Abstract
Dual leucine zipper kinase (DLK), a mitogen-activated protein kinase kinase kinase, controls axon growth, apoptosis and neuron degeneration during neural development, as well as neurodegeneration after various insults to the adult nervous system. Interestingly, recent studies have also highlighted a role of DLK in promoting axon regeneration in diverse model systems. Invertebrates and vertebrates, cold- and warm-blooded animals, as well as central and peripheral mammalian nervous systems all differ in their ability to regenerate injured axons. Here, we discuss how DLK-dependent signalling regulates apparently contradictory functions during neural development and regeneration in different species. In addition, we outline strategies to fine-tune DLK function, either alone or together with other approaches, to promote axon regeneration in the adult mammalian central nervous system.
Keywords: DLK, neural development, degeneration, axon regeneration
See the Glossary for abbreviations used in this article.
Glossary.
- Bad
Bcl2-associated death promoter
- Bax
Bcl2-associated X protein
- Bcl2
B-cell lymphoma 2
- Cdc42
cell division cycle 42
- cebp
CCAAT/enhancer-binding protein
- CLIP
cytoplasmic linker protein
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- DCX
doublecortin
- DRG
dorsal root ganglia
- efa-6
exchange factor for Arf6
- Erk
extracellular signal-regulated kinase
- Fos
FBJ osteosarcoma oncogene
- GABA
gamma-aminobutyric acid
- GEF
guanine exchange factor
- gp130
glycoprotein 130
- GTP
guanosine triphosphate
- hb9
homoebox gene 9
- HNRPK
heterogeneous nuclear ribonucleoprotein K
- IL-6
interleukin 6
- Itch
itchy E3 ubiquitin protein ligase
- JAK
janus kinase
- JIP1/3
JNK-interacting protein 1/3
- JNK
c-Jun amino-terminal kinase
- KLP-7
kinesin-like protein 7
- LIF
leukaemia inhibitory factor
- MAP1B/2/2B
microtubule-associated protein 1B/2/2B
- MAPK
mitogen-activated protein kinase
- MAP2K
mitogen-activated protein kinase kinase
- MAP3K
mitogen-activated protein kinase kinase kinase
- MAPKAP
MAP kinase-activated protein kinase
- MUK
MAPK-upstream protein kinase
- NGF
nerve growth factor
- Nmat2
nicotinamide mononucleotide adenylyltransferease 2
- phr1
Highwire
- PNS
peripheral nervous system
- pten
phosphatase and tensin homologue
- Rac1
Ras-related C3 botulinum toxin substrate 1
- RGC
retinal ganglion cell
- RhoA
Ras homologue family member A
- RPM-1
regulator of presynaptic morphology 1
- SCG10
superior cervical ganglion 10
- Shc
Src homology 2 domain-containing transforming protein
- STAT3
signal transducer and activator of transcription 3
- TIF-IA
transcription initiation factor IA
- UPS
ubiquitin-proteasome system
- Wlds
Wallerian degeneration slow
Introduction
Over the past decades, research involving diverse model organisms has yielded fundamental insights into the molecular and cellular mechanisms of axon growth, degeneration and cell death during neuronal development, as well as neuron degeneration and regeneration failure after injury to the adult nervous system. Neural development is a complex multi-step process, for which a delicate balance exists between cell death against survival and axonal growth against degeneration, which is constantly adjusted from embryonic stages to adult tissue homeostasis. Such a delicate balance also underlies the neuronal response to various insults in the adult. Intracellular signalling cascades integrate a range of extracellular signals to shift the balance in a timely fashion.
The dual leucine zipper kinase (DLK) signalling pathway is one such signalling cascade that regulates several aspects of neural development ranging from axon growth and neuronal migration to apoptosis and axon degeneration in different model organisms [1,2,3,4,5,6]. An important role in controlling both neurodegeneration [7,8,9,10] and regeneration [9,11,12,13,14,15,16] after injury has also emerged for DLK signalling. Thus, DLK might act as a master sensor that initiates apparently contradictory responses under critical conditions during development and after axonal injury.
DLK functions as a MAP3K activating both JNKs and p38α-δ MAPK pathways [17,18]. The MAPK pathways are organized in three sequential modular cascades: MAP3K, MAP2K and the MAPK. Such modular structure allows for fine-tuning of the DLK signalling in response to a myriad of stimuli, ultimately leading to phosphorylation-dependent modulation of numerous downstream targets including transcription factors, cytoskeleton components, membrane transporters and other protein kinases.
In mice, the DLK (also known as LZK, MAP3K12, MUK and ZPK) protein localizes in several areas of the developing nervous system, such as the brain, spinal cord and sensory ganglia [2,19]. In response to oxidative stress and a limited supply of trophic factors, the activation of DLK-dependent signalling cascades leads to rapid neuron degeneration during development [5]. Conversely, DLK deletion protects several classes of neurons from apoptosis in mouse embryos [5,6]. Earlier observations have shown that genetic disruption of DLK results in reduced JNK activity and decreased phosphorylation of several JNK targets, ultimately causing neuronal migration defects as well as incomplete development of axonal tracts including those of the anterior commissure and corpus callosum [2].
DLK expression is also upregulated in response to axonal injury in mice and rats [10,16]. Absence of DLK protects neurons from apoptosis after nerve injuries and in rodent models of neurodegenerative diseases [10,16,20]. Interestingly, loss of DLK also protects distal axons from Wallerian degeneration, thus providing evidence for a role of the DLK pathway in the axon self-destruction programme after injury [7].
Interestingly, a role for DLK in promoting axon regeneration in diverse model systems has emerged. In this regard, the DLK homologues DLK-1 (Caenorhabditis elegans) and Wallenda (Drosophila) have been shown to regulate axon regrowth after injury [11,12] and axon regeneration, respectively [9]. In addition, more recent observations have started to highlight the role of DLK in controlling the regenerative response in the mammalian nervous system [13,15,16]. These studies suggest that a MAPK signal pathway that controls the axonal response to injury might be conserved among model organisms.
However, our understanding of the upstream and downstream components of the DLK pathway remains fragmentary, and it is unknown whether these components are conserved among different organisms and classes of neurons. Here, we discuss evidence gathered from several models, which together support a multifaceted role for DLK-dependent signalling in regulating aspects of neural development, degeneration and regeneration after injury.
