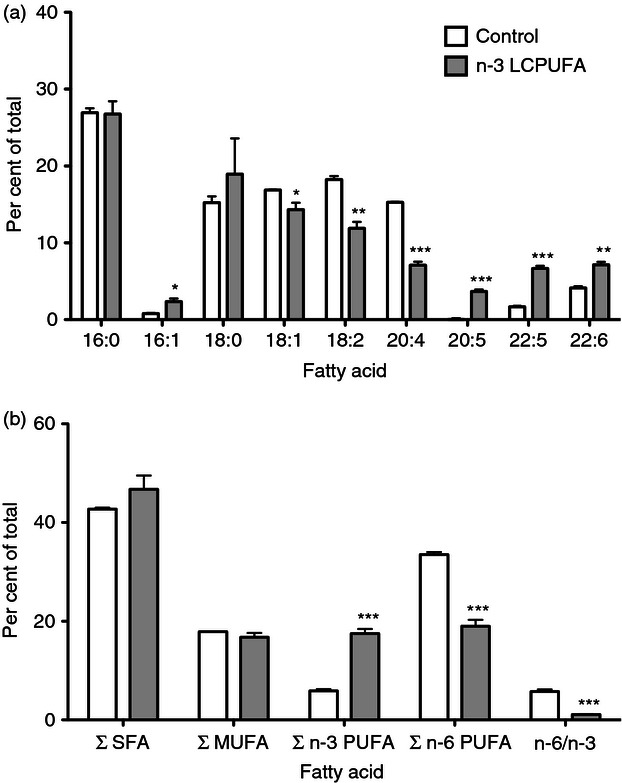
Dendritic cell (DC) eicosapentaenoic and docosahexaenoic acid levels are increased in response to a diet enriched in n-3 long-chain polyunsaturated fatty acids (LCPUFAs). (a) Fatty acid composition of DCs isolated from mice fed control and n-3 LCPUFA diets. (b) The total saturated fatty acid (SFA), monounsaturated fatty acid (MUFA), n-3 PUFA, n-6 PUFA and n-6 : n-3 ratio. Data are from three independent experiments. Asterisks indicate significance from control: *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
This study tested the hypothesis that n-3 LCPUFA-enriched fish oil would suppress DC function, as reported with in vitro studies. First, the data showed that co-stimulatory molecule and cytokine secretion was reduced with fish oil supplementation. Second, n-3 LCPUFAs lowered CD11c expression and phagocytosis. Third, n-3 LCPUFAs lowered surface expression of a T-cell activation marker. Finally, we demonstrated that the observed changes were independent of GM1 microdomain clustering and membrane microviscosity.
LCPUFAs and DC activation
CD80 is a co-stimulatory molecule on the surface of DCs involved in T-cell activation. CD80 up-regulation serves as a measure of DC maturation subsequent to antigen stimulation. In this study, n-3 LCPUFAs decreased the surface expression of CD80 in both the unstimulated and LPS-stimulated states. In addition, n-3 LCPUFAs lowered the amount of TNF-α produced by the DCs. Overall, these findings are in agreement with several in vitro studies. For example, Wang et al. showed that treatment of DCs, produced from culturing peripheral blood human monocytes, with 50 μm EPA or DHA resulted in decreased CD80 and HLA-DR expression, as well as TNF-α and IL-12p70 production, following LPS stimulation.36 We did not measure a change in MHC class II surface expression. A few differences exist between our work and that of Wang et al.20 First, the DCs between the studies were derived from different tissues. Second, with the in vitro study, the cells were treated with the single fatty acids, EPA or DHA, compared with our study, where fish oil contained a mixture of fatty acids including EPA and DHA.
An investigation by Zeyda et al.20 also reported similar findings, where 20 μm EPA treatment decreased CD80 expression and TNF-α and IL-12p40 production from DCs differentiated from human peripheral blood monocytes after 48 hr of LPS stimulation. We used a 24-hr incubation period to ensure that the effects of n-3 LCPUFA supplementation were not lost in the culture conditions, as previously reported.15 An additional study with low-density lipoprotein receptor knockout mice by Nakajima et al. also showed that EPA suppressed DC maturation, lowering CD80, CD86 and CD40 surface expression on DCs.37 A study by Monk et al. compared cytokine secretion from LPS-stimulated DCs isolated from Fat-1 transgenic mice with that from wild-type mice.9 Our data were not in complete agreement with those of Monk et al. They reported that following 24 hr of LPS stimulation, there was an increase in IL-10, a decrease in TGF-β1 and IL-12p40 and no change in TNF-α or IL-6 secretion. The differences between our study and theirs could be a result of the concentration of LPS used (we used 2 μg/ml compared with 10 μg/ml used by Monk et al.) or the different mouse strains. Additional studies on bone-marrow-derived DCs from Zapata-Gonzalez et al.38 showed a down-regulation of co-stimulatory molecules, decreased cytokine secretion and an up-regulation of peroxisome proliferator-activated receptor-γ expression in a DHA-concentration-dependent manner. Together, these studies suggest n-3 LCPUFAs exert immunosuppressive effects on LPS-stimulated DCs in both in vitro and ex vivo studies, albeit at different magnitudes.
