Abstract
Primordial germ cells (PGCs) and somatic cells originate from postimplantation epiblast cells in mice. As pluripotency is lost upon differentiation of somatic lineages, a naive epigenome and the pluripotency network are re-established during PGC development. Here we demonstrate that Prdm14 contributes not only to PGC specification, but also to naive pluripotency in embryonic stem (ES) cells by repressing the DNA methylation machinery and fibroblast growth factor (FGF) signalling. This indicates a critical role for Prdm14 in programming PGCs and promoting pluripotency in ES cells.
Keywords: DNA methylation, FGF signalling, pluripotency, Prdm14 , primordial germ cells
INTRODUCTION
Primordial germ cell (PGC) specification in mice commences in the proximal epiblast cells in response to BMP4 signalling at embryonic day (E) 6.25 just before the onset of gastrulation. By E7.25, approximately 30–40 founder PGCs are established following expression of the three key regulators: Prdm1 (BLIMP1), Prdm14 and Tcfap2c (AP2γ) [1–3]. The segregation of PGCs from neighbouring mesoderm progenitor cells entails repression and reversal of the initiation of the somatic programme, and re-establishment of the pluripotency network in conjunction with changes in chromatin modifications [4, 5].
Expression of Prdm14 is confined to PGCs and pluripotent cells only, where it has a critical role for the regulation of pluripotency genes, and it promotes resetting of the epigenome [3, 6]. Prdm14-deficient PGCs are specified, but fail to proliferate and are eventually lost during migration towards the genital ridges. The mutant PGCs exhibit diminished expression of Sox2 and Stella, and fail to show global histone methylation changes as observed in wild-type PGCs [5]. Prdm14 promotes a naive pluripotent state in differentiation-primed epiblast stem cells [6], while loss of Prdm14 in embryonic stem (ES) cells induces primitive endoderm (PE) fate [7]. PRDM14 is equally important for preventing differentiation of human ES cells, and can enhance somatic cell reprogramming [8, 9].
Here we explored the role of Prdm14 in PGCs and in mouse ES cells. We find that Prdm14 reverses and protects cells from acquiring somatic fates partly by attenuating mitogen-activated protein kinase (MAPK) signalling, thereby stabilizing a naive pluripotent state. Furthermore, Prdm14 represses the DNA methyltransferase machinery, further promoting naive pluripotency.
RESULTS AND DISCUSSION
Loss of PGC-specific gene expression
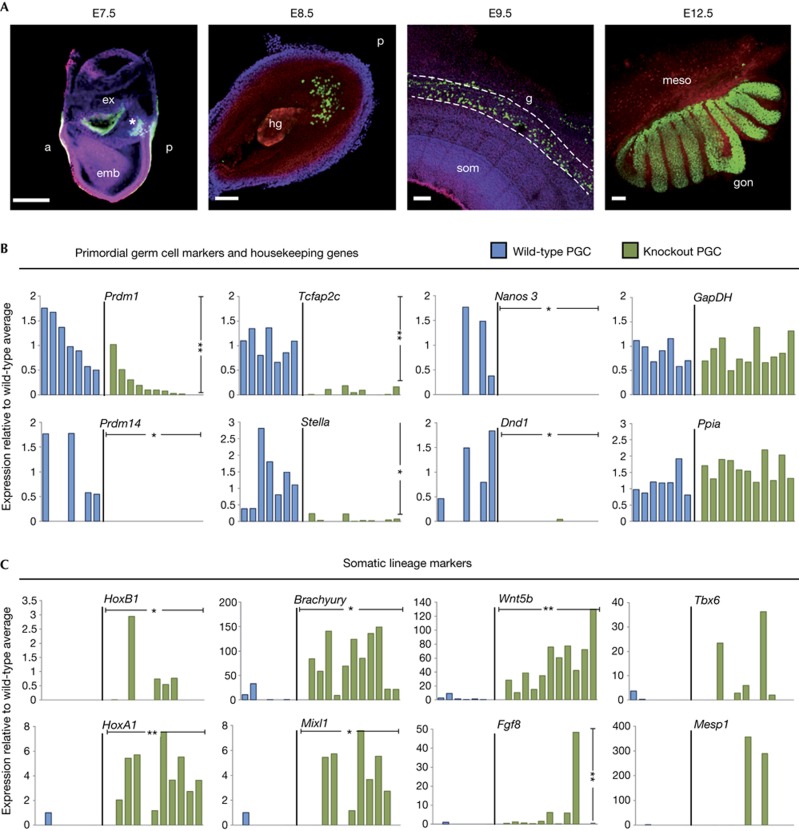
To investigate the consequences of loss of Prdm14 in the germline, we generated Prdm14-null mice (supplementary Fig S1A–C online), and examined gene expression in single-mutant PGCs. While development of wild-type PGCs follows an orderly expression of Prdm1 (BLIMP1), Stella, Tcfap2c, Nanos3 and Dnd1 (Fig 1A,B), Prdm14-null PGCs showed diminished expression of these genes (Fig 1B; supplementary Fig S1D,E online), as well as of regulators of pluripotency (supplementary Fig S1F).