Neural development
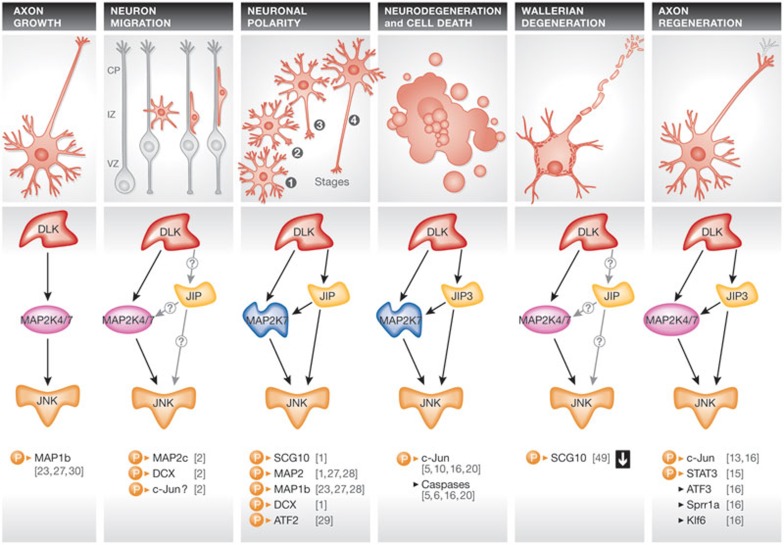
The functionality of the nervous system relies on its correct development. Early stages of neural development are characterized by neuronal migration followed by extensive growth of axons and dendrites, and later growth by synapse formation and refinement of functional connections within a neuronal network. Experimental evidence reveals different roles for the DLK–JNK signalling pathway in vivo during neural development including axon formation and neuronal migration, as well as neuronal apoptosis and axon degeneration (Fig 1; [1,2,3,5,6]).
Figure 1.
DLK pathways controlling contradictory responses in mammalian neurons. Under certain circumstances, DLK initiates a coordinated sequence of phosphorylation events culminating in the activation of JNK activity. On activation, JNK can phosphorylate various intracellular targets. Interaction with JIPs directs JNK activity towards specific neuronal responses. ATF2/3, activating transcription factor 2/3; CP, cortical plate; DCX, doublecortin; DLK, dual leucine zipper kinase; IZ, intermediate zone; JIP, JNK-interacting protein; JNK, c-Jun amino-terminal kinase; Klf6, Kruppel-like factor 6; MAP1b/2c, microtubule associated protein 1b/2c; MAP2K4/7, mitogen-activated protein kinase kinase 4/7; SCG10, superior cervical ganglion 10; Sprr1a, small proline-rich protein 1A; STAT3, signal transducer and activator of transcription 3; VZ, ventricular zone.
Axon growth and neuronal migration
Axon formation and neuronal polarization are fundamental steps during neural development that allow for directional transmission of information within the fully developed nervous system [21,22]. The DLK protein localizes to axons, and it is found in several areas of the developing mammalian nervous system such as the brain, spinal cord and sensory ganglia [2,19]. Dlk null mice have neuronal migration defects and hypoplasia of several axonal tracts including those of the anterior commissure and the corpus callosum [2,3,23]. A reduction in the number of axons is also seen in the lateral olfactory tract, cingulum and internal capsule [2]. Most dlk mutant mice eventually die during the perinatal period. Defects similar to those seen in dlk mutant mice are also found on conditional deletion of phr1, one of the ubiquitin ligases upstream from DLK [3]. However, DLK protein levels are unchanged in phr1 mutant brains, suggesting that the defects in phr1 mutants are not due to changes in DLK expression.
DLK induces JNK activity in vitro [18]. In mammals, activated JNK phosphorylates a wide range of downstream targets, including nuclear substrates (transcription factors and hormone receptors, HNRPK and TIF-IA) and non-nuclear substrates involved in protein degradation (E3 Itch), apoptosis (Bcl2 family members Bax and Bad), signal transduction (JIP1, Shc) and cell motility (keratin 8, DCX, MAP1B and MAP2B, tau, SCG10, kinesin, paxillin; [24]). In the absence of DLK, JNK activity and the phosphorylation of several JNK targets decreases during mouse brain development [2]. Importantly, forced expression of active JNK1 rescues axon formation defects caused by DLK silencing in cultured mouse cortical neurons [1].
In mammals there are three jnk genes (jnk1, jnk2 and jnk3), and in total, at least ten splice variants originate from alternative splicing events. Thus, assessing the consequences of jnk deletion on axon formation in vivo has been problematic [25,26]. Nevertheless, genetic deletion of a single family member, jnk1, is sufficient to alter the integrity of the neuronal cytoskeleton, resulting in disrupted axonal tract formation in the mouse developing neocortex [27,28].
Greater defects are seen in dlk;jnk1 double-mutant mice than dlk and jnk1 single-mutant mice [1], further supporting the hypothesis that DLK–JNK signalling is actively involved in neural development. Several axonal tracts (for example, corpus callosum and anterior commissure), and neuronal structures (for example, internal capsule, hippocampus, plexiform layers and glomerular layer) are either significantly reduced or absent in dlk;jnk1 double mutants [1]. By contrast, the peripheral nervous system and a few other brain structures develop normally in dlk;jnk1 double-mutant mice.
Axon formation has been extensively studied by using cultured hippocampal and cortical neurons [22]. Whilst the JNK protein is uniformly distributed, active phospho-JNK localizes to the axon compartment of cultured embryonic rat hippocampal neurons [29]. Importantly, such compartmentalized expression is present through all subsequent stages of development. It is probable that DLK-mediated local activation of MAP2Ks constrains JNK activity to neurites that are beyond the critical length for axon specification in cultured embryonic rat hippocampal neurons [29]. In line with this hypothesis, a study has shed light on how DLK-mediated activation of MAP2K7 might position JNK signalling modules in the neurite shaft to control microtubule bundling in cultured embryonic mouse hippocampal neurons [30]. Moreover, JNK inhibition through pharmacological and dominant-negative approaches results in axon formation defects without affecting dendrites in cultured embryonic rat hippocampal neurons [29]. Thus, in accordance with the in vivo findings, activation of DLK–JNK signalling is crucial for axonogenesis, as well as the maintenance of neuronal polarity in cultured cells.
Cytoskeleton components provide structural support for growing axons. Continuous remodelling of the actin-based cytoskeleton, together with changes in microtubule stability, influence neuronal polarization [31,32,33]. Several microtubule regulators including SCG10, MAP2, tau, MAP1B, CLIPs and DCX influence axon formation [34,35,36,37]. Indeed, silencing of SCG10, DCX and MAP2 microtubule modulators, which serve as substrates for the DLK–JNK pathway, causes an accumulation of multi-polar mouse cortical neurons in vitro [1]. It is interesting to note that moderate microtubule stabilization can overcome stage-specific defects seen in the polarization of cultured cortical neurons from dlk;jnk1 mutant mice [1].
