Abstract
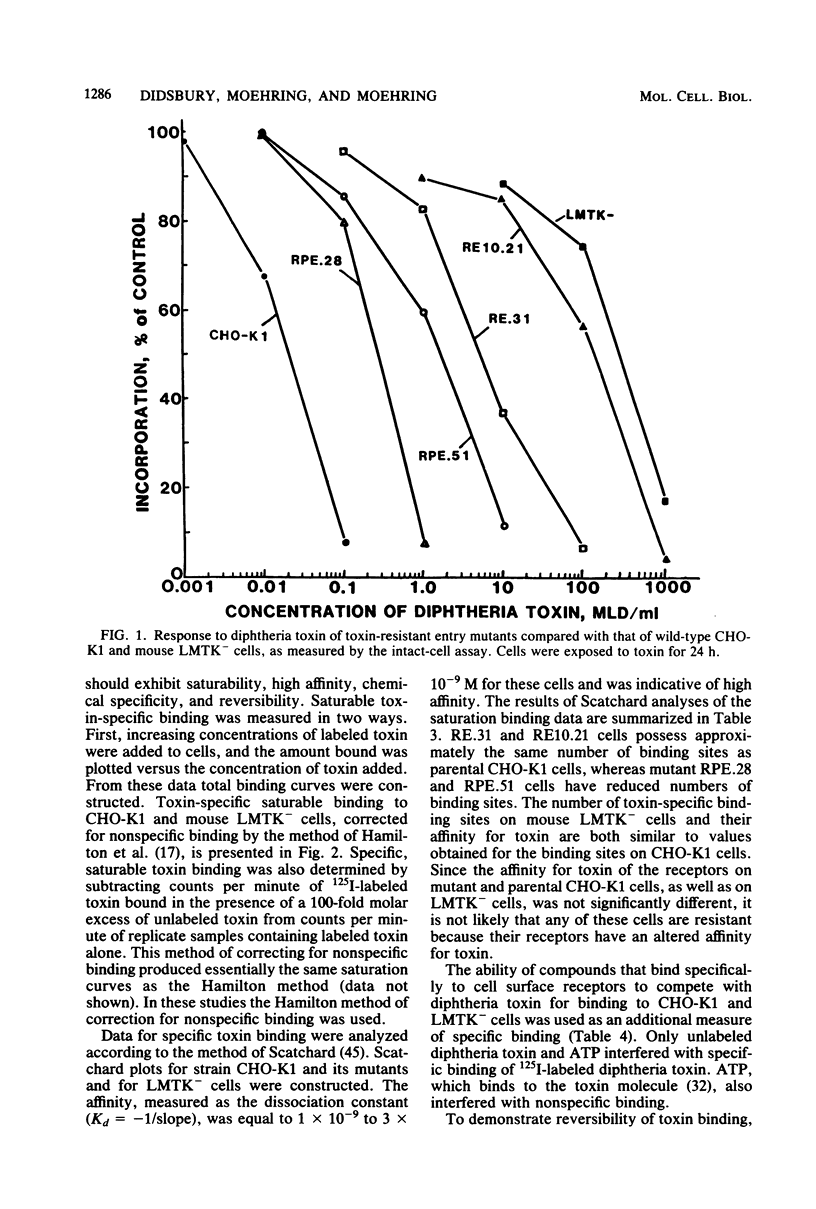
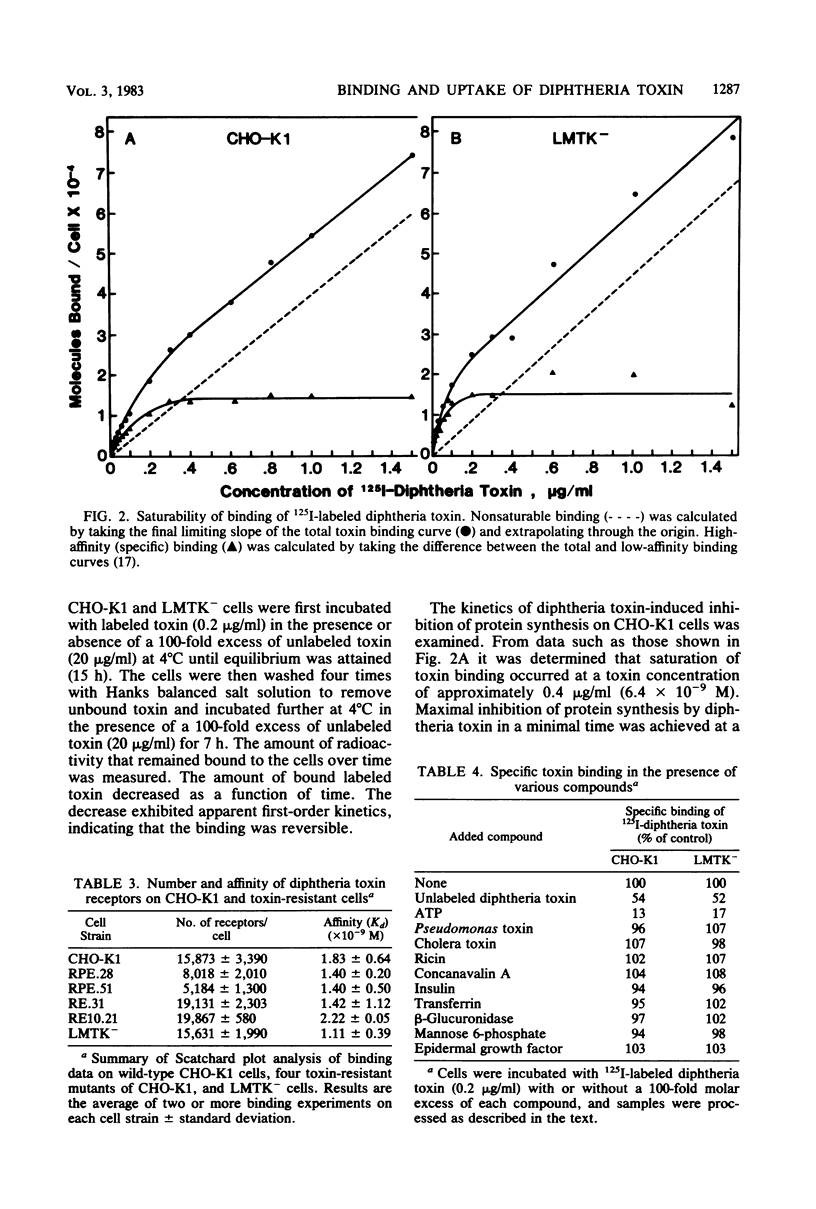
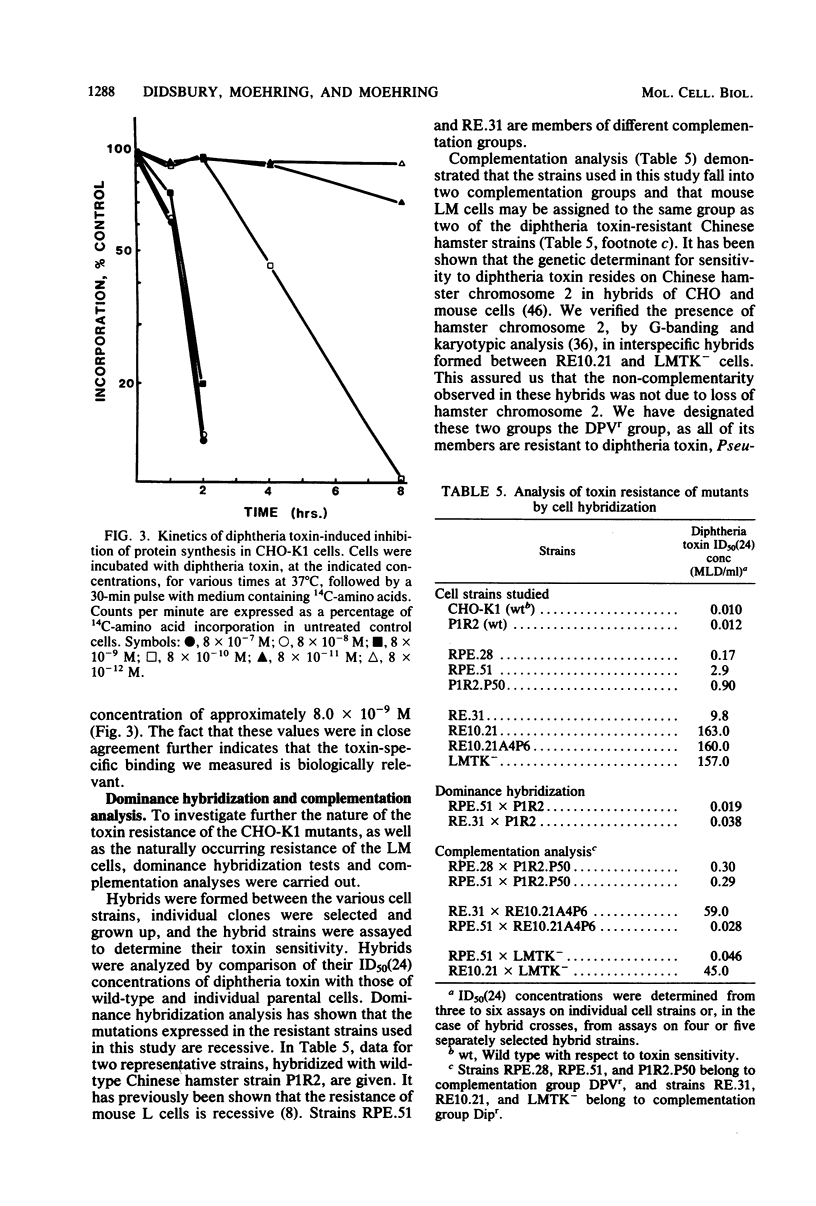
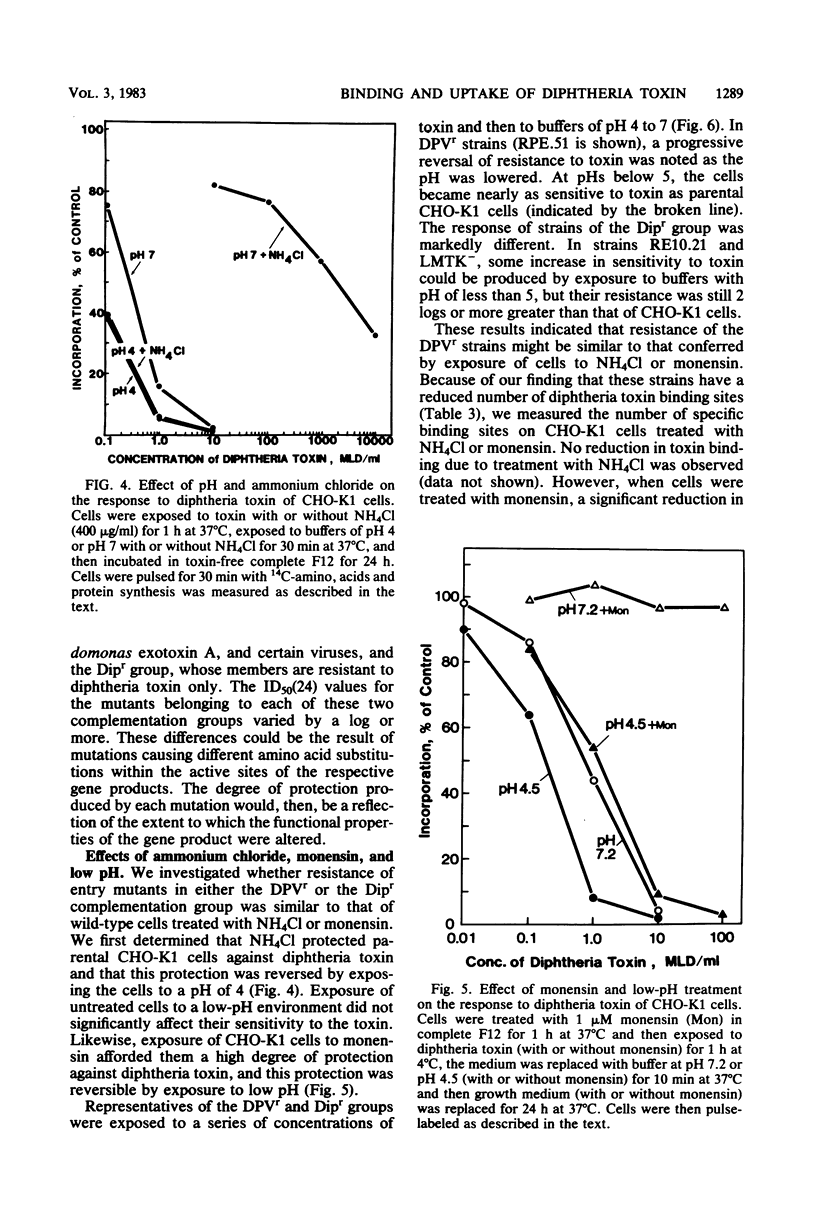
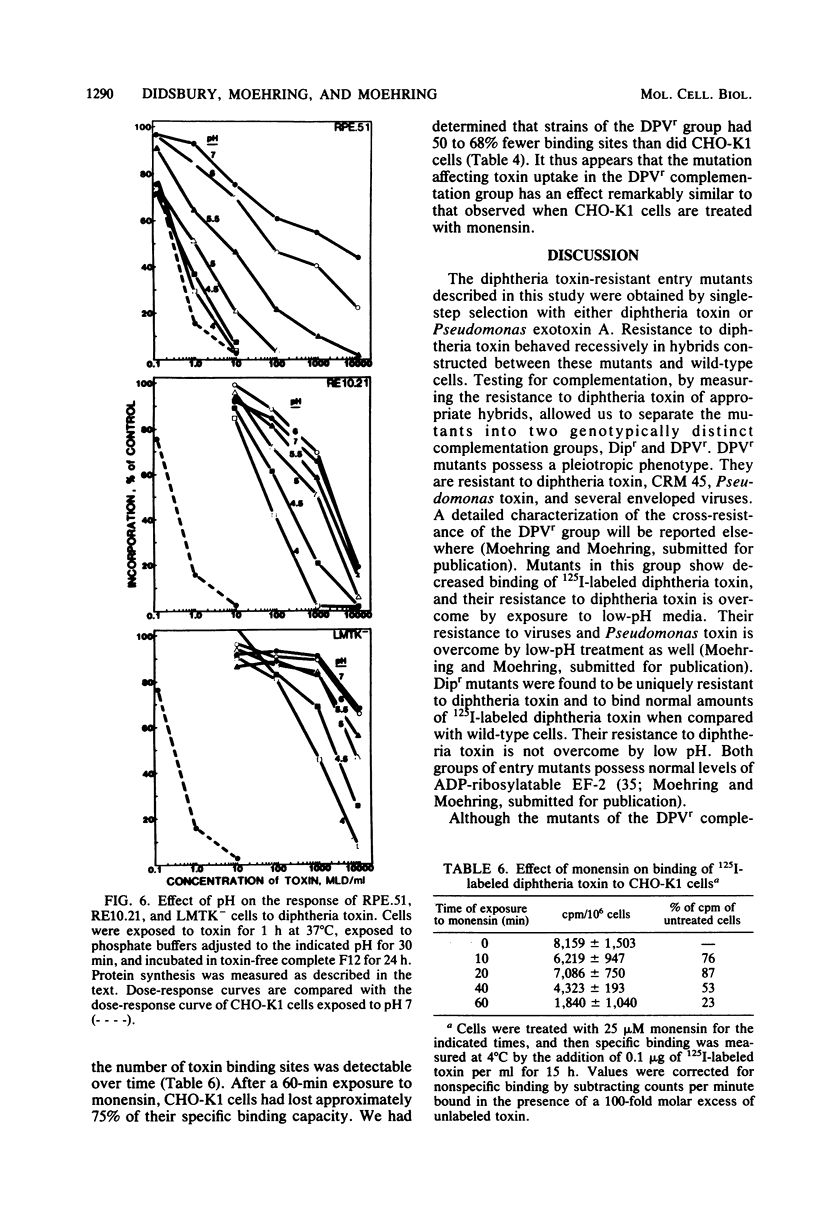
We investigated two phenotypically distinct types of diphtheria toxin-resistant mutants of Chinese hamster cells and compared their resistance with that of naturally resistant mouse cells. All are resistant due to a defect in the process of internalization and delivery of toxin to its target in the cytosol, elongation factor 2. By cell hybridization studies, analysis of cross-resistance, and determination of specific binding sites for 125I-labeled diphtheria toxin, we showed that these cell strains fall into two distinct complementation groups. The Dipr group encompasses Chinese hamster strains that are resistant only to diphtheria toxin, as well as mouse LM cells. These strains possess a normal complement of high-affinity binding sites for diphtheria toxin, but these receptors are unable to deliver active toxin fragment A to the cytosol. Cells of the DPVr group have a broader spectrum of resistance, including Pseudomonas exotoxin A and several enveloped viruses as well as diphtheria toxin. In these studies, which investigate the resistance of these cells