Figure 1.
Loss of germline-specific expression and gain of somatic fate markers in Prdm14 mutant PGCs. (A) Stages of wild-type PGC development (PGCs marked by GFP in green). The founder population of PGCs forms a cluster (E7.5; Prdm1-GFP, marked by asterisk), enters the hindgut region (E8.5; Stella-GFP), migrates through the gut (E9.5; Oct4-ΔPE-GFP) and enters into the gonads (E12.5; Oct4-ΔPE-GFP) to continue their development into gametes. (scale bars=100 μm). (B, C) Gene expression levels relative to wild-type average, which is set to 1 (where expression was absent in all wild-type samples, expression levels are relative to knockout average) of individual wild-type (blue) or knockout (green) PGCs. Single cells were ordered according to levels of Prdm1 expression, with values normalized with Arbp. Transcript levels are shown for PGC genes and two housekeeping genes (Gapdh and Ppia; B) and somatic lineage markers (C). Welch’s t-test was used to calculate statistical significances of differences in expression levels (vertical bars) between wild-type and mutant PGCs. The χ2 test was used to determine statistical significances for expression frequency differences (horizontal bars). (*P<0.05; **P<0.01). a, anterior; E, embryonic day, emb, embryonic and ex, extra-embryonic regions; GFP, green fluorescent protein; hg, hindgut; g, gut; som, somites; meso, mesonephros; gon, gonad; PE, primitive endoderm; p, posterior; PGC, primordial germ cells.
Interestingly, genes associated with a somatic fate, such as HoxB1 and HoxA1, were derepressed in mutant PGCs at E8.5 (Fig 1C). Mutant PGCs showed, in particular, strong expression of primitive streak genes like Brachyury and Mixl1, which is in line with their shared developmental history with the neighbouring mesodermal cells. Notably, Wnt5b is strongly induced in mutant cells (Fig 1C), which is consistent with the location of PGCs posteriorly to the primitive streak. However, we did not observe upregulation of extra-embryonic endoderm genes in mutant cells (supplementary Fig S1G online), despite previous reports that Prdm14 represses them in ES cells [6].
Together, these results demonstrate that loss of Prdm14 causes loss of PGC identity by E8.5, which was less evident in the previous analysis at E7.5 [3]. Most notably, mutant cells acquire gene expression that is characteristic of adjacent somatic cells, indicating that Prdm14 is crucial for PGC specification by promoting expression of germ cell genes while repressing somatic genes.
Prdm14 modulates FGF signalling and DNA methylation
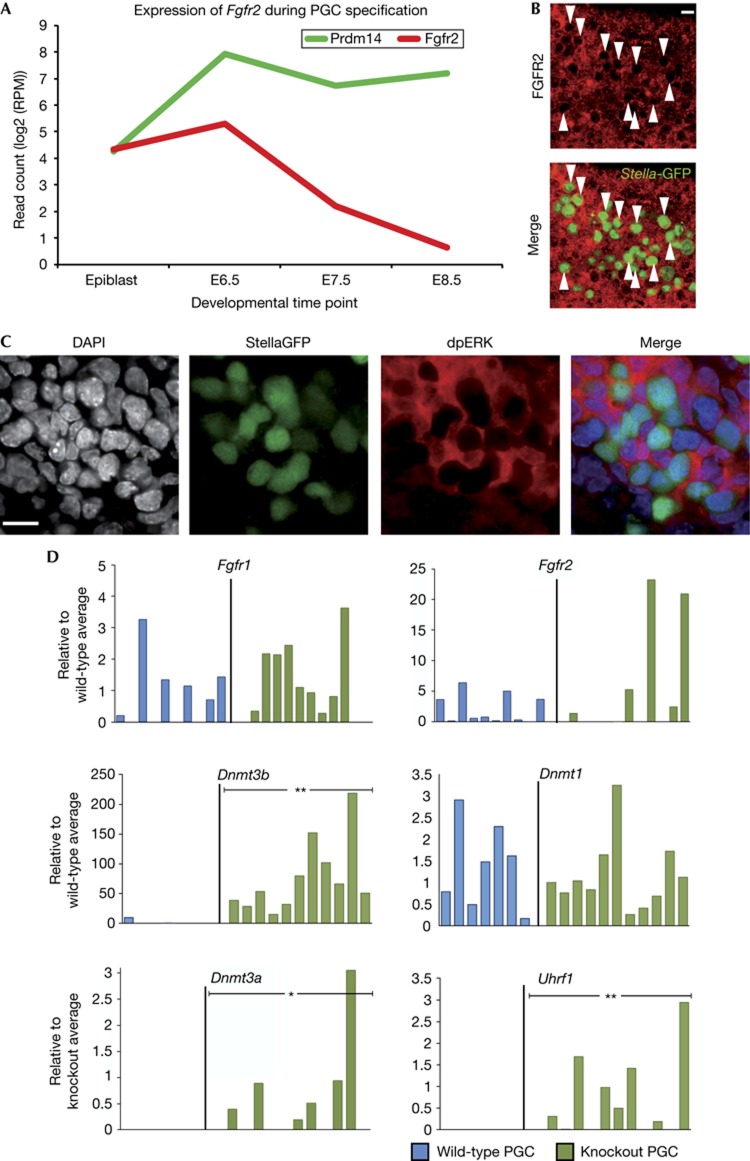
As initiation of lineage priming and perturbation of the pluripotency network are evoked by FGF signalling in ES cells [10], we examined the status of this pathway in PGCs. Indeed, single-cell transcriptome profiling of wild-type PGCs showed that Fgfr2 is specifically downregulated at the onset of Prdm14 expression (Fig 2A), which was confirmed by whole-mount immunostainings for FGFR2 in E8.5 embryos (Fig 2B). Intriguingly, PRDM14 was shown to bind and repress Fgfr2 in ES cells [7], suggesting a potentially direct regulation in PGCs as well.