In addition to controlling axon formation, the DLK–JNK pathway is recognized as a crucial regulator of radial migration during mouse corticogenesis [2,4,38]. Radial and tangential migration of newly generated neurons contributes to neocortex formation and is often associated with axon formation [39,40]. The expression of DLK and phospho-JNKs is higher in the mouse cortical intermediate zone than in the ventricular zone and cortical plate at embryonic day (E) 16. A reduction in DLK protein levels and JNK activity is seen as soon as differentiating cortical neurons reach the sub-plate zone [4]. Without affecting radial glia cell architecture, dlk gene disruption results in incorrect positioning of neurons throughout the cortical plate in vivo. Moreover, JNK pharmacological inhibition alters the migration rate of cortical neurons in slice cultures [2]. Absence of DLK as well as JNK inhibition delays radial migration of neuronal cells, disrupting lamination of the mouse cortical plate [2]. Interestingly, DLK overexpression in neural precursor cells leads to an accumulation of neurons in the subventricular zone at E16. By contrast, overexpression of a DLK mutant lacking kinase activity does not have an impact on neuronal migration, indicating that DLK kinase activity is essential for this phenotype [4].
Taken together this collection of results suggests that temporally and spatially controlled DLK–JNK signalling is required for axon growth and corticogenesis during mammalian neural development.
Neuronal apoptosis and axon degeneration
During development, an excess of neurons is generated. However, only those that make stable and functional connections survive. Thus, the accuracy and establishment of functional connections within a neuronal network requires not only axon and dendrite growth, but also neuronal apoptosis and axon degeneration. In fact, axon degeneration is a major mechanism responsible for large-scale elimination of exuberant projections and unstable synaptic contacts [41].
Extracellular factors including NGF are necessary for the survival of sympathetic and sensory neurons. Consistent with an active role in modulating the stress response, the absence of DLK protects cultured mouse embryonic DRG sensory neurons from cell death and axon degeneration induced by NGF withdrawal—a condition that mimics in vivo competition for trophic factors [5]. DRG neurons in vivo are present in similar numbers in dlk mutants and control littermates at E12.5. After developmental apoptosis has occurred, however, a significant decrease in the number of DRG neurons is seen in control mice but not in dlk mutants at E17.5. Thus, DLK is a positive regulator of developmental apoptosis in mouse DRG sensory neurons [5]. DLK activates stress-induced JNK signalling without affecting JNK basal activity in these neurons. Such complex regulation is achieved by interaction with the scaffold protein JIP3 to form a specific signalling module together with MAP2K, directing JNK activity towards precise functions (Fig 1; [5]). In projecting axons, the DLK protein is also found at the growth cone [42]. Therefore, DLK might be transported retrogradely to activate stress pathways in the nucleus [5]. It has been shown that DLK–JNK positively regulates neuronal apoptosis and axon degeneration in a c-Jun-dependent and -independent manner, respectively [5].
When approaching target cells, multiple neurons compete to form synapses. In particular, the establishment of neuromuscular connections controls the survival of spinal motor neurons. As a result of competition, about 50% of spinal motor neurons die during embryonic development. More than twice as many spinal motor neurons are found in dlk mutant mice compared with control animals [5,6]. As the number of hb9-positive cells committed to becoming spinal motor neurons is comparable to that in control mice at E12.5, it is probable that the absence of DLK reduces apoptosis rather than enhancing motor neuron specification. Interestingly, a similar number of spinal motor neurons to that observed during embryonic development is also found at six months of age in dlk mutants, with no signs of neural atrophy [6]. Genetic deletion of bax, a proapoptotic member of the Bcl2 family, significantly suppresses apoptosis during mouse neural development [43]. Although rescued from apoptosis, bax-deleted motor neurons show clear signs of atrophy during the perinatal period. By contrast, dlk mutant motor neurons are apparently healthy, supporting the hypothesis that DLK promotes additional cell-autonomous responses including axon degeneration in conditions within which apoptosis does not occur [5,6]. Collectively, these results provide evidence for a role of DLK in controlling developmental apoptosis and axon degeneration in different classes of neurons.
Degenerative responses to insults
Neuronal degeneration occurs not only during development, but also in response to various insults including neurotoxicity, demyelination, ischaemia and trauma as well as in neurodegenerative diseases. Morphological changes in neuronal cell bodies in response to stress include displacement of the nucleus towards the periphery of the pericaryon, swelling of the cell body and spreading of large Nissl bodies due to fragmentation of rough endoplasmic reticulum. Axons are vulnerable, highly specialized structures that require maintenance through their entire lifespan. In fact, changes in body size, body movement and ageing constantly challenge the integrity of axonal structures. As a consequence of axonal lesion, loss-of-maintenance factors probably trigger an axonal self-destruction programme called Wallerian degeneration in the distal stump. It is worth noting that Wallerian degeneration after axonal injury should be distinguished from developmental axon pruning. Despite the fact that the two phenomena share several substrates, the mechanisms differ [44]. Studies have underscored a role for DLK-dependent signalling in promoting apoptosis and axon degeneration under different experimental conditions including models of Wallerian degeneration and neurodegeneration (Fig 1).
Cell body response
Recent findings offer an intriguing basis for a possible involvement of DLK-mediated signalling in the pathophysiology of neurodegenerative diseases such as Parkinson disease and optic neuropathies.
By using a viral-mediated delivery approach, dominant-negative forms of DLK suppress neuronal apoptosis in dopamine neurons in a 6-OHDA mouse model of Parkinsonism [20]. After DLK inhibition, neuroprotective and trophic support seems to correlate with inhibition of c-Jun phosphorylation in dopamine neurons [20]. Thus, DLK might activate JNK to induce neuronal cell death through phosphorylation of c-Jun in this model. Given that DLK inhibition has no effect on nigrostriatal projections, these results support the idea that molecular pathways responsible for neuronal apoptosis differ from those mediating axonal degeneration.
More recently, a functional genomic screen has identified DLK as a crucial mediator of neuronal cell death in mammalian models of optic neuropathies, such as glaucoma and after optic nerve injury. After screening an extensive library of 1,869 short interfering RNAs, targeting more than 600 kinases, knockdown of DLK and its downstream substrate MAP2K7 have been found to protect cultured mouse RGCs from cell death [10]. Injuries to the optic nerve cause considerable RGC death after two weeks. DLK conditional deletion promotes mouse RGC survival after optic nerve injury in vivo [10,16]. Once again, the increase in survival correlates with a decrease in JNK, c-Jun phosphorylation and activated caspase 3. In response to optic nerve lesion, DLK seems to be upregulated within one day in RGC axons and within three days in the RGC body, raising the possibility that DLK might trigger a stress response leading to cell death [10,16]. In line with this hypothesis, whilst DLK overexpression accelerates RGC death, overexpression of kinase-dead DLK promotes RGC survival [10]. Moreover, DLK pharmacological inhibition using tozasertib, a protein kinase inhibitor, results in rat RGC survival after optic nerve transection and in a glaucoma model induced by increasing intra-ocular pressure [10]. However, the broad action of tozasertib might result in inhibition of other kinases in addition to DLK. Therefore, developing more specific inhibitors should be a priority in order to validate and extend these highly relevant findings into more clinically applicable strategies.
