Abstract
n-Butyrate deriving from bacterial fermentation in the mammalian intestine is a key determinant in gastrointestinal homeostasis. We examined the effects of this short-chain fatty acid and Toll-like receptor 2 (TLR) and TLR4 engagement on inflammatory/immunity-associated genes, cyclo-oxygenases (COXs), prostaglandins (PGs) and leukotrienes (LTs) in human monocytes. Before RNA isolation, freshly isolated human monocytes were co-incubated for different time-points with 1 mm n-butyrate alone or in combination with bacterial stimuli. Based on a knowledge-driven approach, a signature of 180 immunity/inflammation-associated genes was picked and real-time PCR analysis was performed. Pathway analysis was carried out using a web-based database analysing program. Based on these gene expression studies the findings were evaluated at the protein/mediator level by Western blot analysis, FACS and ELISA. Following co-incubation with n-butyrate and lipopolysaccharide, key enzymes of the eicosanoid pathway, like PTGS2 (COX-2), TXS, ALOX5, LTA4H and LTC4S, were significantly up-regulated compared with stimulation with lipopolysaccharide alone. Furthermore, release of the lipid mediators PGE2, 15d-PGJ2, LTB4 and thromboxane B2 was increased by n-butyrate. Regarding signalling, n-butyrate had no additional effect on mitogen-activated protein kinase and interfered differently with early and late phases of nuclear factor-κB signalling. Our results suggest that among many other mediators of eicosanoid signalling n-butyrate massively induces PGE2 production by increasing the expression of PTGS2 (COX-2) in monocytes following TLR4 and TLR2 activation and induces secretion of LTB4 and thromboxane B2. This underscores the role of n-butyrate as a crucial mediator of gut-specific immunity.
Keywords: eicosanoids, gene regulation, monocytes, n-butyrate
Introduction
Despite continuous exposure to antigens, gastrointestinal immunity normally guarantees mucosal welfare, differentiating between potential pathogens and the commensal flora. In case of disturbance, intestinal homeostasis becomes dysbalanced and, for example, inflammatory bowel disease can ensue. The extensive and dynamic interactions between the symbionts and the immune system are key to colonic homeostasis and health, and require tight regulation of pro-inflammatory and anti-inflammatory immune reactions. Several types of immune cells, as well as the inimitable specific environment are involved in the establishment of this particular system;1 however, little is known about specific factors that guide the establishment of this unique local environment.
Short-chain fatty acids (SCFAs), like acetate, propionate or n-butyrate, are organic acids produced in the gut by the resident colonic microflora through breakdown of carbohydrates.1,2 The production of SCFAs by bacterial fermentation also allows the supply of energy from dietary fibre that is not digested in the small intestine. It has been estimated that SCFAs might contribute up to 15% of the total caloric requirements of the human body. Furthermore, SCFAs are pivotal for maintaining mucosal homeostasis in the gastrointestinal tract.3–6 n-Butyrate exerts multiple biological effects on a variety of cell types leading to immune modulation, cell cycle inhibition, induction of programmed cell death and cellular differentiation. It potently regulates inflammatory reactions by modulating cytokine production, kinase activity and transcription factors in various immune cell populations.7,8 Hence, it has been shown that n-butyrate differentially affects pro-inflammatory and anti-inflammatory cytokine production.8 Furthermore, n-butyrate prevents lipopolysaccharide (LPS) -induced maturation of dendritic cells, resulting in a reduced capability to stimulate T cells.9 Many of the effects of n-butyrate are attributed to inhibition of histone deacetylation and of nuclear factor-κB (NF-κB) transactivation; however, the complete spectrum of the molecular mode of actions responsible for the immunomodulatory effects of this SCFA is still not fully elucidated.
Originally recognized for their potential to govern vascular homeostasis and platelet aggregation, eicosanoids like prostaglandins (PGs) and leukotrienes (LTs) have also been implicated in several immunopathological processes, like inflammation, allergy and autoimmune diseases, as well as in cancer.10,11 Therefore, much interest has focused on the effects of these lipid mediators with antigen presenting cells, B cells and T cells.10,12,13 Despite their unquestionable impact on functions of myeloid and lymphoid cells of the innate and adaptive immune system, little is known about the regulation of these important mediators by particular local conditions in specific organ systems.
In the present study we aimed to get further insight into the regulation of eicosanoid metabolism by n-butyrate in human monocytes. Based on insights from a multigene signature approach evaluating a broad range of inflammation-related genes we focused here on the modulation of the expression of eicosanoid pathway-related genes after microbial activation and concomitant interference with n-butyrate. We found that in bacterially activated human monocytes activated by Toll-like receptor 2 (TLR2) and TLR4 ligation n-butyrate potentiated the expression of cyclo-oxygenase 2 (COX-2) along with increased PGE2 expression. The implications of these findings are discussed.
Materials and methods
Media and reagents
RPMI-1640, supplemented with 2 mm l-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin and 10% fetal calf serum were purchased from PAA (Pasching Austria). The sodium salt of n-butyric acid, TLR4 ligand LPS from Escherichia coli 0111:B4 and TLR2 ligand Staphylococcus aureus Cowan strain A cells were purchased from Sigma (Deisenhofen, Germany). The dose of LPS used in our assays was 100 ng/ml and the n-butyrate dose was 1 mm if not indicated differently.
Isolation of peripheral blood mononuclear cells
Human peripheral blood mononuclear cells were isolated from buffy coats (provided by the Austrian Red Cross) by density gradient centrifugation with Lymphoprep (Axis-Shield PoC AS, Oslo, Norway). Subsequently, monocytes were isolated from peripheral blood mononuclear cells by magnetic cell sorting using anti-CD14-conjugated magnetic beads purchased from Miltenyi Biotec (Bergisch-Gladbach, Germany). The purity of the monocytes was verified via FACS analysis on a FACSCalibur. Purity of isolated monocytes in all experiments was > 95% (data not shown).
Multigene signature approach for real-time PCR-based gene expression profiling
We here used a validated multigene signature approach to investigate transcriptional programmes triggered by n-butyrate and LPS alone or in combination. Based on the knowledge-driven approach of innate immune cell biology and inflammatory process data mining, a signature of immunity/inflammation-associated genes was assembled. TaqMan® array covering immunity/inflammation-related genes (pre-designed; Applied Biosystems, La Jolla, CA) were used as part of the self-designed 180-gene signature.
This signature contained targets involved in immune response and inflammation, and included many upstream signalling molecules (kinases and phosphatases in hierarchical levels), transcription factors, and the downstream chemokines and cytokines. PTGS2 (also known as COX-2), a key enzyme in the biosynthesis of prostanoids, and other molecules central to eicosanoid signalling were also included on the array. Additionally, the housekeeping genes and internal standard (18S) were used as reference genes to exclude potential inter-sample and inter-plate variations.