Figure 2.
Prdm14-deficient PGCs fail to repress Fgfr2 and DNA methyltransferases. (A) Average changes in transcript levels of Prdm14 and Fgfr2 over the course of PGC specification determined by single-cell RNA sequencing of two wild-type cells. (B) Whole-mount immunostaining for FGFR2 (red) and PGCs, marked by a StellaGFP reporter (green, arrowheads) in an E8.5 wild-type embryo (scale bar=15 μm). (C) Whole-mount immunostaining for PGCs, marked by StellaGFP (green), and phosphorylated ERK (red) in wild-type embryos at E8.75 (scale bar=15 μm). (D) Expression of FGF receptors or genes involved in the regulation of DNA methylation in single wild-type (blue) or knockout (green) PGCs. Expression was normalized with Arbp and is shown relative to the average wild-type level, which was set to 1 (where expression was absent in all wild-type samples, expression levels are relative to knockout average). Statistical significances by χ2 test. (*P<0.05, **P<0.01). DAPI, 4,6-diamidino-2-phenylindole; E, embryonic day; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; GFP, green fluorescent protein; PGC, primordial germ cells.
We next examined extracellular signal-regulated kinase (ERK) phosphorylation as an indicator of MAPK pathway activity in migratory PGCs and found that while hindgut cells show strong phosphorylation of ERK, there was essentially no ERK phosphorylation in PGCs, further supporting the notion of PRDM14-induced repression of the MAPK pathway (Fig 2C). In addition, some mutant PGCs showed increased levels of Fgfr2, indicating that this might be due to the absence of Prdm14 (Fig 2D). Based on these observations, it is possible that the loss of Prdm14 causes increased sensitivity to FGF signalling, which could explain changes in gene expression in mutant PGCs and their subsequent elimination.
Development of PGCs is accompanied by the onset of DNA demethylation [5], which in part allows for reversal of the epigenetic silencing of genes at the postimplantation epiblast stage, notably of key germline genes [11–13]. Accordingly, DNA methyltransferases are downregulated in wild-type PGCs [4]. In contrast, de novo methyltransferases, Dnmt3b in particular, exhibit expression in mutant PGCs (Fig 2D). Also, repression of Uhrf1, the essential co-factor for the Dnmt1 maintenance methyltransferase, observed in wild-type PGCs [4], does not occur in mutant cells. Therefore, Prdm14 appears to be implicated in the repression of DNA methylation in the germline, which in turn may allow for the expression of germline genes [11], such as Dazl, Tex19.1, Rhox9 and Sycp3. Our analysis suggests that Prdm14 is involved in the downregulation of Fgfr2 and repression of ERK activation, as well as the repression of DNA methylation, which together may ensure repression of the somatic programme in PGCs and re-expression of genes of the germ cell lineage.