Altogether, these observations suggest that activation of a DLK stress-induced pathway leads to proapoptotic JNK activation in models of CNS degeneration.
Axonal response
In addition to changes in neuronal cell bodies, axonal injuries trigger an active self-destruction programme called Wallerian degeneration in the distal part of the axon. Preventing Ca2+ influx, inhibiting protein degradation and overexpressing Nmnat2 and the chimaeric Wlds delay Wallerian degeneration [45,46,47]. Despite progress, our understanding of the molecular pathways that regulate this process remains limited [48]. Absence of the DLK homologue Wallenda preserves axons from degeneration in a Drosophila model of olfactory receptor–neuron axotomy [7]. In cultured mouse embryonic DRG neurons, DLK-deficient axons have a marked decrease in degeneration when compared with controls after axotomy. Similar results are also seen after in vivo sciatic nerve transection in adult mice [7]. As already mentioned, axon degeneration occurs in response to various insults including neurotoxicity. Absence of DLK also protects cultured DRG axons from vincristine-induced fragmentation, further supporting the hypothesis that DLK signalling might function in an axon self-destruction programme [7]. Although DLK can activate both JNK1–3 and p38α-δ MAPK pathways in response to injury, pharmacological inhibition of JNK within the first three hours after axotomy is sufficient to significantly inhibit axon fragmentation in cultured DRG neurons [7]. These results suggest that early JNK activity is necessary for the commitment to degenerate after injury, before breakdown occurs. A more recent study provides a better understanding of a JNK-controlled downstream mechanism that commits axons to degenerate after injury. Experimental evidence has shown that the JNK substrate SCG10, a microtubule-binding protein, is important in axon maintenance. Early after injury, SCG10 protein levels dramatically decrease in the distal axon compartment of both cultured mouse DRG neurons and in adult sciatic nerves. Of note, a decrease in SCG10 expression occurs within the first few hours after injury, before any sign of axon fragmentation in vitro. Moreover, SCG10 knockdown using short hairpin RNA lentiviral constructs accelerates degeneration of cultured DRG neurons after axonal injury [49]. Unlike NMNAT2, absence of SCG10 does not trigger axon degeneration, supporting the idea that loss of SCG10 might represent a functionally important step during the commitment to axon degeneration [49].
Compared with DLK inhibition, Wlds expression and Ca2+ chelation are more effective in protecting injured axons, suggesting that activation of DLK-mediated signalling is acting in parallel with other responses. A striking loss-of-function phenotype has been characterized in fruit flies carrying mutations in Highwire (rpm-1 in C. elegans and phr1 in mice), a gene encoding an E3 ubiquitin ligase that is also an upstream regulator of Wallenda. Highwire mutations markedly inhibit axon degeneration in a Drosophila model of Wallerian degeneration [8]. Intriguingly, NMNAT has been identified as a target downstream of Highwire. At post-transcriptional levels, Highwire promotes NMNAT downregulation in the distal stump of injured axons independently from an effect on Wallenda [8]. Even though earlier studies have demonstrated that inhibition of the UPS results in delayed axon degeneration in Drosophila [44,50,51], findings suggest that Highwire downregulates NMNAT in a UPS-independent manner [8]. In the absence of Highwire, increased NMNAT protein levels are indeed required and are sufficient to inhibit axon degeneration in several classes of neurons and developmental stages in Drosophila [8]. The mechanism by which Highwire regulates NMNAT remains to be explored in future studies.
Taken together, these results suggest that DLK functions in an internal neuronal signalling pathway to promote axon degeneration after injury.
Regenerative response to injury
Injuries in the adult mammalian CNS lead to permanent functional impairments, severely hindering daily activities and decreasing the quality of life. Adult CNS neurons not only fail to regenerate, but also have a reduced sprouting ability, both of which contribute to the limited functional recovery after injury [52]. However, regeneration spontaneously occurs in most invertebrates, cold-blooded animals including amphibians, fish, reptiles and in the mammalian PNS. Why do some species successfully regenerate whilst others fail? What are the molecular pathways accounting for the differences in the growth ability of PNS and CNS neurons?
Animal models such as C. elegans offer several experimental advantages for studying axon growth and regeneration failure [53,54]. Given the short life cycle, large progeny number, detailed genetic map and low maintenance costs, more and more laboratories use C. elegans as a model organism for extensive genetic screening and to study signal transduction pathways. Several candidate genes identified in worm, fruit fly and zebrafish models, with a role in promoting or inhibiting regeneration, await testing in rodents. Although some signalling pathways might be conserved, injury-related changes to the neuronal environment and immune system differ among species. Nonetheless, translational studies using mammalian models have resulted in exciting findings, highlighting a crucial role for DLK-dependent mechanisms in regulating the regenerative response to injury [15,16]. In the following paragraphs, we provide an overview of the DLK-dependent regenerative response to injury and discuss its implications.
MAPK signalling in axon regeneration
Many studies have demonstrated a role for a conserved MAPK pathway in axon regeneration using different animal models [11,12,13,14,15]. Although C. elegans and Drosophila neurons spontaneously regenerate after laser axotomy, growth cones never form in dlk-1 and wallenda mutants [9,11,12]. The transformation of severed axonal ends into growth cone-like structures is one of the crucial steps in mounting a successful regenerative response [55]. Growth cones are specialized structures at the leading edge of developing and regenerating axons. Continuous polymerization and depolymerization of actin filaments, together with changes in microtubule dynamics, allows growth cones to guide axons to reach their specific cellular targets during neural development and axonal regeneration. Whether signalling pathways required for neuronal development are also activated during axon regeneration is still a matter of debate [56,57].
