SUMMARY
Interleukin-1 (IL-1) is a key regulator of inflammation and ischaemic brain injury, but the contribution of central and peripheral sources of IL-1 to brain injury is not well understood. Here we show that haematopoietic-derived IL-1 is a key driver of ischaemic brain injury. Wild type (WT) mice transplanted with IL-1αβ-deficient bone marrow displayed a significant (40%) reduction in brain injury induced by focal cerebral ischaemia compared with WT mice transplanted with WT bone marrow. This was paralleled by improved neurological outcome and the almost complete absence of splenic-derived, but not liver-derived, IL-1α after stroke in WT mice lacking haematopoietic-derived IL-1. IL-1αβ knockout (KO) mice transplanted with IL-1αβ-deficient bone marrow showed a 60% reduction in brain injury compared with WT mice receiving WT bone marrow. Transplantation of WT bone marrow in IL-1αβ KO mice resulted in a similar level of blood-brain-barrier injury to that observed in WT mice receiving IL-1αβ-deficient bone marrow. Cerebral oedema after brain injury was reduced in IL-1αβ KO recipients irrespective of donor-derived IL-1, but a lack of haematopoetic IL-1 has also been associated with smaller brain oedema independently of recipient status. Thus, both central and haematopoietic-derived IL-1 are important contributors to brain injury after cerebral ischaemia. Identification of the cellular sources of IL-1 in the periphery could allow targeted interventions at these sites.
INTRODUCTION
Neuroinflammatory changes in cerebral ischaemia and other central nervous system (CNS) diseases are driven by both activated glia and peripheral immune cells (Ransohoff and Brown, 2012; Denes et al., 2010a). Interleukin-1 (IL-1), a master cytokine and key inflammatory mediator, is a major contributor to ischaemic brain injury (Denes et al., 2011a; Dinarello, 2011). IL-1 also contributes to chronic diseases that are primary risk factors for cerebrovascular disease and key drivers of clinical outcome after stroke, such as diabetes, hypertension and obesity (Denes et al., 2011a; Dinarello, 2011). Hence, IL-1 of both central and peripheral origin is likely to influence CNS disease, but the cellular sources and relative contribution of endogenous peripheral versus central IL-1 to brain injury are still unclear.
In acute, experimental brain injury, microglia are considered to be the main source of central IL-1, of which IL-1β is the best studied (Davies et al., 1999; Hanisch, 2002). However, IL-1 protein levels in microglia are very low in the first 4 hours after cerebral ischaemia in mice, and the main early isoform expressed in the brain is IL-1α (Luheshi et al., 2011). Indeed, the time profile for the evolution of the infarct does not match the increases in cytokine mRNA and protein expression in the brain (Lambertsen et al., 2012). Thus, peripherally-derived endogenous IL-1 might contribute to ischaemic brain injury, in both the presence and absence of comorbidities. To date, no functional studies have tested this hypothesis. To address this, we developed a chimeric mouse model in which haematopoietic-derived IL-1 is eliminated selectively, enabling us to study the role of central and blood-borne IL-1 in acute brain injury. Both brain and haematopoietic sources of IL-1 were found to contribute to ischaemic injury in mice, suggesting, at least in part, that inflammation and neuronal death after cerebral ischaemia could be limited by blocking peripheral IL-1 actions independently of drug delivery into the brain.