Regulation of gene expression in ES cells
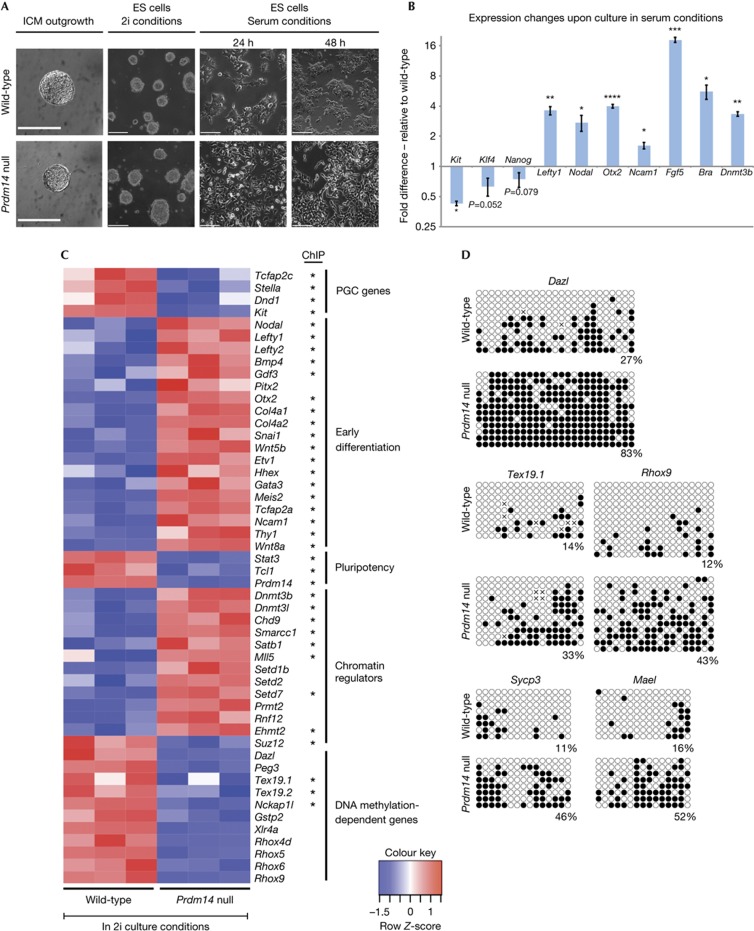
To further explore the molecular basis of the role of Prdm14, we turned to the more tractable ES cells where Prdm14 plays a role in promoting pluripotency [6–9]. We first derived Prdm14-deficient ES cells in 2i culture conditions containing MEK and glycogen synthase kinase-3 inhibitors (Fig 3A) [14, 15]. While their maintenance was not affected by the loss of Prdm14 in these conditions, transfer to classical culture conditions with serum and leukaemia inhibitory factor (LIF) induced their differentiation (Fig 3A,B), confirming that Prdm14 has a role in the maintenance of pluripotent ES cells, which is not overtly evident in 2i culture conditions. We therefore sought evidence for the global impact of loss of Prdm14 in these ES cells by microarray analysis, which indeed revealed significant differences (Fig 3C; supplementary Table S1 online).
Figure 3.
Control of lineage marker expression and DNA methylation by Prdm14 in ES cells. (A) Derivation of Prdm14-null ES cells in 2i culture conditions and morphological changes triggered by a shift to serum culture conditions (scale bar=190 μm for ICM outgrowths and 240 μm for all other images). (B) Gene expression changes induced by culture in serum conditions for 48 h in Prdm14-null ES cells. Values are relative to wild-type cells and normalized to Gapdh (n=three independent biological repeats). Error bars represent the standard error of the mean (s.e.m.) and statistical significance was assessed by t-test (*P⩽0.05; **P⩽0.01; ***P⩽0.001; ****P⩽0.0001) (C) Heatmap of genes with significant expression differences (FDR<0.01) between wild-type and Prdm14-deficient ES cells in 2i culture conditions. Genes determined to be direct target genes by ChIPseq for PRDM14 in mouse ES cells [7] are highlighted by an asterisk. (D) Bisulphite sequencing comparing promoter DNA methylation of DNA methylation-dependent genes in wild-type and Prdm14-null ES cells cultured in 2i conditions. ES, embryonic stem; FDR, false discovery rate; ICM, inner cell mass; PGC, primordial germ cell; s.e.m., standard error of the mean.
First, we found that genes that are essential for early PGC development, such as Tcfap2c, Dnd1 and Kit along with the early PGC marker Stella, showed reduced expression levels in the absence of Prdm14. This is consistent with our findings that Prdm14-deficient PGCs lose expression of germ cell genes. Second, we found that Prdm14-deficient ES cells in 2i showed higher expression levels of early differentiation markers (Fig 3C, supplementary Fig S2 online). Genes such as Nodal, Lefty1, Lefty2 and Bmp4 that promote developmental progression in the postimplantation epiblast and are usually repressed in ES cells are upregulated in the absence of Prdm14. In addition, other differentiation markers that are only expressed during lineage acquisition, like Wnt5b, Snai1, Hhex and Ncam1, were upregulated, which is reminiscent of the upregulation of somatic genes in mutant PGCs.