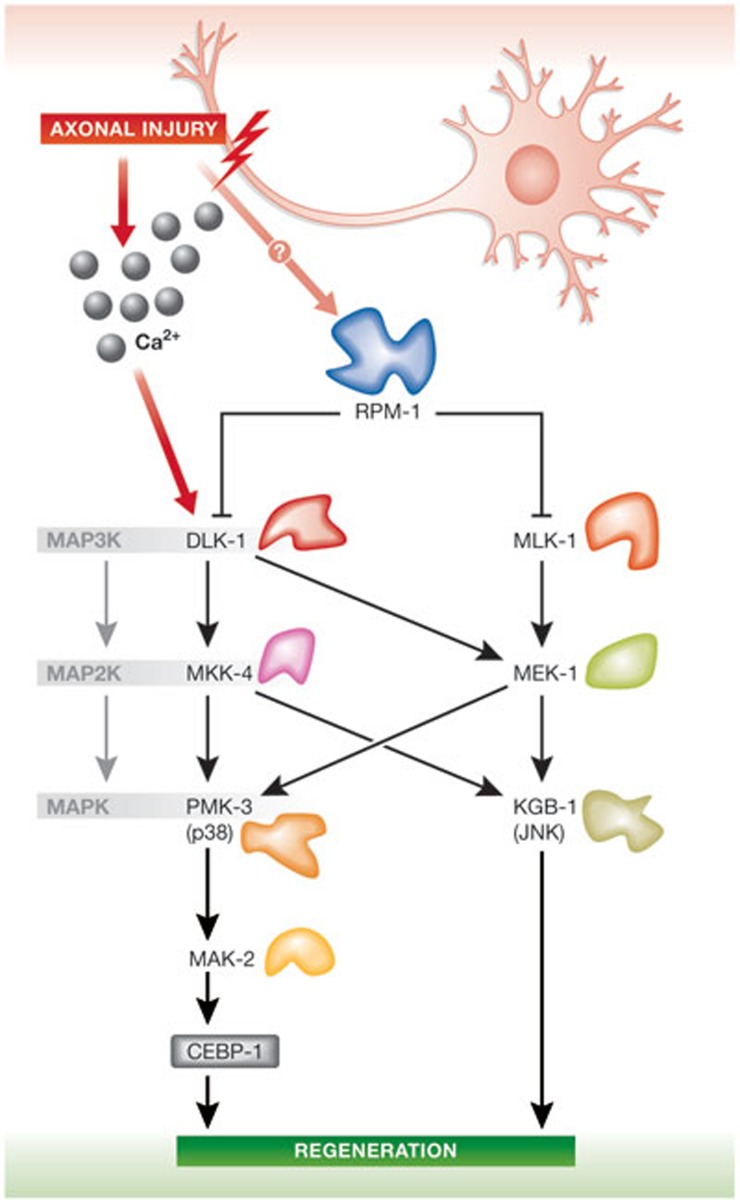
Numerous building blocks are necessary during the assembly of a new growth cone after injury. Raw materials are recycled from axonal debris, transported along the axon or synthesized locally. The DLK-1 pathway regulates cebp-1 mRNA stability through the MAPKAP kinase MAK-2 in C. elegans [12]. It has been shown that growth cones do not form in C. elegans cebp-1 mutants, suggesting that regulation of cebp-1 mRNA stability and translation is an important step to promote axon regrowth after injury (Fig 2; [12]). When axons successfully regenerate, a dynamic growth cone replaces transient filopodia that emerge from the axonal stump within hours after injury. Specialized structures such as filopodia and lamellipodia emerge from growth cones, serving as anchor points both to sustain growth and actively integrate guidance cues of the extracellular environment. Real-time imaging experiments suggest that DLK-1 signalling might be required for the filopodia to growth cone transition in C. elegans [11]. Furthermore, restricted expression of DLK-1 in GABA motor neurons rescues regenerative failure seen in C. elegans dlk-1 null mutants, suggesting that DLK-1 functions in a cell-autonomous manner [11]. Through highly conserved ubiquitin-mediated protein degradation, the DLK-1 pathway is negatively regulated by RPM-1 [42,58,59]. Consistently, defects similar to those found in dlk-1 mutants are also seen after RPM-1 overexpression [11]. Loss-of-function mutations in the DLK-1 downstream targets MAP2K mkk-4 and p38 MAPK homologue pmk-3 result in regeneration defects in C. elegans, further supporting the finding that activation of the entire signalling pathway is required for axon regeneration in C. elegans [11]. The MAP3K MLK-1 pathway has also been found to control axon regeneration of C. elegans GABA motor neurons. In fact, mutations in mlk-1 or its downstream target mek-1 cause regeneration defects [14]. Moreover, neurons fail to regenerate when carrying mutations in the MAPK kgb-1/jnk, a MEK-1 downstream target [14]. Crosstalk between DLK-1- and MLK-1-signalling pathways can explain differences in phenotype severity. In fact, results suggest that a coordinated activation of JNK and p38 MAPK pathways is required for axon regeneration in C. elegans (Fig 2; [14]).
Figure 2.
Two MAPK pathways promoting axon regeneration in Caenorhabditis elegans. Injury signals including Ca2+ influx trigger activation of DLK-1 and the DLK-1–MKK-4–PMK-3 pathway. In parallel to the DLK-1 pathway, the MLK-1–MEK-1–KGB-1 pathway is also activated. Whilst DLK-1 can activate both MKK-4 and MEK-1, MLK-1 can only activate MEK-1. CEBP-1, CCAAT/enhancer-binding protein 1; DLK-1, dual leucine zipper kinase 1; JNK, c-Jun amino-terminal kinase; MAPK, mitogen activated protein kinase; MAP2K, mitogen-activated protein kinase kinase; MAP3K, mitogen-activated protein kinase kinase kinase; MKK-4, MAP kinase kinase 4; RPM-1, regulator of presynaptic morphology 1.
Interestingly, delayed DLK-1 expression results in limited regeneration [11], indicating that DLK-1 must function shortly after injury, presumably in close relation to other phenomena. In fact, a successful regenerative response requires concomitant activation of multiple events. For example, axotomy triggers rapid entry of extracellular ions through opening of the plasma membrane [60]. In Aplysia, Ca2+ influx represents an important step to activate programmes leading either to the formation of a new growth cone or a retraction bulb [61]. Recent work has shed light on how DLK-1 activity is triggered in response to injury in C. elegans [62,63]. Changes in Ca2+ concentration regulate the switch between an inactive and active DLK-1 protein complex [63]. In mammals, however, DLK lacks the domain that allows activity regulation through Ca2+ binding. LZK, another mammalian orthologue, has such a domain, but its role in axon growth and regeneration is not known and deserves attention for future studies.
Taken together, these results demonstrate that DLK-1 initiates a regenerative response to axonal injury by simultaneous activation of JNK and p38 MAPK pathways in non-mammalian model systems.
Microtubule dynamics
Considerable attention has been focused on understanding how the actin and microtubule cytoskeleton network regulates axon growth during neural development as well as after axonal injuries in model organisms. The microtubule network of mature axons is composed of stable and labile microtubules [64], and is normally maintained in a stabilized steady state. In mammals, microtubules disassemble after axonal injury, hindering the regrowth of injured CNS axons [65]. When severed, most mammalian CNS axons form retraction bulbs and die-back, retracting from the site of injury. Dystrophic end bulbs are filled with a disorganized microtubule network [65]. Of note, moderate microtubule stabilization results in axon regeneration within the injured CNS [66,67].
Although progress has been made in temporally characterizing the axonal response to injury, the molecular pathways underlying the morphological changes remain elusive.