RESULTS
Central and haematopoietic-derived IL-1 both contribute to ischaemic brain injury
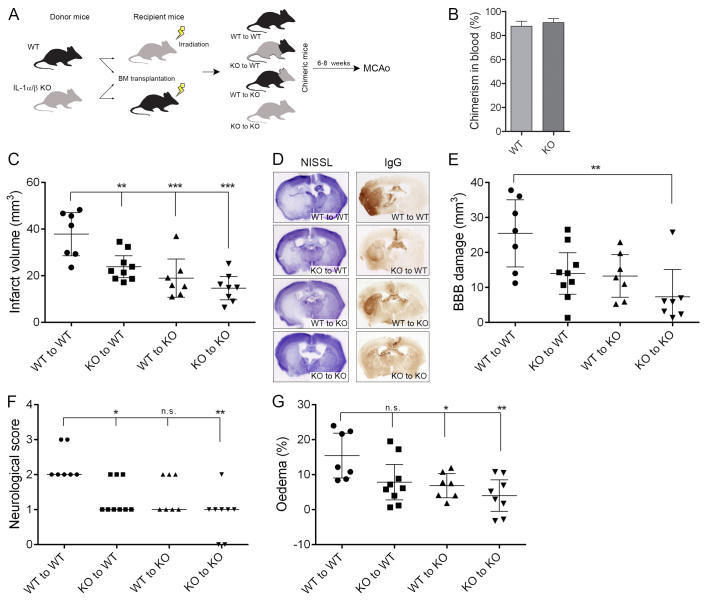
Bone marrow transplantation (Fig. 1A) was successful, as shown by the fact that blood chimerism was 88-91% in wild-type (WT) and IL-1αβ knockout (KO) recipients based on flow cytometric data (Fig. 1B) and no difference in total leukocyte numbers or relative proportion of different leukocyte subsets was found among groups prior to middle cerebral artery occlusion (MCAo) (data not shown).
Fig. 1.
Both central and haematopoietic-derived IL-1 are important contributors to brain injury after cerebral ischaemia. (A) Wild-type (WT) and IL-1αβ-deficient (KO) mice were subjected to whole body irradiation followed by transplantation of WT or IL-1αβ KO bone marrow. After 6–8 weeks of recovery, MCAo was performed. (B) Chimerism in blood as assessed by relative proportion of donor-derived (GFP+) leukocytes within the circulating CD45-positive cell population was uniform in WT and KO mice. (C) Infarct volume is significantly reduced in mice receiving KO bone marrow or in KO mice transplanted with WT bone marrow compared with WT mice reconstituted with WT bone marrow. (D) Representative images showing cresyl violet (Nissl)-stained brain sections or BBB injury (IgG infiltration). (E) Quantification of BBB injury based on IgG infiltration to the brain parenchyma. (F) Neurological deficit scores showing improved outcome in mice receiving IL-1αβ KO bone marrow. (G) Brain oedema is significantly reduced in mice lacking central IL-1. *P<0.05, **P<0.01, ***P<0.01, one-way ANOVA followed by Tukey’s multiple post-hoc comparison (C,E,G) or Kruskal-Wallis test followed by Dunn’s multiple comparison (F). Data are expressed as mean ± s.e.m. (B; n=9), mean and 95% confidence interval (C–E) or median (F). n.s., non-significant.
WT mice transplanted with IL-1αβ KO bone marrow (KO to WT) displayed a 40% reduction in infarct size compared with the injury seen in WT mice receiving WT bone marrow (WT to WT), whereas IL-1αβ KO mice transplanted with WT bone marrow (WT to KO) showed a 50% reduction compared with the WT to WT (Fig. 1C,D). Mice completely lacking IL-1 (KO to KO) showed a 60% reduction in infarct size compared with WT to WT mice, indicating that both central and haematopoietic-derived IL-1 are important contributors to brain injury after cerebral ischaemia. Only mice lacking both central and haematopoietic-derived IL-1 showed significantly (70%) reduced BBB injury (Fig. 1D,E) compared with fully IL-1-competent (WT to WT) mice, whereas similar levels of blood-brain barrier (BBB) breakdown was observed in WT mice transplanted with IL-1αβ KO bone marrow and KO mice transplanted with WT bone marrow (45% and 48%, respectively; Fig. 1D,E). Mice lacking haematopoietic-derived IL-1 displayed improved neurological function at 24 hours reperfusion (Fig. 1F), whereas a significant reduction in brain oedema was observed in mice lacking central IL-1 irrespective of transplantation of WT or IL-1αβ KO bone marrow (Fig. 1G). Two-way ANOVA confirmed that WT recipients had reduced brain oedema independently of donor-derived IL-1 (P=0.0065); however, lack of haematopoietic-derived IL-1 was also associated with some reduction in brain oedema irrespectively of recipient genotype (P=0.0196).