One major limitation of the aforementioned studies, including our own work, is that the studies have relied on ex vivo measurements. To address the impact of fish oil on inflammatory responses, future studies will require in vivo measurements with appropriate doses of LPS and several rodent models of acute and chronic inflammation. These are highly relevant given that fish oil's immunosuppressive effects may be beneficial for select inflammatory conditions; yet, pose a significant risk in response to bacterial or viral infections. Indeed, Schwerbrock et al. 39 demonstrated that administration of fish oil can decrease mouse survival in response to influenza infection.
LCPUFAs and phagocytosis
CD11c expressed on DCs acts as a receptor for the complement component iC3b and is involved in cell adhesion.34,40,41 For the first time, we showed that CD11c surface expression was reduced with n-3 LCPUFA supplementation. Given that CD11c has been shown to be involved in phagocytosis, we also discovered a reduction in phagocytosis.34,35 These results are not in agreement with an in vitro study by Kong et al.17 They reported that 50 μm DHA treatment resulted in decreased expression of co-stimulatory surface markers and cytokine production from bone-marrow-derived DCs but observed no change in FITC-dextran uptake.
DC antigen presentation
Blockage of CD80 on DCs leads to a 24% decrease in naive CD4+ T-lymphocyte activation.42 Concomitant with the CD80 reduction, we anticipated antigen presentation to naive CD4+ T lymphocytes with n-3 LCPUFA-modified DCs would also be reduced as reported for rat DCs.43 Although we did not observe a change in IL-2 or IFN-γ production from the CD4+ T lymphocytes, we did measure a decrease in CD69 surface expression. It is unclear why DCs from mice fed fish oil decreased CD69 surface expression, yet had no impact on CD25 expression or cytokine secretion. Interestingly, we have made the same observation with a lower dose of n-3 LCPUFAs modelling human intake at 2 g per day (data not shown).
Our data were consistent with a human study by Kew et al.44 that showed a reduction in CD69 expression on T cells isolated from humans consuming DHA and activated with concanavalin A for 24 hr. Similar to our study, Kew et al. measured no change in IL-2 or IFN-γ secretion. In addition, the data were consistent with recent work from our laboratory that showed B lymphocytes isolated from fish-oil-fed mice exhibited lowered antigen presentation as measured by decreased IL-2 secretion from CD4+ T lymphocytes.14 Suppressing aspects of T-cell stimulation with both DCs and B lymphocytes could have an additive effect. This would then contribute toward suppression in CD4+ T-lymphocyte activation, probably suppressing Th1 and promoting Th2 immunity.
Membrane microdomain organization of DCs
Although we confirmed an increase in EPA and DHA uptake by DCs, we did not observe a change in membrane reorganization based on induced GM1 microdomain formation and microviscosity. Some studies have previously shown that increased levels of EPA and DHA are not always associated with a decrease in membrane order.45 Interestingly, we did discover that the uptake of the polarity-sensitive dye was increased suggesting that n-3 LCPUFAs could be increasing membrane permeability. Overall, these findings suggest that the immunosuppressive effects are occurring independent of changes in lipid microdomain clustering and are possibly a result of events occurring further downstream in the signalling cascade. Our data do not rule out the possibility that fish oil is targeting other key aspects of membrane organization such as lipid–protein spatio-temporal distribution. For example, it is possible, that fish oil could be targeting Toll-like receptor-4 lateral distribution, as demonstrated in vitro.46 We did measure Toll-like receptor-4 surface expression and found no difference between the control and n-3 LCPUFA diets (data not shown). DHA could also be preventing the aggregation of Toll-like receptor-4 with its regulatory proteins such as MD-2 or NADH oxidase, which would contribute toward a decrease in downstream gene activation and cytokine secretion.46 Our rationale for focusing on GM1 microdomains was to compare the data with recent studies by our group and others showing that fish oil targets lipid microdomain clustering in B and T lymphocytes.6,13,14
The data suggest that the effects of n-3 LCPUFAs are dependent on the cell type and function. Comparing DCs with B lymphocytes, LPS stimulation leads to the opposite effects ex vivo. In addition, the effect of n-3 LCPUFAs on lipid microdomain organization and molecular order were vastly different between the cell types. One key difference is that B and T lymphocytes are developed from a common lymphoid progenitor whereas DCs are developed from a common myeloid progenitor. Both cell types are also morphologically different because of differing functional requirements. Dendritic cells are irregularly shaped with protruding dendrites, whereas B and T lymphocytes are spherical in nature. We speculate that inherited morphological differences could explain how n-3 LCPUFA incorporation into the plasma membrane results in different effects on membrane organization.