While the main regulators of pluripotency, such as Oct4, Sox2 and Nanog, were not affected, the pluripotency-associated gene Stat3, which is crucial for inducing and maintaining naive pluripotency [16], and Tcl1, a positive regulator of AKT signalling that promotes proliferation in ES cells and the early embryo [17], were significantly downregulated upon loss of Prdm14 in ES cells (Fig 3C). Moreover, we found histone H3 lysine-9 methyltransferase Ehmt2 to be upregulated, whereas the H3K27 methyltransferase complex component Suz12 was downregulated in the absence of Prdm14. This is of particular interest, because loss of histone 3 lysine-9 dimethylation (H3K9me2) and the upregulation of histone 3 lysine-27 trimethylation (H3K27me3) fail to occur in Prdm14-deficient PGCs [3].
Loss of Prdm14 also caused de-repression of the DNA methyltransferase Dnmt3b and its co-factor Dnmt3l. Importantly, genes including Dazl, Tex19.1 and Tex19.2, as well as the Rhox gene family that are regulated by their promoter DNA methylation [12, 13], were repressed in Prdm14 mutant ES cells, indicating that Prdm14-mediated suppression of DNA methyltransferases is necessary for their expression. We confirmed retention of DNA methylation in the promoter regions of these genes in Prdm14-null cells by bisulphite analysis (Fig 3D).
Taken together, our data suggest that loss of Prdm14 leads to reduced expression of germline genes and induces somatic lineage priming in ES cells, despite culture in 2i conditions. Furthermore, Prdm14 is critical for repression of DNA methyltransferases, particularly of Dnmt3b, to enable expression of germline genes.
Prdm14 maintains naive pluripotency
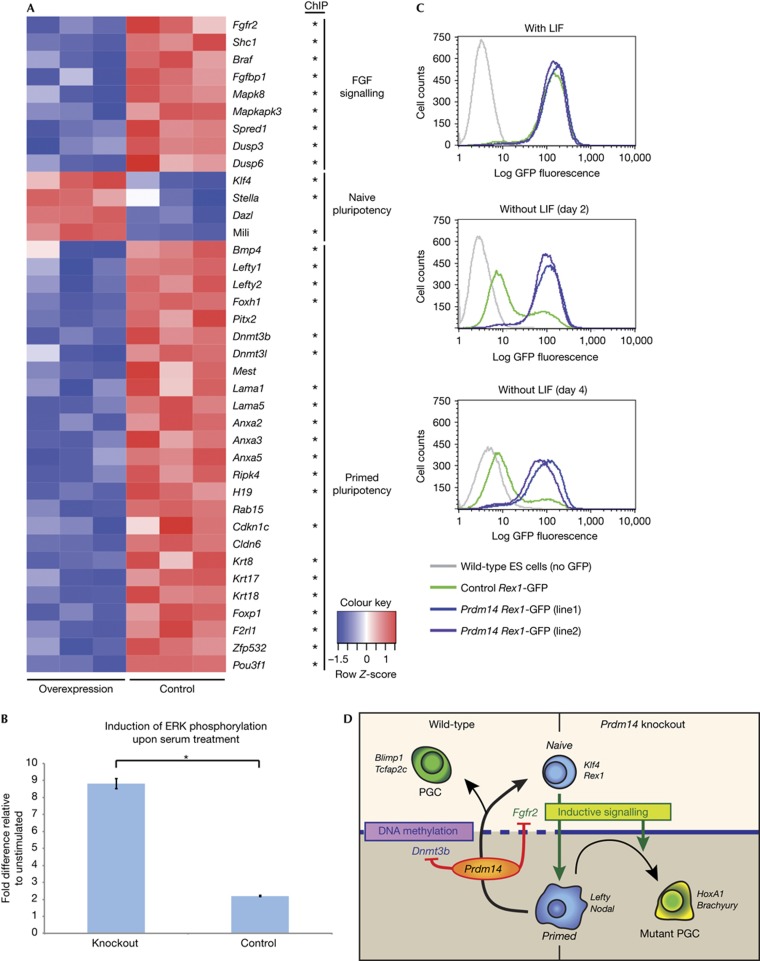
The unexpected upregulation of differentiation markers in Prdm14-deficient ES cells in 2i conditions suggested that Prdm14 might contribute to reducing gene expression heterogeneity in ES cells when cultured in the presence of serum [18]. We therefore examined if Prdm14 gain of function can mimic the effects of 2i culture and reduce heterogeneity in ES cells (Fig 4A; supplementary Table S2 online). We found that genes including Klf4, Mili, Dazl and Stella were upregulated, whereas genes associated with a differentiation-primed state, such as Cldn6, Otx2, Lefty1/2 and Pitx2 [19], were downregulated (Fig 4A; supplementary Fig S3A,B online). In addition, heterogeneity in ES cells with respect to PE fates was also repressed upon Prdm14 overexpression, which is consistent with a previous report [7].
Figure 4.
