Abstract
MicroRNAs (miRNAs) originate from stemloop-forming precursor RNAs found in longer primary transcripts that often contain introns. We show that in plants, those introns, when located 3′ of the stemloop, can promote mature miRNA accumulation, through a mechanism that likely operates at the level of miRNA processing or stability. Reversely, when miRNA production is reduced such as in dicer-like 1 mutants, splicing of introns that promote miRNA processing is considerably increased, pointing to a tight physical and temporal coordination of intron splicing and miRNA processing in plants. Our findings further suggest that miRNA transcripts without introns generated through alternative polyA-site usage might contribute to the differential adjustment of miRNA levels, possibly at a tissue-specific level.
Keywords: miRNA, intron, Arabidopsis
INTRODUCTION
MicroRNAs (miRNAs), a family of small non-coding RNAs, regulate many biological processes by base-pairing to complementary target transcripts and impose a post-transcriptional control that reduces the rate of target protein production [1]. As target regulation is generally of quantitative nature, increased miRNA abundance normally correlates with decreased target levels. Rapid changes in miRNA accumulation, for example on perception of an external stimulus or stress, thus allow the effective adjustment of the levels of often several targets that might participate in the control of the same biological pathway.
Adjustment of miRNA levels can occur at several different steps. Apart from varying the activities of promoters that drive the expression of MIRNA genes, processing of the stemloop-forming precursor transcripts and the stability of mature miRNAs can be modified in several ways. Most of the known proteins with roles in miRNA processing bind to the loop of the MIRNA stemloop and either inhibit (for example, LIN-28A) or promote (for example, KSRP) precursor processing by Dicer enzymes [2]. Stability of miRNAs is often reduced when their Argonaute protein partners are not available, or by exonucleolytic degradation, for example, by SDN proteins in Arabidopsis [3].
Like other RNA PolII transcripts, primary miRNA transcripts often contain introns. These might be part of the host gene when miRNA precursors are encoded within an intron in animals, or they might be adjacent to the miRNA stemloop in plants, where most MIRNA genes form independent transcription units. It had been established early on that sequences outside the miRNA stemloop were largely dispensable for miRNA production [4]. Therefore, it remained mysterious why most plant MIRNA genes are rather long [5–7] and contain elements such as introns, which attract elaborate RNA-modifying activities including via splicing.
RESULTS AND DISCUSSION
To investigate the possible function of introns in plant miRNA transcripts, we selected two representative MIRNA loci: Ath-MIR172a, producing a conserved miRNA present in many monocot and dicot species [8], and Ath-MIR163, which has only been found in the genus Arabidopsis and is thus considered a recently evolved MIRNA gene [9]. Transcripts mapped from these loci include variants with retained and variants with spliced introns (for example, MIR172a variants 2 and 4, Fig 1A [5, 6]). MIR163 additionally produces transcripts that do not contain any intron sequences at all owing to an alternative polyadenylation site (MIR163 variant 2, Fig 1A [10]). Similar to most introns in Arabidopsis MIRNA genes, introns in Ath-MIR172a and Ath-MIR163 are located 3′ of the miRNA stemloop [5, 6].
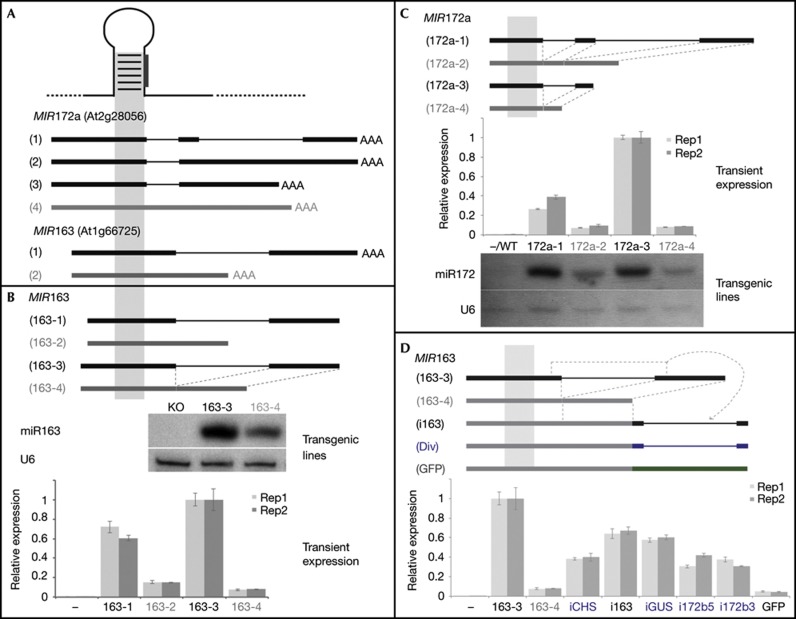
Figure 1.