TRANSLATIONAL IMPACT.
Clinical issue
Brain diseases represent a huge burden on society, and stroke is one of the leading causes of death and morbidity worldwide. Inflammation has been identified as a key contributor to brain injury; however, the mechanisms by which inflammation mediates neuronal cell death are not fully understood. Interleukin-1 (IL-1) is an inflammatory cytokine that plays a major role in both acute and chronic diseases of the central nervous system. Although the main cellular sources of IL-1 have been extensively studied, the relative contributions of central and peripheral (blood)-derived IL-1 to brain injury have not been investigated.
Results
In this study, the authors investigated the role of central and haematopoietic-derived IL-1 in brain injury. To this end, they selectively eliminated IL-1 from blood-derived cells using bone marrow transplantation in a chimeric mouse model of focal cerebral ischaemia. The authors report that IL-1 derived from both sources contributes substantially to ischaemic brain injury. They demonstrate that deletion of blood-derived IL-1 is sufficient to improve neurological outcome of mice, and both central and peripheral IL-1 seem to contribute to the breakdown of the blood-brain barrier after cerebral ischaemia. Centrally-derived IL-1, however, seems to be the main driver of increased cerebral oedema after brain injury.
Implications and future directions
These results provide the first evidence that central and peripheral IL-1 contribute to brain injury. This finding has implications for the development of targeted interventions against detrimental inflammatory actions mediated by IL-1. Promisingly, inflammation and neuronal death after cerebral ischaemia could, at least in part, be limited by blocking peripheral IL-1 actions independently of drug delivery into the brain. Towards this goal, the identification of the exact cellular sources of peripheral IL-1 is now a key priority.
Haematopoietic-derived IL-1 alters systemic inflammatory responses after cerebral ischaemia
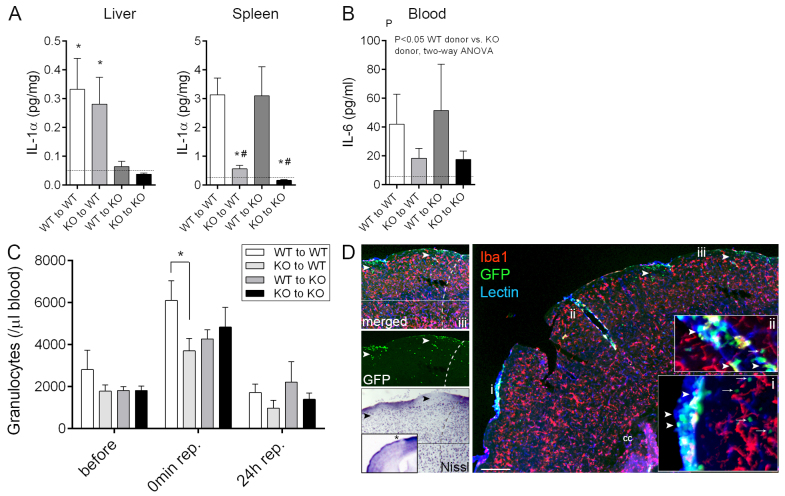
Liver homogenates showed a marked reduction in IL-1α levels in IL-1αβ KO mice irrespective of bone marrow transplant, whereas an almost complete loss of splenic IL-1α was seen in mice transplanted with IL-1αβ KO bone marrow (Fig. 2A). IL-1β concentrations followed a similar trend to IL-1α, although were frequently below the detection limit (5–10 pg/ml) of the assay, as were plasma levels 24 hours after reperfusion (not shown). In contrast, plasma IL-6 levels were significantly reduced (by 55–60%) in mice receiving IL-1αβ KO bone marrow (Fig. 2B; P<0.05, twoway ANOVA vs WT). No other cytokines of the 11 examined [IL-1α, IL-1β, granulocyte colony-stimulating factor (G-CSF), IFNγ, IL-10, IL-17A, keratinocyte chemoattractant (KC), monocyte chemotactic protein-1 (MCP-1), TNFα and RANTES (CCL5)] were altered (data not shown).