Taking advantage of versatile animal models and femtosecond laser axotomy, real-time imaging approaches have begun to explore the role of specific pathways in both growth cone formation and axon regeneration after injury. By activating microtubule growth, C. elegans D-type larval motor neurons rapidly transform proximal axonal stumps into growth cone-like structures in response to laser axotomy [68]. Activation of microtubule growth follows distinct phases in C. elegans mechanosensory axons. First, an increase of dynamic microtubules at the site of injury is accompanied by local downregulation of KLP-7, a depolymerizing kinesin 13 family member that controls steady state conditions [69]. Thereafter, the cytosolic carboxypeptidase CCPP-6A is required for the formation of more stable microtubules during a period of sustained growth [69]. Interestingly, the DLK-1 signalling pathway controls both aspects of microtubule growth in C. elegans mechanosensory axons, as discussed below.
In addition to the DLK-1 pathway, an extensive functional screen has identified several clusters of genes required for axon regrowth in C. elegans, many of which regulate microtubule dynamics [70]. Interestingly, C. elegans GEF efa-6 mutants display increased axon growth within a few hours of injury [70]. GEFs are known to activate several GTPases, including members of the Ras superfamily. Cdc42, Rac1 and RhoA are members of the Rho family of GTPases (a subfamily of Ras), and they control many aspects of cytoskeletal dynamics in mammals [33,71,72]. Morphological analysis of efa-6 mutants in C. elegans has revealed a substantial increase in the number of dynamic microtubules as well as extended periods of persistent growth, all resulting in enhanced axon regrowth compared with wild-type worms [70]. In line with this, earlier work has suggested that EFA-6 limits microtubule growth by decreasing microtubule dynamics in C. elegans [73]. Of note, the efa-6 mutation rescues, at least in part, axon regeneration defects seen in C. elegans dlk-1 mutants, indicating that EFA-6 functions downstream of and/or in parallel to DLK-1 [70]. Other studies had further indicated that a DLK–MAP2K7–JNK1 signalling module regulates microtubule bundling during neurite elongation in cultured mouse hippocampal neurons [30]. The existence of locally activated DLK–JNK modules is intriguing and should stimulate future investigation about the possible relationship of these modules with microtubule cytoskeleton dynamics in more complex mammalian models.
In conclusion, these observations demonstrate that DLK-dependent signalling controls several aspects of microtubule growth in diverse model organisms.
Retrograde signals
Axonal injuries trigger signals that must travel from the site of injury back to the neuronal cell body [74,75,76]. In addition to controlling survival pathways, retrograde transport of injury-activated factors leads to profound changes in the expression of genes related to cytoskeletal dynamics and axon growth [76,77,78]. Therefore, for a neuron, sensing the degree of damage is one of the first priorities in the process of making a decision whether to regenerate or undergo apoptosis. As part of the activation mechanism at the axon terminal, several factors including JNK and Erk are phosphorylated and transported to the nucleus through retrograde motor complexes [75,79,80]. In Drosophila motor neurons, Wallenda-dependent signalling mediates the nuclear response to injury. It has been shown that a Wallenda–JNK–Fos-signalling module controls the sprouting ability of injured Drosophila motor neurons [9]. Most Drosophila motor neurons show signs of growth within 14 h of injury. However, the extent of growth significantly drops after knocking down Wallenda expression and when expressing JNK and Fos dominant-negative mutants [9].
Neuronal cell bodies are often located far away from the damaged area, thus injury-activated signals need to travel considerable distances to trigger a cell body response. When the axonal transport machinery is mutated, the motor neuron response is inhibited, suggesting that Wallenda requires the axonal transport machinery to shuttle injury-activated signals to the nucleus [9].
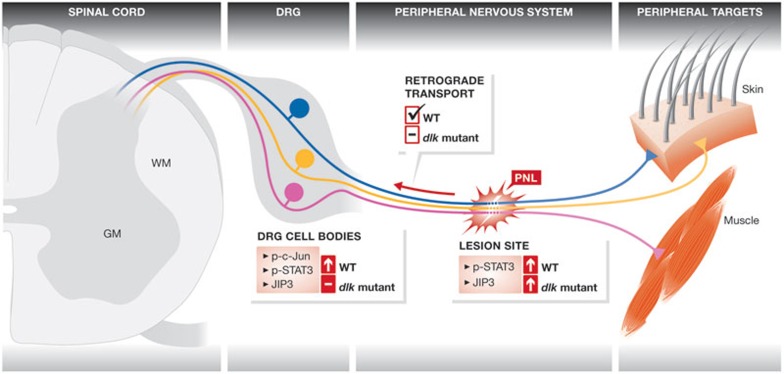
Several positive post-traumatic signals have been identified in mammals. Injury-induced cytokines including LIF, CNTF and IL-6 act through the gp130 receptor complex, upstream from the pro-regenerative JAK–STAT signalling pathway. Recent work has shed light on how mammalian sensory and motor axon regeneration is impaired in the absence of DLK (Fig 3). Data suggest that DLK might be involved in the correct translocation of pro-regenerative signals including phospho-STAT3 and phospho-c-Jun from the injury site to the neuronal cell body [13,15]. At the site of injury, phospho-STAT3 levels are increased normally in dlk null mice. However, retrograde transport of phospho-STAT3 and the adaptor protein JIP3 is abolished in the absence of DLK [15]. In rodents, impairment in retrograde transport does not seem to affect the local assembly of a new growth cone. Hence, absence of DLK might have an impact on growth cone performance rather than on growth cone assembly [15].
Figure 3.
Retrograde transport of injury-activated signals. After peripheral nerve lesion, locally activated regulators are retrogradely transported from the site of injury back to the mouse DRG cell body. Absence of DLK prevents the correct translocation of pro-regenerative signals including phospho-STAT3 and c-Jun needed to activate the intrinsic regeneration programme. p-c-Jun, phosphorylated c-Jun; DRG, dorsal root ganglion; DLK, dual leucine zipper kinase; GM, grey matter; JIP3, JNK-interacting protein 3; PNL, peripheral nerve lesion; STAT3, signal transducer and activator of transcription 3; WT, wild-type; WM, white matter.
Absence of phospho-STAT3 nuclear accumulation is constantly seen in model systems in which regeneration fails [81]. Conversely, increasing experimental evidence suggests that STAT3 activation is required for CNS regrowth [82,83]. It is most probable that activation of gp130–JAK–STAT3 signalling relays injury-activated signals to the nucleus, in which they potentially turn on an intrinsic regenerative response through regulation of gene transcription [84].
After optic nerve injury, DLK-dependent signalling triggers a rapid transcriptional response in mouse RGCs [16]. Interestingly, DLK-induced changes in the expression of proapoptotic and pro-regenerative genes provide further evidence for the contradictory actions of DLK [16]. Although DLK simultaneously activates proapoptotic and regenerative programmes in response to optic nerve injury, the dominant response in regenerative incompetent neurons such as RGC is cell death [10].