Real-time PCR analysis, data visualization and pathway analysis
Total RNA from pre-treated monocytes was isolated using the RNA Miniprep Kit from Stratagene (La Jolla, CA), according to the recommendations of the manufacturer. One microgram of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) to generate cDNA. To identify the housekeeping genes that maintain constant expression levels in our experimental settings, the expression stability of 32 housekeeping genes was pre-evaluated using human TaqMan Gene Expression Endogenous Control Plate (Applied Biosystems). For both TaqMan Gene Expression Endogenous Control and Multigene TaqMan arrays the real-time PCR were performed in the format of 96-well plates on ABI PRISM 7900HT Fast Real-Time PCR System (Applied Biosystems). The cDNA was amplified with TaqMan Universal PCR Master Mix (Applied Biosystems) for 40 cycles using universal cycling conditions (95° for 10 min followed by 40 cycles at 95° for 15 seconds and 60° for 1 min). For profiling of individual control genes such as tumour necrosis factor-α (TNF-α) and interleukin-12p40 (IL-12p40), the primers were designed using primer express 2.x software (Applied Biosystems). Sequences of primers to detect TNF-α were described previously,14the sequences of primers for IL12p40 were forward: CTTCTTCATCAGGGACATCAT CAA, reversed: GGGAGAAGTAGGAATGTGGAGTACTC,probe: FAMCAGGTGGAGGTCAGCTGGGAGTACCC-Tamra. For relative quantification, data were analysed by the ΔΔCT method using SDS 2·3. (Applied Biosystems) and by Data Assist v2·0. Expression levels of target genes were normalized to the average of housekeeping genes.
Network analysis
Ingenuity Pathway Analysis (ipa) software (http://www.ingenuity.com) is a proprietary web-based database that provides information on gene and protein interactions based on the published literature. In this study, the data-driven, n-butyrate-affected eicosanoid-associated gene network was delineated using the ipa software; core analysis was used to identify the most significantly affected biological processes.
Flow cytometry/intracellular staining
For intracellular determination of COX-1 and COX-2 by flow cytometry, stimulated monocytes were fixed with 2% formaldehyde, permeabilized with 0·1% saponin, and stained with anti-COX-1-FITC/anti-COX-2-phycoerythrin (BD, San Jose, CA). For analysis of mitogen-activated protein kinase (MAPK) activation cells were incubated after fixation and permeabilization with antibodies to the phosphorylated forms of the kinases: anti-p-p38 MAPK (pT180/pY182) (BD Biosciences, Franklin Lakes, NJ), anti-p-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), anti-p-SAPK/JNK (Thr183/Tyr185), (both Cell Signaling Technology, Boston, MA). The cells were analysed on a FACSCalibur (BD Biosciences).
Analysis of NF-κB signalling
A total amount of 5 × 106 monocytes (Western blot) and 2 × 106 monocytes (DNA binding) per tube, were pretreated for 1 hr with or without n-butyrate (1 mm) and then stimulated for 5, 10 and 15 min (Western blot) or 60 min (DNA binding) with LPS (30 and 100 ng/ml).
Extract preparation and Western blotting were performed as described previously.15 Antibodies used for the detection of particular signalling molecules were specific for IκB-α (FL), p-IκB-α, NF-κB p-p50 (Ser 337) (all Santa Cruz Biotechnology, Dallas, TX), NF-κB p-p65 (Ser 536), NF-κB p-p105 (Ser 933), pan-actin (all Cell Signaling Technology).
The separation of cytosol and nucleus was executed using a homemade lysis puffer (10 mm HEPES, 10 mm NaCl, 3 mm MgCl2, 1 mm EGTA, 0,05% Nonidet P-40). To protect the nuclei, a 10% sucrose solution was immediately underlayed by the lysis puffer. After centrifugation the cytosolic fraction was taken off and the nuclei were broken with the Complete Nuclear Extraction Puffer from Cayman Chemicals (Ann Arbor, MI). The binding activities of NF-κB p50 and NF-κB p65 were measured with the Transcription Factor Kits for NF-κB p50 and p65 from Pierce Chemicals (Rockford, IL) following the instruction manual. Measurements were made on a luminometer (Labsystem, Helsinki, Finland).
Determination of prostaglandins and leukotrienes by enzyme immunoassay
Enzyme immunoassay kits were used for the quantification of prostaglandins (PGE2, 15-d-PGJ2; Assay Designs, Enzo Life Sciences, Lörrach, Germany) as well as LTB4 and thromboxane B2 (Cayman Chemicals). Tests were performed according to the manufacturers’ recommendations.
Statistics
Statistical analyses were performed using excel and systat12 programs. For Student's t-tests, two-way analysis of variance, and Mann–Whitney U-tests P-values ≤ 0·05 were considered significant.
Results
Alterations in gene expression in response to n-butyrate using inflammation/immunity-related multigene signature
For a deeper insight into the impact of n-butyrate in inflammation/immunity-related reactions we used a multigene signature approach to identify novel targets of this SCFA. The response of human monocytes from peripheral blood to the exposure of n-butyrate alone or in combination with LPS was investigated in vitro by real-time PCR analysis using a pre-designed 180-gene signature (see Supplementary material, Table S1). As specified in the Materials and methods, the major focus was given to inflammation/immunity-related genes. Upon pre-testing of a set of housekeeping genes to identify the best candidate, endogenous controls for normalization, three genes, namely TATA box binding protein (TBP), ubiquitin C (UBC) and ribosomal protein S17 (RPS17), were found to be most stable upon LPS ± n-butyrate treatment and were subsequently used for normalization. Gene expression analysis was performed from cells of two normal donors (donor A and donor B). Our data demonstrated that the reaction of monocytes to LPS ± n-butyrate did not vary substantially between the two individuals, as reflected by the correlation in the results obtained for donors A and B across all genes (conditions: unstimulated r = 0·9838; n-butyrate alone 0·9854, LPS alone r = 0·9568; LPS + n-butyrate r = 0·9518) (see Supplementary material, Fig. S1). To formally prove that n-butyrate exerted anti-inflammatory efficacy in the present experimental setting8,9 we performed expression profiling of IL-12p40 and TNF-α mRNAs (Fig 1a,b). As expected, LPS triggered up-regulation of IL-12p40 and TNF-α, which was strongly inhibited by n-butyrate. Additionally, we confirmed these results on the protein level (data not shown).
Figure 1.