Fig. 2.
Lack of haematopoietic-derived IL-1 results in altered systemic inflammatory responses after cerebral ischaemia. (A) IL-1α production in the liver is dependent on host-derived cells with slow turnover, whereas splenic leukocytes expressing IL-1α are readily replaced by bone-marrow-derived cells within 6-8 weeks after bone marrow transplantation. IL-1α levels in liver and spleen homogenates at 24 hours reperfusion are shown. (B) Plasma IL-6 levels are reduced 24 hours after MCAo in mice receiving IL-1αβ KO bone marrow. Dashed lines in panels A and B indicate the detection limit of the assay. (C) Stroke results in a rapid increase in circulating granulocytes (P<0.01, 45 minutes after the onset of ischaemia compared with prior to occlusion), which is blunted in WT mice transplanted with IL-1αβ KO bone marrow. (D) Recruitment of donor-derived (GFP+, green) leukocytes (arrowheads) to the brain 24 hours after MCAo is most pronounced in the meninges (lectin, blue) and in large cortical blood vessels (lectin, blue). Tissue infiltration of blood-borne cells is seen only in superficial cortical layers (green, arrows) and is minimal deep in the brain parenchyma, with very minor contribution of donor-derived (GFP+) macrophages (Iba1, red). Inserts show a higher magnification of the areas labelled with i and ii. (iii) Recruitment of blood-borne cells into the brain (GFP, arrowheads) is associated with neuronal loss (Nissl, asterisk on insert is showing the area of interest in the ipsilateral hemisphere). Dashed line indicates the boundary of the infarct. Cc, corpus callosum. Scale bar: 200 μm. *P<0.05 versus KO to WT, and KO to KO, respectively (A, left), *P<0.05 versus WT to WT, and #P<0.05 versus WT to KO (A, right), oneway ANOVA followed by Tukey’s multiple post-hoc comparison (A), two-way ANOVA (B), and two-way ANOVA followed by Bonferroni’s multiple comparison (C). n=7–9, data are expressed as mean ± s.e.m.
A rapid (within 45 minutes of onset of ischaemia) twofold rise in granulocyte numbers in response to MCAo was observed in all chimeric groups (P<0.01, two-way ANOVA), which was lower in WT mice receiving IL-1αβ KO bone marrow compared with WT mice transplanted with WT bone marrow (Fig. 2C; two-way ANOVA, Bonferroni’s post-hoc comparison). Increases in circulating granulocytes paralleled a 10- to 20-fold rise in blood plasma KC, IL-6 and MCP-1 levels measured at reperfusion, without any changes seen among different chimeric groups (not shown). Apart from granulocytes, no changes in main leukocyte populations (T cells, B cells, monocytes) were seen in the blood, spleen or bone marrow 24 hours after MCAo in the four chimeric mouse groups.
Donor-derived (GFP+) cells were found mostly around the meninges and in association with larger cerebral blood vessels in the ipsilateral hemisphere 24 hours after MCAo (Fig. 2D). Parenchymal recruitment of GFP+ cells was observed mainly in the superficial layers of the cerebral cortex below the meninges, an area that was spared after MCAo in KO to WT mice as opposed to WT to WT animals (Fig. 2D). Only few blood-borne leukocytes were seen in the deeper cortical layers or in the striatum. Recruited donor-derived cells were mostly granulocytes and some monocyte-like cells were also observed (not shown), but microglia and macrophages (Iba1+) represented less than 4% of donor-derived cells in the brain. Very few donor-derived (GFP+/Iba–) leukocytes were found in the brain parenchyma prior to cerebral ischaemia, or in the contralateral hemisphere after MCAo, and no IgG penetration was observed in the contralateral side, arguing for minimal sustained BBB injury due to irradiation and/or bone marrow transplantation. This is in agreement with our previous data showing that microglial and macrophage repopulation after irradiation conditioning and bone marrow transplant is relatively slow, with 0.7% contribution at 2 weeks post-transplant rising to 10% by 6 months post-transplant (Wilkinson et al., 2013).