Collectively, these results suggest that the ability to activate nuclear responses, through retrograde transport of injury-related signals, might be a major mechanism by which DLK promotes axon regeneration in regeneration-competent neurons.
Strategies to fine-tune DLK signalling
Data from several research groups demonstrate that DLK activation early after injury participates in the nuclear response to injury [9,11,15,16]. How is DLK regulated in response to injury? In C. elegans and Drosophila, RPM-1 and Highwire negatively regulate DLK-1 and Wallenda, respectively. In C. elegans, it is unknown if RPM-1 is regulated in response to injury. However, Highwire expression decreases along the injured axons of Drosophila motor neurons, revealing a potential mechanism for Wallenda activation [9].
In C. elegans, regeneration is modestly enhanced in rpm-1 mutants as well as after DLK-1 overexpression, suggesting that an increase in DLK-1 expression improves the regeneration ability of GABA motor neurons [11]. Given that many factors influence the regenerative response in C. elegans neuron, it is not known whether these findings can be generalized to other classes of neurons and injury paradigm [54,85]. Nonetheless, an absence of Highwire accelerates regeneration within a few hours of injury in Drosophila motor neurons [9]. Together, these results suggest that increasing DLK protein levels directly, or indirectly by inhibiting RPM-1/Highwire, boosts nerve regeneration in both C. elegans and Drosophila.
In contrast to the above-mentioned model organisms, mammalian CNS neurons have a more limited ability to regenerate and a higher probability of undergoing apoptosis when regeneration fails. What are the consequences of an increase in DLK expression in mammalian CNS neurons? DLK overexpression results in increased RGC death in response to injury [10]. Given that injured CNS neurons must survive to regenerate their axons, it is probable that increasing survival might be sufficient to induce them to regenerate [86]. Although absence or inhibition of DLK protects mouse and rat RGC from injury-induced cell death, no signs of regeneration are noticed [10,16]. Furthermore, pten conditional deletion—a strategy to promote RGC regeneration [87]—fails to promote axon regeneration in the absence of DLK [16], thus DLK seems to be a crucial player in the initiation of the regenerative response. Taken together, these results indicate that an increase in DLK expression is not sufficient to induce regeneration in mammalian RGC neurons, however DLK is required for RGC regeneration under specific conditions.
Conclusion
Here we have presented and discussed evidence supporting a role for DLK-dependent signalling in regulating apparently contradictory responses during both neural development and after various insults to the adult nervous system. The ability of DLK to induce apoptosis and axon degeneration is in contrast with its role in promoting axon growth and regeneration. Although progress has been made, our understanding of the mechanisms underlying spatial and temporal activation of DLK in response to a multitude of stresses is still fragmentary (Sidebar A). It is not clear whether the upstream and downstream components of the DLK signalling pathway are conserved in different model organisms. Thus far, our knowledge of the role of DLK in controlling axon regeneration is mostly limited to studies performed in model organisms and systems in which injured axons spontaneously regenerate. It is probable that a context-dependent variability might have an impact on the translation of previous findings to more complex model systems and different injury paradigms. Mammalian CNS neurons, for example, have a limited ability to regenerate. Abortive responses and cell death often lead to permanent neurological deficits due to the failure to re-establish functional connections after CNS trauma, stroke and other types of neurodegenerative disease. Failure of neuroregeneration and repair is partly due to the presence of a hostile environment in the CNS [88,89]. Therefore, future work should aim at understanding how modulation of DLK activity alone, and in combination with other approaches, might aid neuronal survival, CNS repair and regeneration. We believe that progress in this direction will support the development of more applicable strategies to promote repair of the adult mammalian CNS following various insults.
Sidebar A | In need of answers.
How is temporal and spatial activation of DLK controlled in response to cellular stresses and signals in mammals?
What are the context-dependent mechanisms that shift the balance between contradictory DLK-dependent responses?
Does pharmacological inhibition of DLK positively affect the neuron response in models of inflammatory, metabolic, stroke and vascular diseases?
Does the level of DLK activity change in systems that do and do not spontaneously regenerate after injury?

Andrea Tedeschi & Frank Bradke
Acknowledgments
We would like to thank Farida Hellal, David Elliott and Charlotte Coles for critical reading of the manuscript. We apologize to all colleagues whose contributions to this field could not be cited due to space restrictions. The Deutsche Forschungsgemeinschaft, IRP and Wings for Life support research in the Bradke Laboratory.
Footnotes
The authors declare that they have no conflict of interest.
References
- Hirai S, Banba Y, Satake T, Ohno S (2011) Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway. J Neurosci 31: 6468–6480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Cui de F, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S (2006) The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci 26: 11992–12002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Miller BR, Sanes JR, DiAntonio A (2007) The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev 21: 2593–2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Kawaguchi A, Hirasawa R, Baba M, Ohnishi T, Ohno S (2002) MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in telencephalon of mouse embryo. Development 129: 4483–4495 [DOI] [PubMed] [Google Scholar]
- Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW (2011) DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 194: 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Horiuchi M, Wakayama K, Xu J, Bannerman P, Pleasure D, Itoh T (2011) ZPK/DLK, a mitogen-activated protein kinase kinase kinase, is a critical mediator of programmed cell death of motoneurons. J Neurosci 31: 7223–7228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A (2009) A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 12: 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X et al. (2012) The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol 10: e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA (2010) Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 191: 211–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsbie DS et al. (2013) Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci USA 110: 4045–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M (2009) Axon regeneration requires a conserved MAP kinase pathway. Science 323: 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Wu Z, Chisholm AD, Jin Y (2009) The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 138: 1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T (2009) Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun 383: 258–262 [DOI] [PubMed] [Google Scholar]
- Nix P, Hisamoto N, Matsumoto K, Bastiani M (2011) Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci USA 108: 10738–10743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A (2012) Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 74: 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA et al. (2013) DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci USA 110: 4039–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzman LB, Merritt SE, Fan G (1994) Identification, molecular cloning, and characterization of dual leucine zipper bearing kinase. A novel serine/threonine protein kinase that defines a second subfamily of mixed lineage kinases. J Biol Chem 269: 30808–30817 [PubMed] [Google Scholar]
- Gallo KA, Johnson GL (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3: 663–672 [DOI] [PubMed] [Google Scholar]
- Hirai S, Kawaguchi A, Suenaga J, Ono M, Cui DF, Ohno S (2005) Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr Patterns 5: 517–523 [DOI] [PubMed] [Google Scholar]
- Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, Kholodilov N, Burke RE (2008) Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci 28: 672–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polleux F, Snider W (2010) Initiating and growing an axon. Cold Spring Harb Perspect Biol 2: a001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S, Bradke F (2009) Neuronal polarity. Cold Spring Harb Perspect Biol 1: a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K (2010) Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci Res 66: 37–45 [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Kobe B (2006) Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev 70: 1061–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22: 667–676 [DOI] [PubMed] [Google Scholar]