Introns in miRNA precursors correlate with increased miRNA levels. (A) Endogenous transcripts from MIR163 and MIR172a loci. Grey bars indicate transcripts without intron, black bars those with intron. Introns are depicted in thinner lines. (B) MIR163 transgenes (top) and resulting miRNA levels in stable transgenic lines (middle) and N. benthamiana leaves (bottom). (C) MIR172a transgenes (top) and resulting miRNA levels in N. benthamiana leaves (middle) and stable transgenic lines (bottom). (D) Effect of different 3′ introns (blue) and GFP (green) on miR163 accumulation in transient assays. Transgenes are sketched on top. For transient experiments, independent biological replicates are shown in light and dark grey bars, respectively, error bars indicate standard errors of the means of technical triplicates. A pool of 40–60 plants was processed from stable transgenic lines. Div, diverse; GFP, green fluorescent protein; miRNA, microRNA; Rep, replicate.
Higher miRNA levels from intron-containing precursors
We generated transgene variants of the two mapped MIR163 isoforms, 163-1 with a spliceable intron, and 163-2 without intron, and expressed them transiently in Nicotiana benthamiana leaves. We consistently observed higher levels of mature miR163 generated from the longer, intron-containing form (Fig 1B bottom panel). Similarly, expressing a transgenic version of MIR163 in which the entire intron sequence was omitted (163-4) yielded much lower levels of miRNA compared to a transgene containing the intron in its endogenous position (163-3). To rule out any artifactual effects due to transient expression, we introduced these transgenes in an Arabidopsis MIR163 T-DNA knockout background accumulating no mature miR163 (SALK_034556; ‘KO’ in Fig 1B). Analysis of stable transgenic lines showed that miR163 accumulated at higher levels from the intron-containing precursor transcript (Fig 1B, middle panel), confirming the transient expression results.
We then investigated MIR172a, which harbours two introns 3′ of the miRNA stemloop, by generating transgenes terminating downstream of either the first (172a-3 and 172a-4) or the second intron (172a-1 and 172a-2), respectively. In both instances, the intron-containing precursors produced higher levels of mature miRNA compared to their intron-less counterparts (Fig 1C). Because the primary transcript produced from the short MIR172a variant with intron (172a-3) was longer than the transcript from the long variant lacking introns (172a-2), we exclude a positive effect of transcript length on mature miRNA accumulation, and we therefore conclude that the presence of an intron in both the miR172a and miR163 primary transcripts correlates with increased mature miRNA accumulation.
To test if introns in miRNA transcripts contained special features, such as transcriptional enhancers, that would increase miRNA accumulation, we engineered other, unrelated, intron sequences of various lengths (see supplementary Table S1 online for details). These introns were added at the 3′ end of the intron-less MIR163 variant (163-4), a site at which the MIR163 intron could restore mature miRNA accumulation (Fig 1D, i163). As shown in Fig 1D, both the Petunia chalcone synthase-A intron (iCHS; chromdb.org) and the potato LS1 intron (also known as ‘GUS (β-glucuronidase) intron’, iGUS; [11]) could increase miRNA accumulation; this was also observed with MIRNA introns isolated from the MIR172b locus (i172b3, i172b5). Engineering the green fluorescent protein (GFP) coding sequence at the same position had no effect. The levels of mature miRNA were neither correlated with intron length nor with the AT content of the engineered pieces of DNA or U content of introns (see supplementary Table S1, supplementary Fig S1 online). We therefore conclude that increased miRNA accumulation is likely mediated by defined elements that are not specific to introns normally found in MIRNA genes.
A similar positive effect of introns had been previously observed when engineering longer hairpins as the triggers of RNA interference in transgenic plants. Separating the two arms of the hairpin by a spliceable intron indeed correlated with the highest rates of target-gene silencing compared to silencing achieved from constructs in which non-intronic or reverse-intron spacer sequences formed the loop between the two hairpin arms [12, 13]. We thus decided to investigate the mechanisms underlying these positive intron effects, focusing on MIRNA genes.