DISCUSSION
Here we demonstrate for the first time that endogenous IL-1 of both central and haematopoietic origin contributes to ischaemic brain injury, even in the absence of systemic co-morbidity. Identification of the exact cellular source(s) of IL-1 in the haematopoietic system that influence(s) brain injury needs substantially more experimental work, but our results demonstrate that blockade of peripheral IL-1 is a viable option to target brain injury without the requirement of drug delivery to the brain. Because current opportunities to block IL-1 (such as IL-1Ra) do not discriminate between the effects of IL-1α and IL-1β, or central and peripheral IL-1 actions, site- and isoform-specific IL-1 blockade could increase the efficacy of interventions against brain injury while maintaining essential defence mechanisms mediated by IL-1.
Microglia are radio-resistant cells in the CNS (Mildner et al., 2007). We chose an irradiation protocol for bone marrow transplantation, which resulted in minor replacement of microglia by blood-borne cells prior to cerebral ischaemia, and negligible contribution of donor-derived cells to the resident microglial pool was observed within 24 hours reperfusion after cerebral ischaemia. So, this model enabled us to study central IL-1 production separately from haematopoietic IL-1 sources. Because irradiation and bone marrow transplantation can induce changes in the recruitment of leukocytes into the brain (Ajami et al., 2007), we used WT mice transplanted with WT bone marrow to control for the effects of induction of chimerism when comparing with WT littermates receiving IL-1αβ KO bone marrow. Of note, bone marrow transplantation did not result in the replacement of all haematopoietic-derived IL-1-producing cells in the periphery, as evidenced by maintenance of IL-1 production in the liver in WT recipients irrespective of donor-cell-derived IL-1αβ expression. Thus, it is possible that IL-1 from tissue-resident cells with slow turnover in the periphery could also contribute to brain injury. Nevertheless, previous data strongly indicate the involvement of brain-derived IL-1 to brain injury. Central (largely microglial) IL-1 levels increase in response to brain injury, IL-1Ra reaches therapeutic concentrations in the brain after peripheral administration and reduces brain injury, and central administration of both IL-1Ra and IL-1β neutralising antibodies reduces brain injury in different experimental models (Lambertsen et al., 2012; Luheshi et al., 2011; Relton and Rothwell, 1992; Yamasaki et al., 1995; Allan et al., 2005; Clausen et al., 2008; Greenhalgh et al., 2010; Clausen et al., 2011; Denes et al., 2011a; Galea et al., 2011). Therefore, we believe that, in our studies, IL-1 actions that are independent of the cells replaced by bone marrow transplantation are at least in part due to central IL-1 production, although the causal role of different central IL-1 sources in brain injury needs to be investigated further.