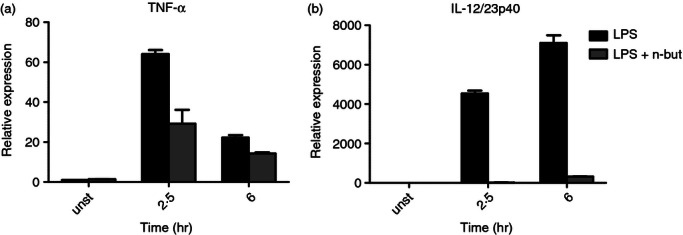
Time kinetics of tumour necrosis factor-α (TNF-α) and interleukin-12 p40 (IL-12p40) expression in response to lipopolysaccharide (LPS) ± n-butyrate. Freshly isolated monocytes were stimulated with LPS (100 ng/ml) ± n-butyrate (1 mm) for 2·5 and 6 hr. Expression profiles were analysed using the ΔΔCT method for relative quantification. Expression levels of TNF-α and IL-12p40 mRNAs were normalized to the average of housekeeping genes and shown relative to unstimulated (unst) cells in the absence of n-butyrate. Results are depicted as mean ± SD values found in monocytes from the donor shown in Fig. 2. The same experiments were performed for another independent donor and showed similar results (data not shown).
Gene expression was analysed at two different time-points (2·5 and 6 hr) after treatment with LPS (100 ng/ml) alone or in combination with n-butyrate (1 mm). As gene regulation was qualitatively similar after 2·5 and 6 hr and differed only with regard to the extent of expression, subsequent results are shown only for the longer stimulation period. Treatment with LPS ± n-butyrate using the indicated concentrations had no influence on cell viability (data not shown).
According to our results, 88% of genes were found to be expressed in monocytes at detectable levels. Compared with untreated cells, 37/27% of genes (donor A/donor B, respectively) were modulated by n-butyrate alone on the mRNA level with at least twofold change in their expression, 27/17% of which were up-regulated and 10/10% were down-regulated upon n-butyrate treatment. Existence of n-butyrate-unresponsive genes, in turn, argues for specific interference of n-butyrate with particular signalling pathway(s).
The top 10 up-regulated genes were PLCD1, ADRB1, PTGS2/COX-2, PDE4B, IRF8, PARD6A, CREB3L4, PIK3R2, GNA11 and MYL9 (up-regulated in the range of 6·0-fold to 19·3-fold) and the top 10 down-regulated genes were PLA2G7, FN1, FAS, IL10, PPARG, PTGER3, ACE, CTLA4, ANXA3 and ACACA (down-regulated in the range of 0·02-fold to 0·32-fold). Furthermore, n-butyrate, when combined with LPS, was able to modulate the LPS-triggered response in monocytes. Hence, after 6 hr of treatment, expression levels of 31/29% of genes (donor A/donor B) were enhanced and of 15/17% were down-regulated. For these treatment conditions, PIK3R2, CD86, LTA4H, ADRB1, LTB4R2, PIK3CD, IRF8, LIF, PLCD1, PTGS2 and ANXA1 were among the most up-regulated (in the range of 7·6-fold to 28·2-fold) and PLA2G7, ACE, FASLG, ANXA3, BCL2L1, HPGD, PTGER3, PPARG and MAP2K6 were among the most down-regulated (in the range of 0·02-fold to 0·21-fold). Hence, enhanced expression of some genes (e.g. PLCD1) was modulated by the action of n-butyrate alone, whereas for other genes (e.g CD86, LTA4H, PTGS2) an additive effect between LPS and n-butyrate was detected; PLA2G7 was found to be the most deregulated.
As each gene might function as an integration point for multiple intracellular signals leading in turn to a wide variety of cellular processes, we used ipa software to delineate the n-butyrate-affected pathways. Here, data analysis revealed prostanoid and leukotriene biosynthetic pathways being among the most affected in human monocytes. In addition to the eicosanoid pathway, other canonical pathways namely ‘roles of macrophages’, ‘fibroblasts and endothelial cells in rheumatoid arthritis’, ‘CD40 signalling’, ‘RANK signalling in osteoclasts’ were modulated in the presence of n-butyrate.
Alterations in eicosanoid pathway-associated genes
The most affected up-regulated genes of the eicosanoid pathway after 6 hr of incubation with n-butyrate alone were found to be ALOX5AP, LTB4R, LTB4R2, PLCD1, PTGS2 and TBXA2R. Following 6 hr co-incubation of cells with LPS alone, the major induced genes were ALOX12, LTB4R2, PLA2G4C, PLA2G7, PTGER2, PTGER3, PTGIR, PTGS2, TBXA2R (Fig. 2a,b).
Figure 2.
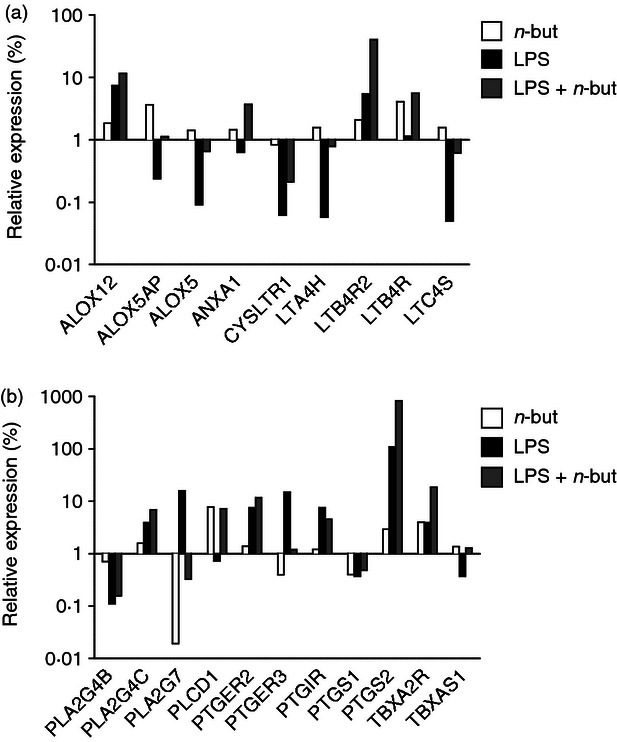
n-Butyrate modulates expression of genes from the canonical eicosanoid pathway. Freshly isolated monocytes were incubated with lipopolysaccharide (LPS; 100 ng/ml) in the presence or absence of n-butyrate (1 mm) for 6 hr. Expression profiles of 19 genes of the eicosanoid pathway were analysed using the ΔΔCT method for relative quantification. Expression levels were normalized to the average of housekeeping genes and shown relative to unstimulated cells in the absence of n-butyrate. Colour code: light grey, n-butyrate versus control; black, LPS versus control, dark grey LPS + n-butyrate versus control. Data are representative of two independent experiments. For better overview, analysed genes were divided into (a) leukotriene-related and (b) prostaglandin-associated genes.