Influence of intron position
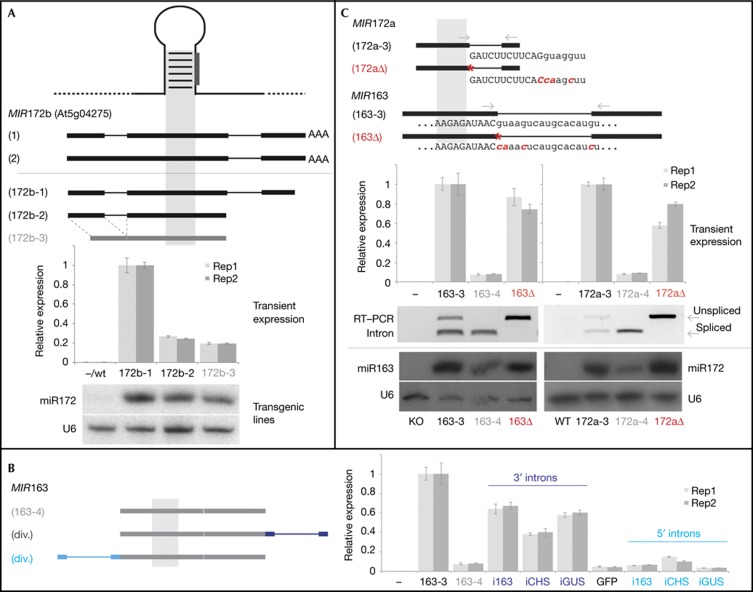
Most introns in endogenous Arabidopsis MIRNA genes localise 3′ relative to the miRNA stemloop, including in cases when primary transcripts comprise longer stretches of 5′ sequence [6]. Therefore, we tested if intron position could influence their competence to increase mature miRNA accumulation. First, we generated MIR172b transgenic variants including or lacking the endogenous 5′ intron found in the miR172b transcript (172b-2 and 172b-3, respectively; Fig 2A). We found that the presence of this 5′ intron had only a minor effect on mature miRNA accumulation. To further investigate a possible intron position effect, we re-engineered three introns that effectively boosted miRNA accumulation when located 3′ of the miRNA stemloop (iCHS, iGUS and i163; Fig 1D), but placed them 5′ as opposed to 3′ relative to the stemloop in the context of the intron-less MIR163 transgene (163-4). In all cases, the 5′ introns had a much weaker effect on mature miRNA accumulation (Fig 2B), suggesting that introns are less effective when located 5′ of the miRNA stemloop.
Figure 2.
Introns enhance miRNA levels when located 3′ of the miRNA stemloop. (A) Endogenous (top) and transgenic (middle) MIR172b variants, and the accumulation of mature miRNA in N. bethamiana leaves and stable transgenic lines (bottom). (B) Effect of 5′ introns (light blue labels, right-most bars) on miR163 accumulation in transient expression assays. The same introns engineered at the 3′ end of MIR163 (dark blue labels) are shown as a control. (C) Effect of splice donor site mutations on miRNA accumulation. Transgenes are sketched on top, including sequences of the 5′ splice junctions and the position of oligonucleotides used for RT–PCR (middle). Red asterisks illustrate the donor site mutation. Northern blots (bottom) were from stable transgenic lines. For transient experiments, independent biological replicates are shown in light and dark grey bars, respectively, error bars indicate standard errors of the means of technical triplicates. A pool of 40–60 plants was processed from stable transgenic lines. miRNA, microRNA; RT–PCR, reverse transcription polymerase chain reaction; Rep, replicate.