Prdm14 represses lineage markers and renders ES cells partially LIF independent. (A) Heatmap of differentially expressed genes (FDR<0.01) in control versus Prdm14 gain of function ES cells cultured in serum. Genes determined to be direct target genes by ChIPseq for PRDM14 in mouse ES cells [7] are highlighted by an asterisk. (B) Induction of ERK phosphorylation in control and Prdm14-null ES cells upon 15 min serum stimulation. Values show fold change compared to levels in unstimulated cells, as determined by quantitative ELISA assay (n= three independent biological repeats). Error bars show standard error of the mean and statistical significance was assessed by t-test (*P⩽0.05). (C) Flow cytometry analysis of Rex1-GFP levels after 2 and 4 days of LIF withdrawal in serum culture conditions in control ES cells compared to two independent ES cell lines overexpressing Prdm14. Data shown are representative of independent biological repeats, with 100,000 cells profiled for each cell line. (D) Model and summary of Prdm14 function in ES cells and PGCs. ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; ES, embryonic stem; FGF, fibroblast growth factor; GFP, green fluorescent protein; FDR, false discovery rate; PGC, primordial germ cell; s.e.m., standard error of the mean.
ES cell priming for differentiation is mediated by fibroblast growth factor (FGF) signalling [10] and its pharmacological inhibition reduces heterogeneity [15]. Strikingly, we found that multiple components of the FGF pathway, such as Fgfr2, Braf and Shc1, which were shown to be direct targets of Prdm14 [7], were downregulated when Prdm14 levels are elevated (Fig 4A, supplementary Fig 3C online). This suggests that the reduction in expression of early differentiation genes could be a consequence of an attenuation of the FGF pathway by PRDM14. Indeed, expression of FGF signalling-induced genes, such as Spred1 and Dusp6 [20], was reduced upon Prdm14 overexpression. Moreover, there are similarities between gene expression changes caused by Prdm14 overexpression and those ascribed to direct inhibition of the FGF signalling pathway [20]. To gain further evidence that Prdm14 acts in part through attenuation of MAPK pathway activation, we determined the induction of ERK phosphorylation by a quantitative enzyme-linked immunosorbent assay (ELISA) assay upon exposure of ES cells to serum. We found that Prdm14-null ES cells show a stronger induction of the pathway compared to wild-type cells (Fig 4B). These findings demonstrate that Prdm14 limits the sensitivity of ES cells to differentiation-inducing signals.
Consistently, we also found that Prdm14 limits the continuous FGF signalling-induced drift of ES cells out of a naive pluripotent state that is commonly observed under standard culture conditions [10], as judged by the expression of Rex1 in ES cells. Thus, the majority of ES cells overexpressing Prdm14 reside within the Rex1-high expression state that indicates naive pluripotency (supplementary Fig S3D online). We also found that the expression of key pluripotency factors, Kruppel-like factor 4 (KLF4) and NANOG, was significantly increased, with a clear reduction of cells showing low expression (supplementary Fig S3E online). Furthermore, we found that constitutive expression of Prdm14 limits spontaneous differentiation of ES cells grown in the presence of LIF, generating mostly undifferentiated colonies with strong alkaline phosphatase staining (supplementary Fig S3F online).
We next tested if constitutive expression of Prdm14 could maintain the pluripotent state in the absence of LIF as judged by the extent of Rex1 expression, which discriminates between naive and differentiation-primed ES cell states [18] (Fig 4C). Within 2 days of LIF withdrawal, the majority of control cells drifted into the Rex1-negative state, and only a minority of them were Rex1 positive after 4 days. In contrast, most of the Prdm14-overexpressing cells remained in the Rex1 positive state after 2 and 4 days of LIF withdrawal; thereafter we observed cell death, which might be due to their inability to differentiate. We also found that while withdrawal of LIF in control cells led to a strong reduction in the expression levels of pluripotency genes, these levels were only slightly diminished in cells with constitutive expression of Prdm14 (supplementary Fig S4A,B online).
In summary, we found that Prdm14 represses components of the FGF signalling pathway, thereby limiting ERK activation. Consequently, genes associated with naive pluripotency are induced and genes characteristic for a differentiation-primed state are repressed in ES cells overexpressing Prdm14.