In comparison, ALOX12, LTB4R2, PLA2G4C, PTGER2, TBXA2R and massively PTGS2 were found to be further up-regulated after 6 hr co-incubation with LPS and n-butyrate (Fig. 2a,b).
Up-regulation of COX-2 by n-butyrate and bacterial activation
To further substantiate alterations in gene expression we first assessed the influence of n-butyrate on the expression of the key enzyme of eicosanoid metabolism COX-2 (PTGS2) at the protein level. Monocytes were incubated with LPS ± n-butyrate for different time periods and expression of COX-1 and COX-2 was assessed by intracellular staining as specified in the Materials and methods. COX-1 was constitutively expressed and not affected by n-butyrate (data not shown). In contrast, expression of COX-2 was up-regulated by LPS. Furthermore, we observed an even more pronounced expression of COX-2 after co-incubation with n-butyrate after between 4 and 8 hr of treatment with the maximum detected after 6 hr (Fig. 3). To find out whether the potent enhancement of COX-2 expression was specific for the TLR4 pathway we investigated the effect of n-butyrate also for TLR2 ligation by S. aureus cells. In this experimental setting we also found a significant up-regulation of COX-2 as verified by Western blot (see Supplementary material, Fig. S2).
Figure 3.
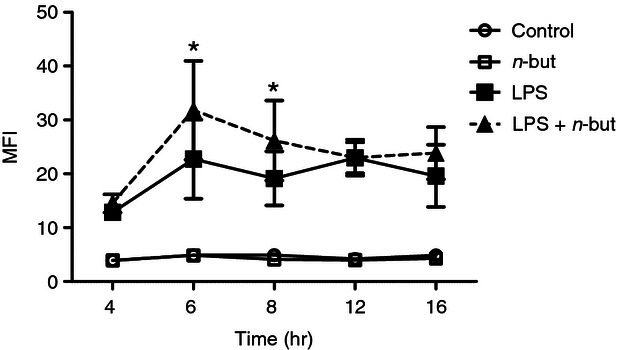
n-Butyrate up-regulates cyclo-oxygenase 2 (COX-2) expression in monocytes. Monocytes were incubated with 1 mm n-butyrate or medium and stimulated with 100 ng/ml lipopolysaccharide (LPS) for the time-points indicated. COX-2 expression was assessed by intracellular staining. Data are shown as summary of three independent experiments. Results are expressed as mean ± SEM, * P ≤ 0·05 (paired Student's t-test)
Alteration of prostaglandin secretion upon co-incubation of n-butyrate and LPS
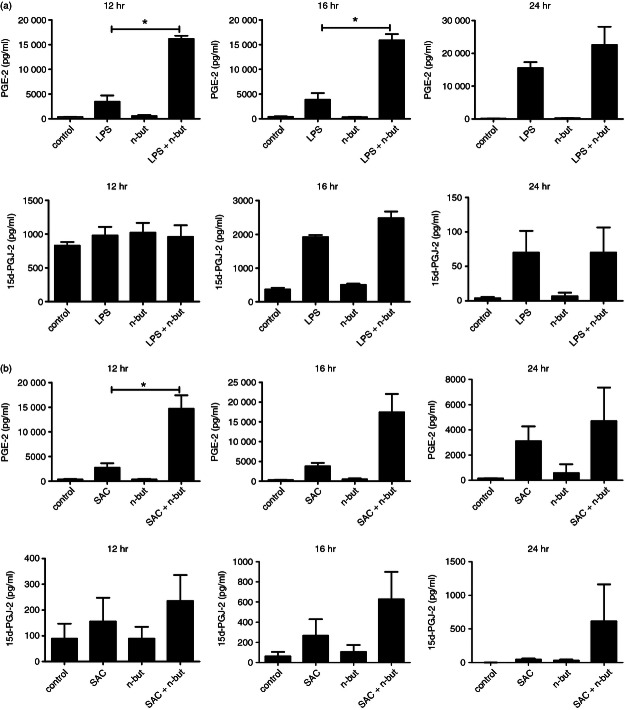
Based on these findings we next elucidated whether enhanced COX-2 expression is accompanied by alterations in the production of mediators related to the eicosanoid pathway downstream of COX-2. To answer this, release of PGE2 and 15d-PGJ2, two prostaglandins with well-known immunomodulatory effects, was analysed after n-butyrate co-treatment with LPS or with S. aureus cells to trigger TLR4 or TLR2, respectively. Release of PGE2 and 15d-PGJ2 was induced after LPS as well as S. aureus cell stimulation (Fig. 4a,b) and was substantially up-regulated after co-incubation with n-butyrate in both cases. Akin to monocytes we found an increased release of prostaglandins following TLR2 and TLR4 activation and co-incubation with n-butyrate into the supernatants of monocyte-derived dendritic cells (data not shown).
Figure 4.
Prostaglandin release by monocytes after bacterial stimulation in response to n-butyrate. Monocytes were stimulated with (a) lipopolysaccharide (LPS; 100 ng/ml) or (b) Staphylococcus aureus cells (SAC; 7·5 μg/ml) and co-incubated with n-butyrate (1 mm) for varying times, and levels of prostaglandins (PGE2 and 15d-PGJ2) were determined in culture supernatants by ELISA. Results are expressed as mean ± SEM of five independent experiments each performed in duplicate. * P ≤ 0·05 (paired Student's t-test)
Effects of n-butyrate and LPS stimulation on leukotriene secretion
Profound up-regulation of genes encoding the key leukotriene synthesizing enzymes was also recorded (Fig. 2a,b), so we next evaluated the impact of n-butyrate on the release of leukotrienes. Here we found that LTB4 and thromboxane B2, both key members of the lipoxygenase pathway, were significantly up-regulated following n-butyrate treatment and LPS activation when compared with LPS stimulation alone (Fig. 5a,b).
Figure 5.