to diphtheria toxin, we demonstrate that they possess a reduced number of specific binding sites for this toxin and behave, phenotypically, like cells treated with the proton ionophore monensin. Their resistance is related to a defect in a mechanism required for release of active toxin from the endocytic vesicle.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzone P., Lesniak M. A., Gorden P., Van Obberghen E., Carpentier J. L., Orci L. Binding, internalization, and lysosomal association of 125I-human growth hormone in cultured human lymphocytes: a quantitative morphological and biochemical study. J Cell Biol. 1980 Nov;87(2 Pt 1):360–369. doi: 10.1083/jcb.87.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Boquet P., Pappenheimer A. M., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976 Sep 25;251(18):5770–5778. [PubMed] [Google Scholar]
- Chang T., Neville D. M., Jr Demonstration of diphtheria toxin receptors on surface membranes from both toxin-sensitive and toxin-resistant species. J Biol Chem. 1978 Oct 10;253(19):6866–6871. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagan R. P., Chen S., Ruddle F. H. Genetic analysis of the cell surface: association of human chromosome 5 with sensitivity to diphtheria toxin in mouse-human somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2237–2241. doi: 10.1073/pnas.72.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Membrane receptors. Annu Rev Biochem. 1974;43(0):169–214. doi: 10.1146/annurev.bi.43.070174.001125. [DOI] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Montal M. Insertion of diphtheria toxin into and across membranes: role of phosphoinositide asymmetry. Nature. 1982 Aug 12;298(5875):669–672. doi: 10.1038/298669a0. [DOI] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Effect of ammonium chloride on receptor-mediated uptake of diphtheria toxin by Vero cells. Exp Cell Res. 1981 Aug;134(2):319–327. doi: 10.1016/0014-4827(81)90432-8. [DOI] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagy W. E., Neville D. M., Jr Kinetics of protein synthesis inactivation by diphtheria toxin in toxin-resistant L cells. Evidence for a low efficiency receptor-mediated transport system. J Biol Chem. 1981 Dec 25;256(24):12788–12792. [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen J. H., Maxfield F. R., Hardegree M. C., Habig W. H. Receptor-mediated endocytosis of diphtheria toxin by cells in culture. Proc Natl Acad Sci U S A. 1982 May;79(9):2912–2916. doi: 10.1073/pnas.79.9.