Overall, we show that n-3 LCPUFAs, at a pharmacologically relevant dose, suppress key aspects of splenic DC function. The imaging studies reveal that n-3 LCPUFAs have no effect on DC plasma membrane microdomain clustering and microviscosity. Future studies will be required to understand how n-3 LCPUFAs exert functional effects on varying subsets of DCs, especially in vivo, and the underlying mechanisms of action.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R15AT006122) to S.R.S.
Disclosure
The authors have no competing interests.
References
- 1.Duda MK, O'Shea KM, Tintinu A, et al. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–27. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han YY, Lai SL, Ko WJ, Chou CH, Lai HS. Effects of fish oil on inflammatory modulation in surgical intensive care unit patients. Nutr Clin Pract. 2012;27:91–8. doi: 10.1177/0884533611429796. [DOI] [PubMed] [Google Scholar]
- 3.Luu NT, Madden J, Calder PC, et al. Dietary supplementation with fish oil modifies the ability of human monocytes to induce an inflammatory response. J Nutr. 2007;137:2769–74. doi: 10.1093/jn/137.12.2769. [DOI] [PubMed] [Google Scholar]
- 4.Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trebble TM, Stroud MA, Wootton SA, et al. High-dose fish oil and antioxidants in Crohn's disease and the response of bone turnover: a randomised controlled trial. Br J Nutr. 2005;94:253–61. doi: 10.1079/bjn20051466. [DOI] [PubMed] [Google Scholar]
- 6.McMurray DN, Jolly CA, Chapkin RS. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model. J Infect Dis. 2000;182(Suppl 1):S103–7. doi: 10.1086/315909. [DOI] [PubMed] [Google Scholar]
- 7.Guermouche B, Yessoufou A, Soulimane N, Merzouk H, Moutairou K, Hichami A, Khan NA. n-3 fatty acids modulate T-cell calcium signaling in obese macrosomic rats. Obes Res. 2004;12:1744–53. doi: 10.1038/oby.2004.216. [DOI] [PubMed] [Google Scholar]
- 8.Chapkin RS, Arrington JL, Apanasovich TV, Carroll RJ, McMurray DN. Dietary n-3 PUFA affect TcR-mediated activation of purified murine T cells and accessory cell function in co-cultures. Clin Exp Immunol. 2002;130:12–8. doi: 10.1046/j.1365-2249.2002.01951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk JM, Jia Q, Callaway E, Weeks B, Alaniz RC, McMurray DN, Chapkin RS. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J Nutr. 2012;142:117–24. doi: 10.3945/jn.111.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse modulate calcium signaling in T cells. J Immunol. 2010;184:5865–73. doi: 10.4049/jimmunol.0904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brix S, Lund P, Kjaer TM, Straarup EM, Hellgren LI, Frokiaer H. CD4+ T-cell activation is differentially modulated by bacteria-primed dendritic cells, but is generally down-regulated by n-3 polyunsaturated fatty acids. Immunology. 2010;129:338–50. doi: 10.1111/j.1365-2567.2009.03163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–51. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- 13.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, Shaikh SR. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J Lipid Res. 2012;53:674–85. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition prevents palmitate-induced apoptosis differentially modifies B cell cytokine secretion in vitro and ex vivo. J Lipid Res. 2010;51:1284–97. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 17.Kong W, Yen JH, Vassiliou E, Adhikary S, Toscano MG, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010;9:12. doi: 10.1186/1476-511X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong W, Yen JH, Ganea D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav Immun. 2011;25:872–82. doi: 10.1016/j.bbi.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Draper E, Reynolds CM, Canavan M, Mills KH, Loscher CE, Roche HM. Omega-3 fatty acids attenuate dendritic cell function via NF-κB independent of PPARγ. J Nutr Biochem. 2011;22:784–90. doi: 10.1016/j.jnutbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Zeyda M, Saemann MD, Stuhlmeier KM, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB activation. J Biol Chem. 2005;280:14293–301. doi: 10.1074/jbc.M410000200. [DOI] [PubMed] [Google Scholar]
- 21.Shaikh SR, Rockett BD, Salameh M, Carraway K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J Nutr. 2009;139:1632–9. doi: 10.3945/jn.109.108720. [DOI] [PubMed] [Google Scholar]