Introns are generally removed from nascent transcripts co-transcriptionally by the spliceosome, and removal of different introns occurs with different efficiencies [14]. Processing of miRNA precursors (hereafter called ‘dicing’) and splicing are tightly coupled processes when miRNA precursors reside within the introns of protein-coding genes. In human cells, the two processes are not strictly interdependent [15, 16], yet alternative splicing events can affect the production of intronic miRNAs in plants [17]. To test if the splicing of adjacent introns affects plant miRNA accumulation, we introduced point mutations at the splice donor sites in both the MIR163 and MIR172a precursor RNAs, which completely abolished splicing of the respective introns (163Δ, 172aΔ; Fig 2C). Mature miRNA levels were slightly reduced in transient assays, but unchanged or rather upregulated in transgenic lines, even if acceptor and putative branch sites were mutated in addition (data not shown). This suggests that the positive effect of introns on mature miRNA accumulation does not strictly require splicing of the respective intron, but is more likely mediated by one or several elements within intronic sequences. The position effect of introns further suggests that such elements should be located 3′ relative to the miRNA stemloop in order to exert their positive effects on miRNA accumulation and that these effects might thus entail a defined spatial and/or temporal order of stemloop and intron production during transcription.
Motifs and mechanisms mediating the intron effect
In plants and animals, several introns have been shown to increase steady-state levels of their host protein-coding transcripts in a poorly understood process termed ‘intron-mediated enhancement of gene expression’ (IME) [18]. Similar to our observations, IME is independent of intron splicing [19] but correlates with the over-representation of short sequence motifs within introns, known to increase gene expression in Arabidopsis and rice [20].
To determine whether increased primary transcript levels could account for higher miRNA accumulation from intron-containing precursors, we introduced MIR163 transgenes with and without intron into dicer-like1 (dcl1) mutants, which are impaired in miRNA processing. The rationale of this approach was that precursor processing would not interfere with primary transcript level measurements. We carried out semiquantitative reverse transciption–polymerase chain reaction (RT–PCR) using oligonucleotides amplifying the nascent transgene MIR163 transcripts before processing (‘stemloop’ in Fig 3A, supplementary Fig S2 online), and also oligonucleotides amplifying a short product in the transcribed terminator region, possibly stabilized after processing. As shown in Fig 3A, the precursor levels were only slightly increased when produced from the intron-containing transcripts (163-5). This suggests that an IME-like mechanism that acts to promote transcript accumulation, is unlikely to account, at least significantly, for the observed increased miRNA levels.
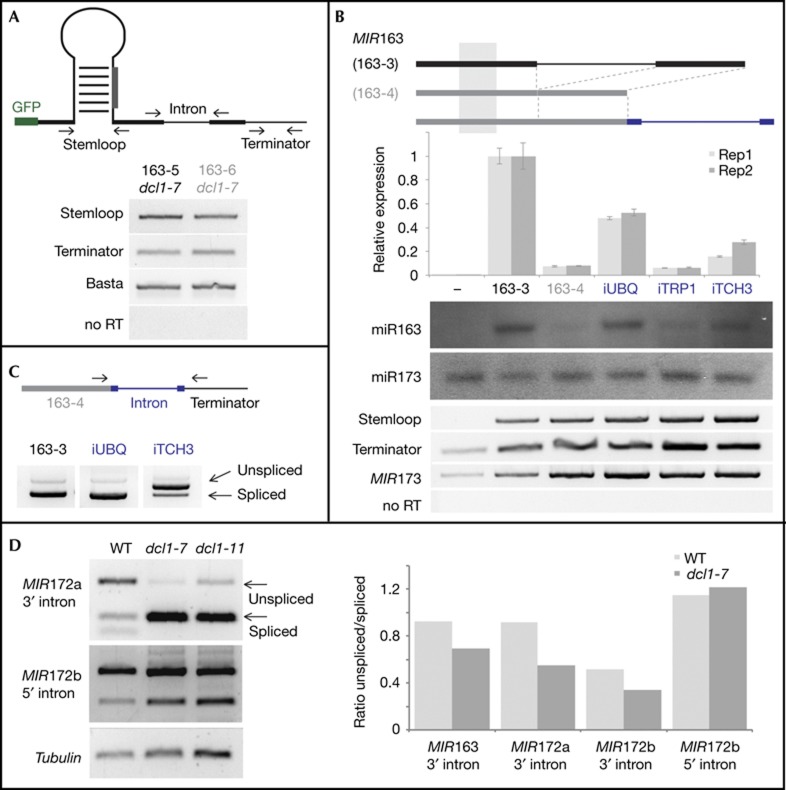
Figure 3.