Our data indicate that haematopoietic-derived IL-1 might influence brain damage via different mechanisms to that of central IL-1, such as by altering leukocyte mobilisation or recruitment after injury. We found that splenic IL-1-competent cells were readily replaced by bone-marrow-derived cells within 6–8 weeks. The splenic reservoir of monocytes and CXCR2-positive granulocytes in the bone marrow are mobilised rapidly in response to injury, infection or stroke (Shi and Pamer, 2011; Denes et al., 2011b) and are candidates for further investigation similarly to platelets that produce IL-1α (Thornton et al., 2010). We have shown that platelet-derived IL-1α drives cerebrovascular inflammation and facilitates the recruitment and transendothelial migration of neutrophils in an IL-1-dependent manner (Thornton et al., 2010). In addition, early recruitment of IL-1-positive, non-microglial inflammatory cells in the ischaemic brain has been reported, and some of these cells have been identified as macrophages (Lambertsen et al., 2012; Clausen et al., 2008), which in part could correspond to the cells recruited to the brain in our model. Collectively, recruitment of platelets, monocytes, macrophages or other cells into the injured brain and/or actions of inflammatory mediators released from these cells inside or outside the brain could contribute to BBB breakdown, brain oedema and neuronal injury. Cell-specific ablation of IL-1 will be essential to identify blood cell populations that contribute to brain injury. Similarly, it will be essential to investigate the contribution of microglia and other resident brain cells to IL-1-mediated central inflammatory responses, which would allow cell-type-specific interventions. Further studies are also needed to examine the role of peripheral-IL-1-mediated actions in different models of cerebral ischaemia, such as after permanent cerebral ischaemia or when the infarct only affects cortical areas, as well as in other models of acute and chronic CNS injury.
Taken together, our results demonstrate for the first time a clear role for both central and haematopoietic-derived IL-1 in brain injury, a finding that could have an impact on the development of targeted cell therapies in CNS diseases. Importantly, by blocking peripheral IL-1 actions, drugs might not need to get through the BBB to exert beneficial effects against IL-1-mediated inflammation and injury in the brain.
MATERIALS AND METHODS
Mice
Male 7- to 9-week-old wild-type (WT) C57BL/6 and IL-1αβ-deficient (IL-1αβ KO, C57BL/6 background, bred in-house) mice were subjected to whole body irradiation (2× 5Gy) followed by transplantation of WT or IL-1αβ KO bone marrow (107 cells/mouse) (Okabe et al., 1997). The irradiation dose was selected to enable effective repopulation of haematopoietic cells (Bigger et al., 2006), but resulted in only minor (1–4%) replacement of hemispheric microglia by donor-derived cells after 6–8 weeks. Mice recovered fully after 6 weeks, as indicated by their body weight gain, peripheral leukocyte numbers and overall fitness. Animals (3%) showing impaired recovery (decreasing body weight, prolonged piloerection, hunched posture or any other sign of illness) 4 weeks after transplantation were excluded from further experiments. All animal procedures were performed under the University of Manchester project license number (40/3076), adhered to the UK Animals (Scientific Procedures) Act (1986) and were in accordance with the ARRIVE guidelines.
Middle cerebral artery occlusion (MCAo)
Focal cerebral ischaemia was induced as described earlier (Dénes et al., 2010b), 6–8 weeks after bone marrow transplantation. Mice were anaesthetised with isoflurane and were subjected to 45 minutes MCAo (left side occluded), using a silicon-coated monofilament with a tip diameter of 210 μm (Doccol Corp., Redlands, CA) and perfused transcardially 24 hours after reperfusion. Successful occlusion of the MCA was confirmed by laser Doppler and no difference has been found among different chimeric groups.
Transcardial perfusion and tissue processing
To isolate peripheral organs, mice were perfused transcardially with saline under isoflurane anaesthesia. Brains were subsequently perfused with 4% paraformaldehyde, post-fixed for 24 hours, cryoprotected in 20% sucrose/PBS and sectioned (20 μm diameter) on a sledge microtome. Organs were homogenised as described previously (Dénes et al., 2010b).
Measurement of infarct volume, BBB damage and neurological deficit
The volume of ischaemic and BBB damage were measured as described previously (Dénes et al., 2010b) and corrected for oedema. IgG immunostaining was used to assess BBB permeability (Dénes et al., 2010b). Neurological deficit was assessed blinded to group identity and according to a neurological grading score of increasing severity of deficit, as described previously (Dénes et al., 2010b). Owing to histological problems, brain sections from a single mouse were not processed for determination of BBB injury (Fig. 1E, KO to KO group).