CONCLUDING REMARKS
Here we provide further insights on the impact of loss of Prdm14 function on PGC development and in ES cells. Our results indicate that the defects in Prdm14 mutant cells arise from a lack of repression of the DNA methyltransferase machinery, and by a failure to attenuate the differentiation-inducing FGF signalling pathway. These conclusions are in agreement with other recent studies that describe the role of Prdm14 concerning pluripotency and self-renewal of ES cells [21, 22]. PRDM14 functions appear to be particularly critical for resetting the epigenome, re-establishment of the pluripotency network and maintenance of germline fate in vivo (Fig 4D). While Prdm14 is critical for PGC development and in promoting pluripotency in ES cells, there was no detectable effect of loss of Prdm14 on pre- or peri-implantation development [3] when pluripotency is established. This might be because all traversed states during development are short lived as cells quickly transit through cell fate specification programmes. This is unlike the prolonged maintenance of pluripotency of ES cells in culture, where negative regulation of FGF signalling helps to inhibit lineage commitment. Notably, the type of lineage deregulation in the absence of Prdm14 was context dependent, suggesting that Prdm14 counteracts the generation of differentiation-primed states in general in ES and PGCs, and not of one specific lineage. It is striking that despite culture in 2i conditions, absence of Prdm14 leads to upregulation of some differentiation markers in ES cells, indicating a possible additional contribution of Prdm14 to the homogenous gene expression profile of naive pluripotency [23]. Our findings highlight a unique function for Prdm14 in regulating signalling and epigenetic states that modulate the balance between pluripotency and differentiation.
METHODS
Generation of Prdm14 heterozygous mice and genotyping. C57BL/6 ES cells heterozygous for Prdm14 were obtained from the EUCOMM repository and injected into E3.5 C57BL/6 host blastocysts. Knockout embryos were obtained by heterozygous crosses. All husbandry and experiments involving mice were carried out according to the local ethics committee and were performed in a facility designated by the Home Office.
Single-cell cDNA library preparation. For isolation of PGCs, embryos at the 4–6 somites stage from timed heterozygous crosses of mice bearing a Stella-GFP reporter were dissected and single PGCs from individual genotyped embryos sorted using a MoFlo MLS high-speed flow sorter (Beckman Coulter). Generation of cDNAs and subsequent amplification were performed as described by Tang et al [24].
ES cell derivation, culture and manipulation. ES cells were derived in 2i conditions as detailed previously [14]. ES cells were cultured either in gelatin-coated dishes under standard culture conditions with serum or in fibronectin-coated dishes under 2i culture conditions, both as described previously [6]. For generation of stable Prdm14 overexpressing ES cell lines, the transcriptional Rex1-GFPd2 (Zfp42-GFPd2) reporter ES cell line [25] was used as parental line. Quantitation of induction of ERK phosphorylation was performed using an ELISA kit (R&D Systems). Bisulphite sequencing was performed as previously described [12].
Quantitative real-time PCR and global gene expression analysis. RNA was extracted using the RNeasy Kit (Qiagen) and cDNA was synthesized using SuperScript III reverse transcriptase (Life Technologies). All quantitative PCR runs were performed and analysed as detailed previously [6]. For genome-wide expression analysis, 1 μg of total RNA was used for labelling and hybridization to Mouse WG-6 Expression BeadChips (Illumina). Differential expression was calculated using LIMMA v3.6.0, P-values were corrected for multiple testing and probes with P<0.01 were deemed significant. Microarray data are available from the GEO depository under the accession number GSE45509.
Whole-mount immunostaining. Embryos from timed heterozygous matings were dissected and processed for immunostaining as described previously [26]. Images were acquired using a confocal microscope (Olympus) and analysed with Volocity software (Perkin Elmer). Primary antibodies used were as follows: anti-GFP (Nacalai Tesque, GF090R), anti-BLIMP1 (eBiosciences, 14-5963-80); anti-OCT-3/4 (BD Transduction Laboratories, 611203); anti-phosphoERK1/2 (Thr202/Tyr204) (Cell Signalling Technology, #9101); anti-FGFR2 (Sigma-Aldrich, SAB1403815).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We thank P. Neveu for quantitative analysis of immunostainings, N. Miller for flow cytometry, B. Mansfield and C.E. Dumeau for blastocyst injections and C. Lee for help with animal husbandry. Further, we thank M.H. Johnson for advice and comments on the manuscript, A. Gillich for helpful discussions and T. Kalkan and A. Smith for Rex1-GFP reporter ES cells. This work was supported by grants from the Wellcome Trust to N.G., the Human Frontier Science Program and the Wellcome Trust to M.A.S., and from King Abdulla University of Science and Technology and The Austrian Academy of Sciences to JT.