miRNA transcript levels in transgenic WT and dcl1 mutant plants. (A) RT–PCR analysis of RNA extracted from transgenic Arabidopsis dcl1 mutant plants expressing MIR163 variants (bottom); the positions of oligonucleotides are indicated on top. 163-5 and 163-6 transgenes correspond to 163-3 and 163-4, but contain an additional GFP at their 5′ side, which does not alter the intron effect (supplementary Fig S2 online). (B) Effect of IME introns on miRNA accumulation in N. benthamiana (middle). Histograms show independent biological replicates in light and dark grey bars, error bars indicate standard errors of the means of technical triplicates. Northern blots are from an independent experiment. Semiquantitative RT–PCR analyses indicate precursor levels (bottom); miR173 served as a control for equal infiltration. (C) RT–PCR analyses employing oligonucleotides flanking the cloned intron fragments indicate the degree of splicing. (D) RT–PCR analyses conducted with oligonucleotides spanning introns in WT and dcl1 mutants. Quantifications indicate the ratio of unspliced versus spliced products and represent a single biological experiment, independent of the one shown as a gel picture on the left side. A pool of 15–20 plants was processed from dcl1 and control plants. GFP, green fluorescent protein; IME, intron-mediated enhancement; miRNA, microRNA; RT–PCR, reverse transcription–polymerase chain reaction; WT, wild type.
To further exclude an IME-type of regulation, we used three introns that had been shown to strongly (iUBQ, At4g05320), moderately (iTRP1, At5g17990) or weakly (iTCH3, At2g41100) increase PAT1:GUS mRNA accumulation through IME [19]. As in the previous experiments, these introns were engineered at the 3′ end of the otherwise intron-less 163-4 transcript. As shown in Fig 3B, both the UBQ and TCH3 introns could boost miR163 accumulation, while the TRP1 intron had no effect, indicating no correlation between the ability of introns to cause IME, on the one hand, and to promote miRNA accumulation on the other. Furthermore, the MIR163 and MIR172a introns produced very low scores in IMEter tests, which estimate the ability of an intron to stimulate gene expression [20] (supplementary Table S2 online), yet both increased miRNA production very effectively. In addition, IME is most effective from introns located close to the transcription start [21], whereas in our case, 5′ introns were less effective (Fig 2B). Therefore, increased miRNA accumulation from 3′ localised introns is likely mediated by elements distinct from those involved in IME in protein-coding transcripts.
Having established that 3′-adjacent introns can affect mature miRNA accumulation, we tested whether the converse was also true, that is, that miRNA precursor processing could affect splicing of those MIRNA introns. We carried out semiquantitative RT–PCR with oligonucleotides flanking the introns of several endogenous MIRNA genes and monitored the relative accumulation of spliced versus retained intron in two distinct dcl1 Arabidopsis mutants, dcl1-7 and dcl1-11, which display strongly reduced miRNA processing. As shown in Fig 3D, the 3′ introns in MIR163, MIR172a (first intron), and MIR172b—all of which could boost mature miRNA accumulation when located 3′ of a miRNA stemloop (Fig 1)—were spliced more efficiently in dcl1 mutants compared to WT plants. The 5′ intron in MIR172b, which did not affect mature miRNA accumulation remained, by contrast, largely unaffected (Fig. 3D). Slight alterations in spliced miRNA transcript levels were also observed when HYL1, a DCL1 co-factor, was mutated [6].
These findings collectively suggest a negative effect of dicing on 3′ adjacent intron splicing, similar to the situation in mammals, where miRNA-containing introns are spliced less effectively when pri-to-pre-miRNA processing takes place simultaneously [15, 16]. Our data thus support a model in which proteins carrying out the miRNA processing reaction could slow down or hinder splicing reactions that occur in the vicinity [15].
While dicing seems unfavourable to adjacent intron splicing, we did not find convincing evidence suggesting that splicing of adjacent introns is required for their effect on miRNA accumulation. Rendering those introns that could boost mature miRNA levels unspliceable did not abolish their effects or even increased miRNA accumulation in transgenic lines (Fig 2C). Likewise, we identified spliceable introns that were unable to affect miRNA levels, and also found that a similar increase in mature miRNA accumulation could be mediated by introns with very different splicing efficiencies (Fig 3C). We presume, therefore, that factors mediating the intron effect on miRNA accumulation might operate before or during spliceosome recruitment. This hypothesis also takes into account the fact that introns were less effective when located 5′ of the miRNA stemloop, as those factors might no longer be accessible when splicing or spliceosome assembly has already been initiated, before the miRNA stemloop is fully folded.