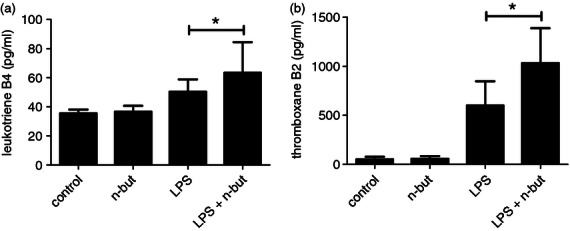
Release of leukotriene B4 and thromboxane B2 in response to n-butyrate after stimulation with lipopolysaccharide (LPS). Monocytes were stimulated with LPS (100 ng/ml) and co-incubated with n-butyrate (1 mm) for 12 hr, and levels of (a) leukotriene B4 and (b) thromboxane B2 were determined in culture supernatant by enzyme immunoassay. Results are expressed as mean ± SEM of nine independent experiments each performed in duplicate. * P ≤ 0·05 (paired student's t test)
n-Butyrate has no additional effect on MAPK activation
Recent studies have indicated that COX-2 expression depended on MAPK signalling including extracellular signal-regulated kinase 1/2 and p3816–18 and therefore we next investigated the impact of n-butyrate on the activation of these crucial signalling pathways. We, therefore, performed a time kinetics study for MAPK activation after bacterial challenge of monocytes in the presence or absence of n-butyrate. Phosphorylation of extracellular signal-regulated kinase 1/2 and p38 could be demonstrated after 30 min stimulation with LPS whereas Jun N-terminal kinase was not affected. Addition of n-butyrate to LPS did not lead to a further up-regulation of any MAPK activation pathways (Fig. 6a, same results after 5 and 15 min). Addition of the specific MAPK/ERK kinase (MEK)1/2 inhibitor UO126 as well as p38 inhibitors SB203580 and SK86002 blocked phosphorylation of the respective MAPK after stimulation with LPS and after stimulation with LPS plus n-butyrate (data not shown). Similar results were obtained, when MAPK activation was assessed by intracellular staining and Western blotting (data not shown).
Figure 6.
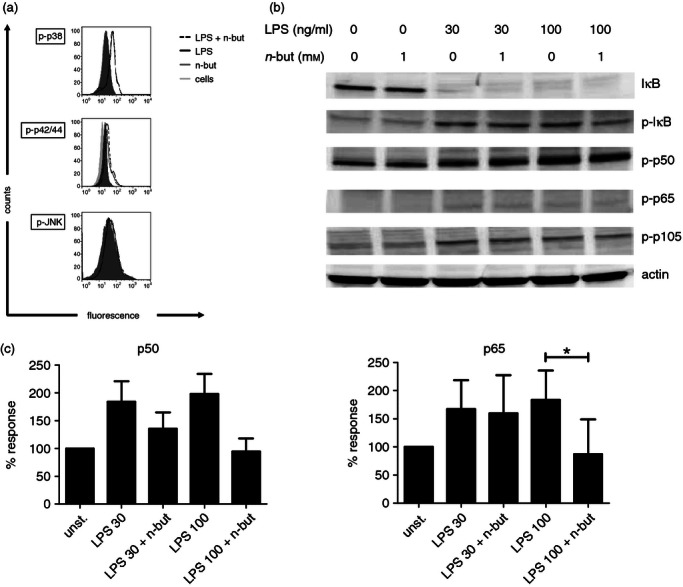
Impact of n-butyrate on mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signalling. (a) Freshly isolated monocytes were stimulated with lipopolysaccharide (LPS; 100 ng/ml) and incubated in the presence or absence of n-butyrate (1 mm). Intracellular staining was performed and the phosphorylated (active) form of the respective MAPK was investigated at 5, 15 (data not shown) and 30 min after LPS stimulation. (b) Monocytes (5 × 106) were pre-treated for 1 hr with or without n-butyrate (1 mm) and then stimulated for 5, 10 (data not shown) and 15 min with LPS (30 and 100 ng/ml). Western blot analysis was performed for the indicated NF-κB signalling molecules. Results are representative of four independent experiments. (c) Monocytes (2 × 106) per probe were pre-treated for 1 hr with or without n-butyrate (1 mm) and then stimulated for 60 min with LPS (30 and 100 ng/ml). DNA binding was assessed for p50 and p65. Results are presented as % binding of nuclear extracts from unstimulated (unst.) monocytes and show mean values ± SEM of four independent experiments. * P ≤ 0·05 (paired Student's t-test)
n-Butyrate has differential effects on NF-κB signalling
Since COX-2 expression also largely depends on NF-κB signalling19–21 we elucidated the impact of n-butyrate on several components of this pathway after LPS activation. We, therefore performed Western blot analyses for NF-κB activation after bacterial challenge of monocytes in the presence or absence of n-butyrate. Results of these experiments clearly showed that phosphorylation and degradation of IκB, as well as phosphorylation of p50 and p65, after stimulation with different concentrations of LPS was unaffected by n-butyrate (Fig. 6b). We next assessed DNA binding activity of NF-κB p50 and NF-κB p65 after stimulation with LPS in the presence or absence of n-butyrate and found that n-butyrate treatment had an inhibitory effect on DNA binding in monocytes (Fig. 6c). Interestingly, phosphorylation of p105, a marker for alternative NF-κB pathway activation, was also unaffected by n-butyrate (Fig. 6b). These findings indicate that n-butyrate appears to differently interfere with early and late phases of NF-κB signalling and might even have the converse effect on different NF-κB signalling pathways.
Discussion
Many recent studies highlight the immunomodulatory potential of the SCFA n-butyrate in various immune cell populations like monocytes, dendritic cells, T cells and mast cells as well as epithelial cells.5,8–10,12,13,22–25 As its presence is largely restricted to the gastrointestinal tract and immunological features of this region have striking similarities to the effects brought about by this physiologically occurring substance there is great interest in its molecular mode of action, which, so far has been poorly understood.