- 22.Rockett BD, Franklin A, Harris M, Teague H, Rockett A, Shaikh SR. Membrane raft organization is more sensitive to disruption by (n-3) PUFA than nonraft organization in EL4 and B cells. J Nutr. 2011;141:1041–8. doi: 10.3945/jn.111.138750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyeregger R, Zeyda M, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids interfere with formation of the immunological synapse. J Leukoc Biol. 2005;77:680–8. doi: 10.1189/jlb.1104687. [DOI] [PubMed] [Google Scholar]
- 24.Zech T, Ejsing CS, Gaus K, de WetB, Shevchenko A, Simons K, Harder T. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 2009;28:466–76. doi: 10.1038/emboj.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim W, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids–physiological relevance of dose. Prostaglandins Leukot Essent Fatty Acids. 2010;6:155–8. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bays H. Rationale for prescription omega-3-acid ethyl ester therapy for hypertriglyceridemia: a primer for clinicians. Drugs Today (Barc) 2008;44:205–46. doi: 10.1358/dot.2008.44.3.1166387. [DOI] [PubMed] [Google Scholar]
- 27.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(Suppl 2):S171–84. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Arora M, Yarlagadda M, Oriss TB, Krishnamoorthy N, Ray A, Ray P. Distinct responses of lung and spleen dendritic cells to the TLR9 agonist CpG oligodeoxynucleotide. J Immunol. 2006;177:2373–83. doi: 10.4049/jimmunol.177.4.2373. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med. 2011;208:1917–29. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan JK, Periasamy P, O'Neill HC. Delineation of precursors in murine spleen that develop in contact with splenic endothelium to give novel dendritic-like cells. Blood. 2010;115:3678–85. doi: 10.1182/blood-2009-06-227108. [DOI] [PubMed] [Google Scholar]
- 31.Loike JD, Silverstein SC. A fluorescence quenching technique using trypan blue to differentiate between attached and ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J Immunol Methods. 1983;3:373–9. doi: 10.1016/0022-1759(83)90097-2. [DOI] [PubMed] [Google Scholar]
- 32.Owen DM, Rentero C, Magenau A, Abu-Siniyeh A, Gaus K. Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc. 2012;7:24–35. doi: 10.1038/nprot.2011.419. [DOI] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 34.Malhotra V, Hogg N, Sim RB. Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3) Eur J Immunol. 1986;16:1117–23. doi: 10.1002/eji.1830160915. [DOI] [PubMed] [Google Scholar]
- 35.Keizer GD, Te Velde AA, Schwarting R, Figdor CG, De Vries JE. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987;17:1317–22. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Hao Q, Li QR, Yan XW, Ye S, Li YS, Li N, Li JS. Omega-3 polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition. 2007;23:474–82. doi: 10.1016/j.nut.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Nakajima K, Yamashita T, Kita T, et al. Orally administered eicosapentaenoic acid induces rapid regression of atherosclerosis via modulating the phenotype of dendritic cells in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1963–72. doi: 10.1161/ATVBAHA.111.229443. [DOI] [PubMed] [Google Scholar]
- 38.Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, Diaz-Delfin J, de Madariaga MA, Domingo JC. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPARγ:RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008;84:1172–82. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]
- 39.Schwerbrock NM, Karlsson EA, Shi Q, Sheridan PA, Beck MA. Fish oil-fed mice have impaired resistance to influenza infection. J Nutr. 2009;139:1588–94. doi: 10.3945/jn.109.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myones BL, Dalzell JG, Hogg N, Ross GD. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988;82:640–51. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ihanus E, Uotila LM, Toivanen A, Varis M, Gahmberg CG. Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: characterization of the binding sites on ICAM-4. Blood. 2007;109:802–10. doi: 10.1182/blood-2006-04-014878. [DOI] [PubMed] [Google Scholar]
- 42.Dilioglou S, Cruse JM, Lewis RE. Function of CD80 and CD86 on monocyte- and stem cell-derived dendritic cells. Exp Mol Pathol. 2003;75:217–27. doi: 10.1016/s0014-4800(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 43.Sanderson P, MacPherson GG, Jenkins CH, Calder PC. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J Leukoc Biol. 1997;62:771–7. doi: 10.1002/jlb.62.6.771. [DOI] [PubMed] [Google Scholar]
- 44.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am J Clin Nutr. 2004;79:674–81. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 45.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 46.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]