Bielewicz et al, in an accompanying paper [22], observed a much more pronounced reduction of mature miRNA levels when mutating the splice donor site in MIR163 transgenes. This discrepancy might be due to the different point mutations that were engineered to render those introns unspliceable: while both successfully abolished completion of the splicing reaction, they might have affected recruitment of binding factors differently. Alternatively, we suspect that the use of different promoters from which the transgenes were expressed (35S versus endogenous MIR163 promoter) might have contributed to this effect, possibly also by affecting the spectrum of recruited proteins. Bielewicz et al observed that miR161 levels were affected only to a milder degree when expressing splice donor-site mutated precursor transcripts from the 35S promoters, compared to miR163 versions expressed from their own promoter elements, which supports the latter explanation. Nevertheless, further experiments will be required to clarify this issue.
Introns have been shown to increase transgene stability by protecting them from the recruitment of RNA–DEPENDENT RNA POLYMERASE 6 (RDR6) and subsequent degradation of the resulting double-stranded RNA [23]. Protection correlates with the efficiency of intron splicing and therefore likely employs a set of factors that differ from the ones involved in intron-mediated miRNA increase. Nonetheless, in a similar way that splicing might alter the relative composition of factors acting on nascent RNAs, which might disfavour the recruitment of RDR6 [23], the presence or absence of factors binding to intronic motifs might alter the availability of miRNA-producing or -stabilizing proteins.
We have shown that the specific location of some, but not all, introns 3′ of miRNA stemloops in precursor transcripts can increase the levels of the respective mature miRNA. Using the dcl1 background, we have ruled out an effect on miRNA primary transcript stability, suggesting that increased processing of miRNA precursor or reduced turnover of mature miRNA might underpin these properties of intron-containing miRNA transcripts. Although the precise mechanisms involved are yet to be elucidated, at least one biological role can be foreseen for the phenomenon reporter here. Indeed, use of alternative polyadenylation sites, such as those within introns, has been observed for a number of Arabidopsis MIRNAs [6], generating transcripts of different length. We have shown that in the case of MIR163, this can have a principal effect on mature miRNA accumulation. Tissue-specific or stress-induced variations of polyA-site usage could thus modulate the extent of miRNA-mediated regulation of gene expression in a spatiotemporal manner.
METHODS
Strains and growth conditions. All Arabidopsis thaliana plants used in this study were of the Col-0 ecotype. dcl1-7, and dcl1-11 mutants have been described [24, 25]. Plants were grown in growth chambers in long-day conditions (16 h light, 8 h dark) at 21 °C.
Transgenes and expression assays. Transgenes were engineered into the pB2GW7 binary plasmid containing a 35S promoter [26] and mobilised into Agrobacterium strain GV3101. Oligonucleotides and templates are listed in supplementary Table S4 online. Transient assays in N. benthamiana leaves (labelled ‘Transient expression’ in figures) were performed as described [4] with a starting OD600 of 0.5; leaves were harvested after 3 days. MiR163 (163-3) was co-infiltrated and served as a control for equal infiltration for all experiments analysing miR172 levels, 172a-3 served as a control for miR163 experiments except in Fig 3B bottom panel where miR173 was used. Stable Arabidopsis transformants were generated for selected lines (labelled ‘Transgenic lines’).
RNA analyses. RNA was extracted from pooled inflorescences (Arabidopsis) (n=15–20 in dcl1 experiment in Fig 3D; rest n=40–60) or four infiltrated leaves (N. benthamiana) using Trizol. Reverse transcription was performed with Superscript III (Invitrogen) or RevertAid (Thermo). MiRNA abundance was quantified by RT–PCR with oligo-dT and specific stemloop primer using SYBR-Green (Thermo) on a CFX system (Bio-Rad). Relative expression was normalised to MIR163 and MIR172a respectively (see above). Experiments were repeated and two biological replicates are represented as independent experimental series in the figures. The means of technical triplicates are shown (± standard error of the means). Details are listed in supplementary Methods online. Semiquantiative RT–PCR was performed in Fig 3 with standard reagents. Band intensities were quantified with ImageJ in Fig 3D. Small RNA northern blots were performed as described [4]. Oligonucleotides used for probes and PCRs are listed in supplementary Table S4 online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank Michael Christie for critical reading of the manuscript, the Arabidopsis stock center, Scott Poethig and Ying-Tang Lu for seeds, Chris Brosnan, Peter Brodersen and Arturo Mari for many helpful discussions, and Patrice Salome for help with qRT–PCR analysis. R.S. was supported by a Human Frontiers Post-doctoral Fellowship.