In this study, we show that the bacterial metabolite n-butyrate substantially influences the monocytic gene regulation of several members of the eicosanoid pathway and potentiates the release of prominent prostaglandins and leukotrienes. Following co-incubation with n-butyrate and LPS key enzymes of the eicosanoid pathway like PTGS2 (COX-2), ALOX5, LTA4H and LTC4S were significantly up-regulated compared with stimulation with LPS alone. Similarly, mRNA levels coding for leukotriene receptors LTB4R2 and CYSLTR and functional prostaglandin receptors TBXAR2 and PTGER2 were increased by n-butyrate. In accordance with the up-regulation in enzyme expression, release of the lipid mediators PGE2, 15d-PGJ2, LTB4 and thromboxane B2 was increased by n-butyrate. Eicosanoids exert their effects via binding to their respective receptors, which are expressed on various immune and endothelial cells. All of these receptors belong to the group of G-coupled receptors and trigger increase or decrease in the rate of second messengers cAMP and Ca2+.26,27 These proximal signals activate downstream kinase cascades, which leads to alterations in cellular activities, ranging from changes in motility to transcriptional activation.12,28
Previous studies have resulted in highly divergent results depending on the experimental setup, so our major concern was to test the impact of n-butyrate in a model using primary human monocytes stimulated with TLR2 and TLR4 agonists, which resembles the stimulatory conditions in the gastrointestinal tract. Previously it has been shown on the one hand that this bacterial fermentation product inhibits COX-2 activation in HT-29 and other colon cancer cell lines.29,30 On the other hand, it has been found that n-butyrate potentiates LPS-induced COX-2-induced gene expression at the transcriptional level in murine macrophages.31 Furthermore Iida et al. have shown that butyric acid increases expression of COX-1 and COX-2 in rat osteoblasts and induces PGE2 production.32
Prostaglandins exert a broad range of functions in pain and inflammation, and are effective in modulating the induction of adaptive immune responses. Previous results reveal that these mediators and their receptors exert pro-inflammatory and anti-inflammatory activities, having both immune activating and inhibitory properties.33 Interestingly, Scher et al. indicated that PGE2, the classic representative of a pro-inflammatory lipid mediator, also has anti-inflammatory properties similar to the classical anti-inflammatory prostaglandin 15d-PGJ2.34 The impact of PGE2 on dendritic cell biology seems to vary, depending on the stage of maturation, and ranges from suppression of differentiation when present during early stages of development35 to promotion of maturation in already developed dendritic cells.36–38 Moreover, it has recently been shown that PGE2 and COX-2 are able to redirect the differentiation of human dendritic cells towards stable myeloid-derived suppressor cells.39 Prostaglandin E2-induced inhibition of dendritic cell differentiation and function seems to be also a key mechanism implicated in cancer-associated immunosuppressive mechanisms.40 Other lines of evidence show that eicosanoids, in particular PGE2, also regulate macrophage inflammatory function. In a zymosan-stimulated mouse macrophage model it has been demonstrated that PGE2 down-regulates TNF-α production and up-regulates anti-inflammatory IL-10 through prostaglandin receptor signalling.41 Recent data even indicate a role of PGE2 and SOCS1 as an intestinal immune tolerance mechanism distinct from IL-10 and regulatory T cells.42 It has been shown in mice by Nataraj et al. that ligation of EP2, the receptor for PGE2 encoded by the gene PTGER2 directly inhibits T-cell proliferation, thereby regulating the cellular immune response.43 Another study by Bryn et al. showed that COX-2-derived PGE2 suppresses the T-cell-mediated immune response by inducing Foxp3+ T regulatory cells.44 Further evidence for its inhibitory effect on T-cell activation comes from recent studies identifying PGE2 as a T-cell stop signal antagonist.45
Moreover, PGE2 appears also to regulate B-cell proliferation and associated malignancies involving tumour suppressor PTGER4.46 In autoimmune disease, it is suggested that PGE2 affects the release of autoantibodies via inhibiting T suppressor cells.12 Prostaglandin E2 acts in an inhibitory manner on immature and developing B cells47 but in contrast, it seems that PGE2 enhances the proliferation of mature B cells.48 Furthermore, PGE2 induces immature B-cell apoptosis, but does not induce cell death in mature B cells. The PGE2 regulates the activity of mature B cells by enhancing immunoglobulin-class switching and modulates the activation of B cells and stimulates the production of IgG1 and IgE in LPS-stimulated and IL-4-stimulated B cells by a cAMP-dependent mechanism, thereby inducing T helper type 2 responses.
The same complexity and multifunctionality as observed for prostaglandins was shown for leukotrienes.49 These mediators play prominent roles in the pathogenesis of various inflammatory diseases, mainly in asthma, irritable bowel disease and rheumatoid arthritis.50 Their impact on the cardiovascular and neuroendocrine system as well as on leucocyte activation (LTB4) and bronchoconstriction (LTC4 and LTD4) is well established.26,27,51,52 In various animal models it has been shown that leukotrienes can influence the peristaltic action of the intestine. Leukotrienes are key immunomodulators mediating the cross-talk between different cell types in inflammation and cancer. However, the roles of these eicosanoids in such processes and the mechanisms beyond seem to be diverse and complex. This diversity is a result of their variability in occurrence, composition, targets and G-protein-coupled signalling.11,28,53 Their specific action is considered tissue-specific and organ-specific and depends on the cell-type-specific expression of their receptors as well as their local production. The exact role of leukotrienes in the intestine, however, remains to be elucidated.49 In the gut, LTB4 is considered to function as a neutrophil chemoattractant and to enhance their adherence to the endothelium,54 while LTD4 has been reported to have a proliferative effect, and to increase mucus production and vascular permeability.55 Leukotrienes are synthesized in response to a large spectrum of various infectious agents and enhance the capacity of macrophages and other immune cells to ingest and kill microbes and to produce antimicrobial mediators. In animal models of infection, genetic or pharmacological interference with leukotriene synthesis or signalling massively impairs local microbial clearance.56 In summary, these data imply that certain levels of leukotrienes are indispensable to control microbial invaders and to maintain local immune reactivity not only in the lung but also in the gastrointestinal tract.
Similar to prostanoids, the SCFA n-butyrate brings about interference with immune cell activation at key stages of immune cell activation inhibiting dendritic cell maturation and consequent T-cell actions. Previous studies demonstrated that pre-treatment of human peripheral blood mononuclear cells or monocytes as well as monocyte-derived dendritic cells with this agent resulted in a dose- and time-dependent down-regulation of their capability to stimulate T-cell responses.8,9,22,56–61 Therefore, it is tempting to speculate that n-butyrate itself, or through induction of mediators like eicosanoids, may contribute to the generation of an anti-inflammatory immune responsiveness. As the presence of n-butyrate is largely restricted to the gastrointestinal tract and immunological features of this region have striking similarities to the effects brought about by this physiologically occurring substance, further elucidation of the underlying principles appears promising.
There are several potential transcriptional regulatory elements in the promotor region of the COX-2 gene including a peroxisome proliferator response element, two cAMP response elements, a sterol response element, two NF-κB sites, an SP1 site, a CAAT enhancer binding protein motif, two AP-2 sites, an E-box, and a Tata box.63 Previous studies have shown that cAMP response element-binding protein (CREB) and NF-κB are particularly important in LPS-induced COX-2 transcription indicating that p65/p50 heterodimer together with CREB is required for an early phase of rapid induction and the p50 homodimer together with CREB is crucial during later phases.63 Testing the impact of n-butyrate treatment on LPS triggering, we found that the early phase of NF-κB signalling including IκB phosphorylation, IκB degradation and phosphorylation of p65 and p50 was completely unaffected. The late phase of the classical NF-κB pathway, as indicated by p65/p50 DNA binding, however, was profoundly inhibited. These finding are in agreement with previous studies25,64–66 showing inhibition of NF-κB signalling by n-butyrate. Furthermore, we were able to demonstrate that phosporylation of p105, the precursor for the formation of p50 homodimer, was also sustained. We therefore show that the classical NF-κB pathway is inhibited by n-butyrate whereas the alternative way, using p105 activation, might still be active. As COX-2 expression crucially depends on p50 homodimer binding to distinct promotor sites,19–21 this pathway might also be responsible for up-regulation of COX-2 expression under the conditions used in the present study. Further investigations will have to elucidate the exact molecular mechanisms leading to this potential converse effect of n-butyrate on different NF-κB signalling pathways.