Measurement of inflammation
Plasma samples, and liver and spleen homogenates were processed for cytokine measurement using CBA Flex Sets (BD Biosciences, UK). Eleven key inflammatory cytokines were assessed: IL-1α, IL-1β, G-CSF, IFNγ, IL-6, IL-10, IL-17A, KC, MCP-1, TNFα and RANTES (CCL5). The detection limit for each cytokine was 5–10 pg/ml. Spleen, bone marrow and blood cells were isolated and stained with appropriate combinations of CD45-PerCP-Cy5.5, Ly6c-PerCP-Cy5.5, CD4-PE-Cy7, CD8-PE, CD3-APC, CD19-PE-Cy7, MHCII-APC (eBioscience, UK) and Ly6g-PE (1A8, BD Biosciences, UK) following Fc receptor blockade (eBioscience). Donor-derived WT cells were identified based on expression of green fluorescent protein (GFP) and were used to assess chimerism in WT and IL-1αβ KO recipients. Cells were acquired on an LSRII flow cytometer, using FACS Diva software (BD Biosciences, UK). Immunostaining was performed on free-floating brain sections using combinations of chicken anti-GFP (Invitrogen), rabbit anti-Iba1 (WAKO, Germany) and rabbit anti-neutrophil serum (SJC, kindly provided by Drs Daniel Anthony and Sandra Campbell, University of Oxford, Oxford, UK). Sections were incubated in primary antibody overnight followed by adequate fluorochrome (Alexa-Fluor-594, Alexa-Fluor-488)-conjugated antibodies (Invitrogen). Donor-derived microglia and macrophages were visualised using immunofluorescence against GFP and Iba1, and chimerism presented as percentage of GFP+ cells within the Iba1-positive microglial population. Biotinylated tomato lectin (Sigma-Aldrich, UK) was used to visualise blood vessels and meninges, followed by streptavidin-Alexa-Fluor-350 conjugate (Invitrogen). Images were collected on a Zeiss Axioskop or an Olympus BX51 microscope using a CoolSNAP ES camera (Photometrics, UK) through MetaVue software (Molecular Devices, UK), and processed using ImageJ and Adobe Photoshop softwares.
Randomization, quantification and statistical analysis
Group sizes were determined by power analysis based on our previous MCAo studies (Denes et al., 2010b; Denes et al., 2011b). Mice were randomly assigned to groups prior to bone marrow transplantation and were randomised in blocks for the induction of cerebral ischaemia (to ensure that all chimeric groups are represented in every day of surgery). All quantitative assessments were performed in a blinded manner. Four mice were excluded from analysis pre hoc, owing to surgical artifacts or failure to meet pre-established criteria for the MCAo model in our laboratory. Statistical analysis was performed by one-way or two-way ANOVA followed by Tukey’s or Bonferroni’s post-hoc multiple comparison for normally distributed data, and Kruskal-Wallis test followed by Dunn’s multiple comparison for the analysis of neurological scores, using GraphPad Prism 5 software. P<0.05 was considered statistically significant.
Footnotes
COMPETING INTERESTS
N.J.R. is the Non-Executive Director of AstraZeneca but the company has no involvement in this work.
AUTHOR CONTRIBUTIONS
A.D., S.M.A., N.J.R. and B.B. conceived and designed the experiments; A.D. and F.W. performed the experiments; A.D. and M.C. analysed the data; B.B. and F.W. contributed research tools; A.D., S.M.A. and N.J.R. wrote the paper.
FUNDING
We are grateful for funding provided by the Medical Research Council (NR MRC Research Professorship) and the European Union’s Seventh Framework Programme (FP7/2008-2013) under grant agreements no. 201024 and no. 202213 (European Stroke Network; to N.J.R. and A.D.).