- Brecht S et al. (2005) Specific pathophysiological functions of JNK isoforms in the brain. Eur J Neurosci 21: 363–377 [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M (2003) JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell 4: 521–533 [DOI] [PubMed] [Google Scholar]
- Bjorkblom B, Ostman N, Hongisto V, Komarovski V, Filén JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET (2005) Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci 25: 6350–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva AA, Atkins CM Jr, Copenagle L, Banker GA (2006) Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci 26: 9462–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltrin D, Fusco L, Witte H, Moretti F, Martin K, Letzelter M, Fluri E, Scheiffele P, Pertz O (2012) Growth cone MKK7 mRNA targeting regulates MAP1b-dependent microtubule bundling to control neurite elongation. PLoS Biol 10: e1001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG (1999) The role of local actin instability in axon formation. Science 283: 1931–1934 [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42: 897–912 [DOI] [PubMed] [Google Scholar]
- Witte H, Neukirchen D, Bradke F (2008) Microtubule stabilization specifies initial neuronal polarization. J Cell Biol 180: 619–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng J, Takei Y, Harada A, Nakata T, Chen J, Hirokawa N (2001) Synergistic effects of MAP2 and MAP1B knockout in neuronal migration, dendritic outgrowth, and microtubule organization. J Cell Biol 155: 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman P, Barg J, Rindzoonski L, Ginzburg I (1993) Subcellular localization of tau mRNA in differentiating neuronal cell culture: implications for neuronal polarity. Neuron 10: 627–638 [DOI] [PubMed] [Google Scholar]
- Bilimoria PM, de la Torre-Ubieta L, Ikeuchi Y, Becker EB, Reiner O, Bonni A (2010) A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J Neurosci 30: 16766–16776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neukirchen D, Bradke F (2011) Cytoplasmic linker proteins regulate neuronal polarization through microtubule and growth cone dynamics. J Neurosci 31: 1528–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Chihama K, Nabeshima Y, Hoshino M (2003) The in vivo roles of STEF/Tiam1, Rac1 and JNK in cortical neuronal migration. EMBO J 22: 4190–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Murakami F (2002) In vitro analysis of the origin, migratory behavior, and maturation of cortical pyramidal cells. J Comp Neurol 454: 1–14 [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7: 136–144 [DOI] [PubMed] [Google Scholar]
- Luo L, O'Leary DD (2005) Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28: 127–156 [DOI] [PubMed] [Google Scholar]
- Lewcock JW, Genoud N, Lettieri K, Pfaff SL (2007) The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56: 604–620 [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD (1998) Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18: 1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DD, Luo L (2006) Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron 50: 883–895 [DOI] [PubMed] [Google Scholar]
- Mack TG et al. (2001) Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci 4: 1199–1206 [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305: 1010–1013 [DOI] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR (2010) Wallerian degeneration, wlds, and nmnat. Annu Rev Neurosci 33: 245–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA (2012) Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol 196: 7–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE et al. (2012) SCG10 is a JNK target in the axonal degeneration pathway. Proc Natl Acad Sci USA 109: E3696–E3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z (2003) Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron 39: 217–225 [DOI] [PubMed] [Google Scholar]
- MacInnis BL, Campenot RB (2005) Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol Cell Neurosci 28: 430–439 [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z (2011) Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci 34: 131–152 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chisholm AD (2011) Axon regeneration mechanisms: insights from C. elegans. Trends Cell Biol 21: 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME (2012) Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci 13: 183–193 [DOI] [PubMed] [Google Scholar]
- Filbin MT (2006) Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci 361: 1565–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci 7: 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Wairkar YP, Johnson SL, DiAntonio A (2006) Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 51: 57–69 [DOI] [PubMed] [Google Scholar]
- Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y (2005) Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120: 407–420 [DOI] [PubMed] [Google Scholar]
- Yoo S, Nguyen MP, Fukuda M, Bittner GD, Fishman HM (2003) Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca(2+)-regulated proteins. J Neurosci Res 74: 541–551 [DOI] [PubMed] [Google Scholar]
- Kamber D, Erez H, Spira ME (2009) Local calcium-dependent mechanisms determine whether a cut axonal end assembles a retarded endbulb or competent growth cone. Exp Neurol 219: 112–125 [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD (2010) Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 30: 3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Jin Y (2012) Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron 76: 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FJ, Pienkowski TP, Baas PW (1993) Regional differences in microtubule dynamics in the axon. J Neurosci 13: 856–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertürk A, Hellal F, Enes J, Bradke F (2007) Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci 27: 9169–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellal F et al. (2011) Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science 331: 928–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D (2011) Taxol facilitates axon regeneration in the mature CNS. J Neurosci 31: 2688–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A (2004) Neurosurgery: functional regeneration after laser axotomy. Nature 432: 822. [DOI] [PubMed] [Google Scholar]
- Ghosh-Roy A, Goncharov A, Jin Y, Chisholm AD (2012) Kinesin-13 and tubulin posttranslational modifications regulate microtubule growth in axon regeneration. Dev Cell 23: 716–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L et al. (2011) Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron 71: 1043–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, Brakebusch C, Bamburg JR, Bradke F (2007) Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci 27: 13117–13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S et al. (2010) Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci 30: 6930–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SM, Christensen SN, Bowerman B (2010) Caenorhabditis elegans EFA-6 limits microtubule growth at the cell cortex. Nat Cell Biol 12: 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Cavalli V (2008) Nerve injury signaling. Curr Opin Neurobiol 18: 276–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V, Kujala P, Klumperman J, Goldstein LS (2005) Sunday Driver links axonal transport to damage signaling. J Cell Biol 168: 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K et al. (2012) Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 31: 1350–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Skene JH (1997) A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci 17: 646–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M et al. (2002) Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci 3: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M (2005) Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron 45: 715–726 [DOI] [PubMed] [Google Scholar]
- Hanz S et al. (2003) Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40: 1095–1104 [DOI] [PubMed] [Google Scholar]
- Schwaiger FW et al. (2000) Peripheral but not central axotomy induces changes in Janus kinases (JAK) and signal transducers and activators of transcription (STAT). Eur J Neurosci 12: 1165–1176 [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 25: 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M (2011) In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci USA 108: 6282–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A (2011) Tuning the orchestra: transcriptional pathways controlling axon regeneration. Front Mol Neurosci 4: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD (2007) Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci USA 104: 15132–15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Barres BA (2000) The relationship between neuronal survival and regeneration. Annu Rev Neurosci 23: 579–612 [DOI] [PubMed] [Google Scholar]
- Park KK et al. (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322: 963–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J (2007) The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17: 120–127 [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]