In conclusion, we have demonstrated that n-butyrate potently up-regulates expression of key enzymes and receptors of the eicosanoid pathway when activated via bacterial stimulation, leading to an increased release of PGE2, 15d-PGJ2, LTB4 and thromboxane B2. Through selective induction of several eicosanoid mediators and up-regulation of its receptors we speculate that such effects of SCFAs might contribute to the generation of the gut intrinsic milieu, thereby specifically regulating the local gastrointestinal immune response.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Names of investigated genes.
Figure S1. Correlation of gene expression for lipopolysaccharide (LPS) activated CD14 monocytes from donor A and B.
Figure S2. n-Butyrate up-regulates cyclo-oxygenase 2 (COX-2) expression in monocytes after both lipopolysaccharide (LPS) and Staphylococcus aureus cell (SAC) stimulation as demonstrated by Western blot. Results are representative of four independent experiments.
References
- 1.Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010;11:558–60. doi: 10.1038/ni0710-558. [DOI] [PubMed] [Google Scholar]
- 2.Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–9. [PubMed] [Google Scholar]
- 3.Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol Rev. 1990;70:567–90. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 4.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 5.Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care. 2004;7:563–7. doi: 10.1097/00075197-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–64. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 7.Crew TE, Elder DJ, Paraskeva C. A cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug enhances the growth inhibitory effect of butyrate in colorectal carcinoma cells expressing COX-2 protein: regulation of COX-2 by butyrate. Carcinogenesis. 2000;21:69–77. doi: 10.1093/carcin/21.1.69. [DOI] [PubMed] [Google Scholar]
- 8.Saemann MD, Böhmig GA, Österreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14:2380–2. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 9.Saemann MD, Parolini O, Bohmig GA, et al. Bacterial metabolite interference with maturation of human monocyte-derived dendritic cells. J Leukoc Biol. 2002;71:238–46. [PubMed] [Google Scholar]
- 10.Harizi H, Gualde N. The impact of eicosanoids on the crosstalk between innate and adaptive immunity: the key roles of dendritic cells. Tissue Antigens. 2005;65:507–14. doi: 10.1111/j.1399-0039.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 13.Lee IY, Cho W, Kim J, Park CS, Choe J. Human follicular dendritic cells interact with T cells via expression and regulation of cyclooxygenases and prostaglandin E and I synthases. J Immunol. 2008;180:1390–7. doi: 10.4049/jimmunol.180.3.1390. [DOI] [PubMed] [Google Scholar]
- 14.Mechtcheriakova D, Wlachos A, Sobanov J, et al. Sphingosine 1-phosphate phosphatase 2 is induced during inflammatory responses. Cell Signal. 2007;19:748–60. doi: 10.1016/j.cellsig.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Saemann MD, Weichhart T, Zeyda M, et al. Tamm–Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005;115:468–75. doi: 10.1172/JCI22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–46. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 17.Monick MM, Powers LS, Barrett CW, et al. Constitutive ERK MAPK activity regulates macrophage ATP production and mitochondrial integrity. J Immunol. 2008;180:7485–96. doi: 10.4049/jimmunol.180.11.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pouliot M, Gilbert C, Borgeat P, et al. Expression and activity of prostaglandin endoperoxide synthase-2 in agonist-activated human neutrophils. FASEB J. 1998;12:1109–23. doi: 10.1096/fasebj.12.12.1109. [DOI] [PubMed] [Google Scholar]
- 19.Crofford LJ, Tan B, McCarthy CJ, Hla T. Involvement of nuclear factor κB in the regulation of cyclooxygenase-2 expression by interleukin-1 in rheumatoid synoviocytes. Arthritis Rheum. 1997;40:226–36. doi: 10.1002/art.1780400207. [DOI] [PubMed] [Google Scholar]
- 20.Thomas B, Berenbaum F, Humbert L, Bian H, Bereziat G, Crofford L, Olivier JL. Critical role of C/EBPδ and C/EBPβ factors in the stimulation of the cyclooxygenase-2 gene transcription by interleukin-1β in articular chondrocytes. Eur J Biochem. 2000;267:6798–809. doi: 10.1046/j.1432-1033.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 21.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–66. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 22.Diakos C, Prieschl EE, Saemann M, Novotny V, Bohmig G, Csonga R, Baumruker T, Zlabinger GJ. Novel mode of interference with nuclear factor of activated T-cells regulation in T-cells by the bacterial metabolite n-butyrate. J Biol Chem. 2002;277:24243–51. doi: 10.1074/jbc.M200191200. [DOI] [PubMed] [Google Scholar]
- 23.Diakos C, Prieschl EE, Saemann MD, Bohmig GA, Csonga R, Sobanov Y, Baumruker T, Zlabinger GJ. n-Butyrate inhibits Jun NH2-terminal kinase activation and cytokine transcription in mast cells. Biochem Biophys Res Commun. 2006;349:863–8. doi: 10.1016/j.bbrc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- 24.Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. 2002;130:245–55. doi: 10.1046/j.0009-9104.2002.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schroder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NF κB signalling. Mol Immunol. 2007;44:3625–32. doi: 10.1016/j.molimm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Alanko J, Riutta A, Vapaatalo H. Effects of catecholamines on eicosanoid synthesis with special reference to prostanoid/leukotriene ratio. Free Radic Biol Med. 1992;13:677–88. doi: 10.1016/0891-5849(92)90041-e. [DOI] [PubMed] [Google Scholar]
- 27.Feuerstein G. Autonomic pharmacology of leukotrienes. J Auton Pharmacol. 1985;5:149–68. doi: 10.1111/j.1474-8673.1985.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 28.Harizi H, Corcuff JB, Gualde N. Arachidonic-acid-derived eicosanoids: roles in biology and immunopathology. Trends Mol Med. 2008;14:461–9. doi: 10.1016/j.molmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Daly K, Shirazi-Beechey SP. Microarray analysis of butyrate regulated genes in colonic epithelial cells. DNA Cell Biol. 2006;25:49–62. doi: 10.1089/dna.2006.25.49. [DOI] [PubMed] [Google Scholar]
- 30.Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463–71. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 31.Park GY, Joo M, Pedchenko T, Blackwell TS, Christman JW. Regulation of macrophage cyclooxygenase-2 gene expression by modifications of histone H3. Am J Physiol Lung Cell Mol Physiol. 2004;286:L956–62. doi: 10.1152/ajplung.00338.2003. [DOI] [PubMed] [Google Scholar]