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnell M. H., Stookey M., Draper R. K. Monensin blocks the transport of diphtheria toxin to the cell cytoplasm. J Cell Biol. 1982 Apr;93(1):57–62. doi: 10.1083/jcb.93.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Wellsteed J., Kern H., Harms E., Helenius A. Monensin inhibits Semliki Forest virus penetration into culture cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5297–5301. doi: 10.1073/pnas.79.17.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Schlessinger J., Shechter Y., Pastan I., Willingham M. C. Collection of insulin, EGF and alpha2-macroglobulin in the same patches on the surface of cultured fibroblasts and common internalization. Cell. 1978 Aug;14(4):805–810. doi: 10.1016/0092-8674(78)90336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B., Leppla S. H. Association of diphtheria toxin with Vero cells. Demonstration of a receptor. J Biol Chem. 1978 Oct 25;253(20):7325–7330. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Protection of mammalian cells from diphtheria toxin by exogenous nucleotides. Can J Microbiol. 1979 Mar;25(3):285–290. doi: 10.1139/m79-046. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Characterization of the diphtheria toxin-resistance system in Chinese hamster ovary cells. Somatic Cell Genet. 1979 Jul;5(4):453–468. doi: 10.1007/BF01538880. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Comparison of diphtheria intoxication in human and nonhuman cell lines and their resistant variants. Infect Immun. 1976 Jan;13(1):221–228. doi: 10.1128/iai.13.1.221-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Danley D. E., Moehring J. M. Codominant translational mutants of Chinese hamster ovary cells selected with diphtheria toxin. Somatic Cell Genet. 1979 Jul;5(4):469–480. doi: 10.1007/BF01538881. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Interaction of diphtheria toxin and its active subunit, fragment A, with toxin-sensitive and toxin-resistant cells. Infect Immun. 1976 May;13(5):1426–1432. doi: 10.1128/iai.13.5.1426-1432.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. IV. Isolation of KB cells resistant to diphtheria toxin. Infect Immun. 1972 Oct;6(4):487–492. doi: 10.1128/iai.6.4.487-492.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Selection and characterization of cells resistant to diphtheria toxin and pseudomonas exotoxin A: presumptive translational mutants. Cell. 1977 Jun;11(2):447–454. doi: 10.1016/0092-8674(77)90063-0. [DOI] [PubMed] [Google Scholar]