Author contributions: R.S. designed and performed the experiments and wrote the paper; C.S. performed experiments; S.L. and O.V. participated in fruitful discussions and helped in writing the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucchi M, Briata P, Filipowicz W, Rosenfeld MG, Ramos A, Gherzi R (2009) How to control miRNA maturation? RNA Biol 6: 536–540 [DOI] [PubMed] [Google Scholar]
- Ramachandran V, Chen X (2008) Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321: 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizotto EA, Dunoyer P, Rahm N, Himber C, Voinnet O (2004) In vivo investigation of the transcription, processing, endonucleolytic activity, and functional relevance of the spatial distribution of a plant miRNA. Genes Dev 18: 2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC (2005) Expression of Arabidopsis MIRNA genes. Plant Physiol 138: 2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarzynska B, Sobkowiak L, Pant BD, Balazadeh S, Scheible WR, Mueller-Roeber B, Jarmolowski A, Szweykowska-Kulinska Z (2009) Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs. Nucleic Acids Res 37: 3083–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chia JM, Kumari S, Stein JC, Liu Z, Narechania A, Maher CA, Guill K, McMullen MD, Ware D (2009) A genome-wide characterization of microRNA genes in maize. PLoS Genet 5: e1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus JT, Fahlgren N, Carrington JC (2011) Evolution and functional diversification of MIRNA genes. Plant Cell 23: 431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Sung GH, Spatafora JW, Carrington JC (2004) Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat Genet 36: 1282–1290 [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Watanabe Y (2004) Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA 101: 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L, Rocha-Sosa M (1990) Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet 220: 245–250 [DOI] [PubMed] [Google Scholar]
- Wesley SV et al. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590 [DOI] [PubMed] [Google Scholar]
- Smith NA, Singh SP, Wang MB, Stoutjesdijk PA, Green AG, Waterhouse PM (2000) Total silencing by intron-spliced hairpin RNAs. Nature 407: 319–320 [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R (2009) The spliceosome: design principles of a dynamic RNP machine. Cell 136: 701–718 [DOI] [PubMed] [Google Scholar]
- Kim YK, Kim VN (2007) Processing of intronic microRNAs. EMBO J 26: 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Fujita M, Ohno M (2009) Functional association of the microprocessor complex with the spliceosome. Mol Cell Biol 29: 3243–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K, Liu P, Wu CA, Yang GD, Xu R, Guo QH, Huang JG, Zheng CC (2012) Stress-induced alternative splicing provides a mechanism for the regulation of microrna processing in arabidopsis thaliana. Mol Cell 48: 521–531 [DOI] [PubMed] [Google Scholar]
- Morello L, Breviario D (2008) Plant spliceosomal introns: not only cut and paste. Curr Genomics 9: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB (2002) Requirements for intron-mediated enhancement of gene expression in Arabidopsis. RNA 8: 1444–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB, Elfersi T, Parra G, Korf I (2008) Promoter-proximal introns in Arabidopsis thaliana are enriched in dispersed signals that elevate gene expression. Plant Cell 20: 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB (2004) The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J 40: 744–751 [DOI] [PubMed] [Google Scholar]
- Bielewicz D, Kalak M, Kalyna M, Windels D, Barta A, Vazquez F, Szweykowska-Kulinska Z, Jarmolowski A (2013) Introns of plant pri-miRNAs enhance miRNA biogenesis. EMBO Rep 14: 622–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Croft LJ, Carroll BJ (2011) Intron splicing suppresses RNA silencing in Arabidopsis. Plant J 68: 159–167 [DOI] [PubMed] [Google Scholar]
- Zhang JF, Yuan LJ, Shao Y, Du W, Yan DW, Lu YT (2008) The disturbance of small RNA pathways enhanced abscisic acid response and multiple stress responses in Arabidopsis. Plant Cell Environ 31: 562–574 [DOI] [PubMed] [Google Scholar]
- Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS (2004) SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev 18: 2368–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.