- 32.Iida T, Kawato T, Tanaka H, et al. Sodium butyrate induces the production of cyclooxygenases and prostaglandin E in ROS 17/2.8 osteoblastic cells. Arch Oral Biol. 2011;56:678–86. doi: 10.1016/j.archoralbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Narumiya S. Prostanoids and inflammation: a new concept arising from receptor knockout mice. J Mol Med (Berl) 2009;87:1015–22. doi: 10.1007/s00109-009-0500-1. [DOI] [PubMed] [Google Scholar]
- 34.Scher JU. Pillinger MH. J Investig Med: The anti-inflammatory effects of prostaglandins; 2009. [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 36.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 37.Landi A, Babiuk LA, van DrunenLittel-vandenHurkS. Dendritic cells matured by a prostaglandin E2-containing cocktail can produce high levels of IL-12p70 and are more mature and Th1-biased than dendritic cells treated with TNF-α or LPS. Immunobiology. 2011;216:649–62. doi: 10.1016/j.imbio.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor α cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells towards stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Yamagata N, Yadav R, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Investig. 2003;111:727–35. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinomiya S, Naraba H, Ueno A, et al. Regulation of TNFα and interleukin-10 production by prostaglandins I2 and E2: studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochem Pharmacol. 2001;61:1153–60. doi: 10.1016/s0006-2952(01)00586-x. [DOI] [PubMed] [Google Scholar]
- 42.Chinen T, Komai K, Muto G, et al. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat Commun. 2011;2:190. doi: 10.1038/ncomms1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nataraj C, Thomas DW, Tilley SL, Nguyen MT, Mannon R, Koller BH, Coffman TM. Receptors for prostaglandin E2 that regulate cellular immune responses in the mouse. J Clin Investig. 2001;108:1229–35. doi: 10.1172/JCI13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryn T, Yaqub S, Mahic M, Henjum K, Aandahl EM, Tasken K. LPS-activated monocytes suppress T-cell immune responses and induce FOXP3+ T cells through a COX-2-PGE2-dependent mechanism. Int Immunol. 2008;20:235–45. doi: 10.1093/intimm/dxm134. [DOI] [PubMed] [Google Scholar]
- 45.Wiemer AJ, Hegde S, Gumperz JE, Huttenlocher A. A live imaging cell motility screen identifies prostaglandin E2 as a T cell stop signal antagonist. J Immunol. 2011;187:3663–70. doi: 10.4049/jimmunol.1100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murn J, Alibert O, Wu N, Tendil S, Gidrol X. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205:3091–103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garrone P, Galibert L, Rousset F, Fu SM, Banchereau J. Regulatory effects of prostaglandin E2 on the growth and differentiation of human B lymphocytes activated through their CD40 antigen. J Immunol. 1994;152:4282–90. [PubMed] [Google Scholar]
- 48.Brown DM, Warner GL, Ales-Martinez JE, Scott DW, Phipps RP. Prostaglandin E2 induces apoptosis in immature normal and malignant B lymphocytes. Clin Immunol Immunopathol. 1992;63:221–9. doi: 10.1016/0090-1229(92)90226-e. [DOI] [PubMed] [Google Scholar]
- 49.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007;357:1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 50.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–6. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 51.Reims A, Redfors S, Sjovall H, Strandvik B. Cysteinyl leukotrienes are secretagogues in atrophic coeliac and in normal duodenal mucosa of children. Scand J Gastroenterol. 2005;40:160–8. doi: 10.1080/00365520410009564. [DOI] [PubMed] [Google Scholar]
- 52.Shahbazian A, Heinemann A, Peskar BA, Holzer P. Differential peristaltic motor effects of prostanoid (DP, EP, IP, TP) and leukotriene receptor agonists in the guinea-pig isolated small intestine. Br J Pharmacol. 2002;137:1047–54. doi: 10.1038/sj.bjp.0704958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma JN, Mohammed LA. The role of leukotrienes in the pathophysiology of inflammatory disorders: is there a case for revisiting leukotrienes as therapeutic targets? Inflammopharmacology. 2006;14:10–6. doi: 10.1007/s10787-006-1496-6. [DOI] [PubMed] [Google Scholar]
- 55.Paruchuri S, Hallberg B, Juhas M, Larsson C, Sjolander A. Leukotriene D4 activates MAPK through a Ras-independent but PKCε-dependent pathway in intestinal epithelial cells. J Cell Sci. 2002;115:1883–93. doi: 10.1242/jcs.115.9.1883. [DOI] [PubMed] [Google Scholar]
- 56.Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;174:589–94. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- 57.Bohmig GA, Csmarits B, Cerwenka A, Alaei P, Kovarik J, Zlabinger GJ. Induction of alloantigen-specific hyporesponsiveness in vitro by the short-chain fatty acid N-butyrate. Transplantation. 1995;59:1500–3. doi: 10.1097/00007890-199505270-00029. [DOI] [PubMed] [Google Scholar]
- 58.Bohmig GA, Krieger PM, Saemann MD, Ullrich R, Karimi H, Wekerle T, Muhlbacher F, Zlabinger GJ. Stable prodrugs of n-butyric acid: suppression of T cell alloresponses in vitro and prolongation of heart allograft survival in a fully allogeneic rat strain combination. Transpl Immunol. 1999;7:221–7. doi: 10.1016/s0966-3274(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 59.Bohmig GA, Krieger PM, Saemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234–43. doi: 10.1046/j.1365-2567.1997.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bohmig GA, Wekerle T, Saemann MD, Kovarik J, Zlabinger GJ. Induction of alloantigen-specific hyporesponsiveness in vitro by n-butyrate: antagonistic effect of cyclosporin A. Transpl Int. 1996;9(Suppl 1):S318–22. doi: 10.1007/978-3-662-00818-8_79. [DOI] [PubMed] [Google Scholar]
- 61.Kovarik JJ, Tillinger W, Hofer J, Holzl MA, Heinzl H, Saemann MD, Zlabinger GJ. Impaired anti-inflammatory efficacy of n-butyrate in patients with IBD. Eur J Clin Invest. 2011;41:291–8. doi: 10.1111/j.1365-2362.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 62.Tanabe T, Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins Other Lipid Mediat. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 63.Caivano M, Gorgoni B, Cohen P, Poli V. The induction of cyclooxygenase-2 mRNA in macrophages is biphasic and requires both CCAAT enhancer-binding protein beta (C/EBP β) and C/EBP δ transcription factors. J Biol Chem. 2001;276:48693–701. doi: 10.1074/jbc.M108282200. [DOI] [PubMed] [Google Scholar]
- 64.Yin L, Laevsky G, Giardina C. Butyrate suppression of colonocyte NF-κB activation and cellular proteasome activity. J Biol Chem. 2001;276:44641–6. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]
- 65.Segain JP, Raingeard delaBletiereD, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFκB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, Kotani J. Butyrate and trichostatin A attenuate nuclear factor κB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28:321–8. doi: 10.1016/j.nutres.2008.02.012. (New York, NY) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.