- Moynihan M. R., Pappenheimer A. M., Jr Kinetics of adenosinediphosphoribosylation of elongation factor 2 in cells exposed to diphtheria toxin. Infect Immun. 1981 May;32(2):575–582. doi: 10.1128/iai.32.2.575-582.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Proia R. L., Hart D. A., Holmes R. K., Holmes K. V., Eidels L. Immunoprecipitation and partial characterization of diphtheria toxin-binding glycoproteins from surface of guinea pig cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):685–689. doi: 10.1073/pnas.76.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Stallings R. L., Siciliano M. J., Adair G. M., Humphrey R. M. Structural and functional hemi- and dizygous Chinese hamster chromosome 2 gene loci in CHO cells. Somatic Cell Genet. 1982 Jul;8(4):413–422. doi: 10.1007/BF01538704. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Harper A. A. Reconstitution of diphtheria toxin from two nontoxic cross-reacting mutant proteins. Science. 1972 Feb 25;175(4024):901–903. doi: 10.1126/science.175.4024.901. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kartenbeck J., Helenius A. Fusion of Semliki forest virus with the plasma membrane can be induced by low pH. J Cell Biol. 1980 Oct;87(1):264–272. doi: 10.1083/jcb.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Maxfield F. R., Pastan I. H. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]