REFERENCES
- Ajami B., Bennett J. L., Krieger C., Tetzlaff W., Rossi F. M. (2007). Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 [DOI] [PubMed] [Google Scholar]
- Allan S. M., Tyrrell P. J., Rothwell N. J. (2005). Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 5, 629–640 [DOI] [PubMed] [Google Scholar]
- Bigger B. W., Siapati E. K., Mistry A., Waddington S. N., Nivsarkar M. S., Jacobs L., Perrett R., Holder M. V., Ridler C., Kemball-Cook G. (2006). Permanent partial phenotypic correction and tolerance in a mouse model of hemophilia B by stem cell gene delivery of human factor IX. Gene Ther. 13, 117–126 [DOI] [PubMed] [Google Scholar]
- Clausen B. H., Lambertsen K. L., Babcock A. A., Holm T. H., Dagnaes-Hansen F., Finsen B. (2008). Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J. Neuroinflammation 5, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen F., Hånell A., Israelsson C., Hedin J., Ebendal T., Mir A. K., Gram H., Marklund N. (2011). Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 34, 110–123 [DOI] [PubMed] [Google Scholar]
- Davies C. A., Loddick S. A., Toulmond S., Stroemer R. P., Hunt J., Rothwell N. J. (1999). The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J. Cereb. Blood Flow Metab. 19, 87–98 [DOI] [PubMed] [Google Scholar]
- Denes A., Thornton P., Rothwell N. J., Allan S. M. (2010a). Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain Behav. Immun. 24, 708–723 [DOI] [PubMed] [Google Scholar]
- Dénes A., Humphreys N., Lane T. E., Grencis R., Rothwell N. (2010b). Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J. Neurosci. 30, 10086–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A., Pinteaux E., Rothwell N. J., Allan S. M. (2011a). Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovasc. Dis. 32, 517–527 [DOI] [PubMed] [Google Scholar]
- Denes A., McColl B. W., Leow-Dyke S. F., Chapman K. Z., Humphreys N. E., Grencis R. K., Allan S. M., Rothwell N. J. (2011b). Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J. Cereb. Blood Flow Metab. 31, 1036–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117, 3720–3732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J., Ogungbenro K., Hulme S., Greenhalgh A., Aarons L., Scarth S., Hutchinson P., Grainger S., King A., Hopkins S. J., et al. (2011). Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J. Cereb. Blood Flow Metab. 31, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh A. D., Galea J., Dénes A., Tyrrell P. J., Rothwell N. J. (2010). Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br. J. Pharmacol. 160, 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U. K. (2002). Microglia as a source and target of cytokines. Glia 40, 140–155 [DOI] [PubMed] [Google Scholar]
- Lambertsen K. L., Biber K., Finsen B. (2012). Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 32, 1677–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luheshi N. M., Kovács K. J., Lopez-Castejon G., Brough D., Denes A. (2011). Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J. Neuroinflammation 8, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A., Schmidt H., Nitsche M., Merkler D., Hanisch U. K., Mack M., Heikenwalder M., Brück W., Priller J., Prinz M. (2007). Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 [DOI] [PubMed] [Google Scholar]
- Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. (1997). ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 407, 313–319 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M., Brown M. A. (2012). Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton J. K., Rothwell N. J. (1992). Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res. Bull. 29, 243–246 [DOI] [PubMed] [Google Scholar]
- Shi C., Pamer E. G. (2011). Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton P., McColl B. W., Greenhalgh A., Denes A., Allan S. M., Rothwell N. J. (2010). Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood 115, 3632–3639 [DOI] [PubMed] [Google Scholar]
- Wilkinson F. L., Sergijenko A., Langford-Smith K. J., Malinowska M., Wynn R. F., Bigger B. W. (2013). Busulfan conditioning enhances engraftment of hematopoietic donor-derived cells in the brain compared with irradiation. Mol. Ther. 21, 868–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y., Matsuura N., Shozuhara H., Onodera H., Itoyama Y., Kogure K. (1995). Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke 26, 676–680, discussion 681. [DOI] [PubMed] [Google Scholar]