Author contributions: The study was conceived and designed by N.G., J.T. and A.S. N.G. generated the Prdm14 knockout mouse, derived Prdm14-null ES cells and performed analysis of both. J.T. contributed the data on Prdm14 overexpression. J.A.H. performed bisulphite sequencing. S.K., F.T., H.G.L. and E.M. provided critical assistance and advice on mouse husbandry and embryology, single-cell expression analysis, ES cell derivation and culture and experimental design and data analysis, respectively. The manuscript was written by N.G., J.T. and A.S.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ohinata Y et al. (2005) Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 436: 207–213 [DOI] [PubMed] [Google Scholar]
- Weber S et al. (2009) Critical function of AP-2gamma/TCFAP2C in mouse embryonic germ cell maintenance. Biol Reprod 82: 214–223 [DOI] [PubMed] [Google Scholar]
- Yamaji M, Seki Y, Kurimoto K, Yabuta Y, Yuasa M, Shigeta M, Yamanaka K, Ohinata Y, Saitou M (2008) Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat Genet 40: 1016–1022 [DOI] [PubMed] [Google Scholar]
- Kurimoto K, Yabuta Y, Ohinata Y, Shigeta M, Yamanaka K, Saitou M (2008) Complex genome-wide transcription dynamics orchestrated by Blimp1 for the specification of the germ cell lineage in mice. Genes Dev 22: 1617–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y, Yamaji M, Yabuta Y, Sano M, Shigeta M, Matsui Y, Saga Y, Tachibana M, Shinkai Y, Saitou M (2007) Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 134: 2627–2638 [DOI] [PubMed] [Google Scholar]
- Gillich A, Bao S, Grabole N, Hayashi K, Trotter MWB, Pasque V, Magnúsdóttir E, Surani MA (2012) Epiblast stem cell-based system reveals reprogramming synergy of germline factors. Cell Stem Cell 10: 425–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Swigut T, Valouev A, Rada-Iglesias A, Wysocka J (2011) Sequence-specific regulator Prdm14 safeguards mouse ESCs from entering extraembryonic endoderm fates. Nat Struct Mol Biol 18: 120–127 [DOI] [PubMed] [Google Scholar]
- Chia N-Y et al. (2010) A genome-wide RNAi screen reveals determinants of human embryonic stem cell identity. Nature 468: 316–320 [DOI] [PubMed] [Google Scholar]
- Tsuneyoshi N, Sumi T, Onda H, Nojima H, Nakatsuji N, Suemori H (2008) PRDM14 suppresses expression of differentiation marker genes in human embryonic stem cells. Biochem Biophys Res Commun 367: 899–905 [DOI] [PubMed] [Google Scholar]
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith AG (2007) FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134: 2895–2902 [DOI] [PubMed] [Google Scholar]
- Borgel J, Guibert S, Li Y, Chiba H, Schübeler D, Sasaki H, Forné T, Weber M (2010) Targets and dynamics of promoter DNA methylation during early mouse development. Nat Genet 42: 1093–1100 [DOI] [PubMed] [Google Scholar]
- Hackett JA, Reddington JP, Nestor CE, Dunican DS, Branco MR, Reichmann J, Reik W, Surani MA, Adams IR, Meehan RR (2012) Promoter DNA methylation couples genome-defence mechanisms to epigenetic reprogramming in the mouse germline. Development 139: 3623–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Hubé F, Rollin J, Neuillet D, Philippe C, Bouzinba-Segard H, Galvani A, Viegas-Péquignot E, Francastel C (2010) Dnmt3b recruitment through E2F6 transcriptional repressor mediates germ-line gene silencing in murine somatic tissues. Proc Natl Acad Sci USA 107: 9281–9286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J, Jones K, Phillips JM, Newland SA, Roode M, Mansfield W, Smith AG, Cooke A (2009) Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med 15: 814–818 [DOI] [PubMed] [Google Scholar]
- Ying Q-L, Wray J, Nichols J, Morera LB, Doble B, Woodgett J, Cohen P, Smith AG (2008) The ground state of embryonic stem cell self-renewal. Nature 453: 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Burdon T, Chambers I, Smith AG (1998) Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 12: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Koval A, Hallas C, Bichi R, Tresini M, Malstrom S, Russo G, Tsichlis P, Croce CM (2000) Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc Natl Acad Sci USA 97: 3028–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H (2008) Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135: 909–918 [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448: 196–199 [DOI] [PubMed] [Google Scholar]
- Lanner F, Lee KL, Sohl M, Holmborn K, Yang H, Wilbertz J, Poellinger L, Rossant J, Farnebo F (2010) Heparan sulfation-dependent fibroblast growth factor signaling maintains embryonic stem cells primed for differentiation in a heterogeneous state. Stem Cells 28: 191–200 [DOI] [PubMed] [Google Scholar]
- Yamaji M, Ueda J, Hayashi K, Ohta H, Yabuta Y, Kurimoto K, Nakato R, Yamada Y, Shirahige K, Saitou M (2013) PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell 12: 368–382 [DOI] [PubMed] [Google Scholar]
- Leitch HG et al. (2013) Naive pluripotency is associated with global DNA hypomethylation. Nat Struct Mol Biol 20: 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks H et al. (2012) The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149: 590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Nordman E, Li B, Xu N, Bashkirov VI, Lao K, Surani MA (2010) RNA-seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc 5: 516–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith AG (2011) Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13: 838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohinata Y, Sano M, Shigeta M, Yamanaka K, Saitou M (2008) A comprehensive, non-invasive visualization of primordial germ cell development in mice by the Prdm1-mVenus and Dppa3-ECFP double transgenic reporter. Reproduction 136: 503–514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.