Abstract
SNAP-25 is a key component of the synaptic-vesicle fusion machinery, involved in several psychiatric diseases including schizophrenia and ADHD. SNAP-25 protein expression is lower in different brain areas of schizophrenic patients and in ADHD mouse models. How the reduced expression of SNAP-25 alters the properties of synaptic transmission, leading to a pathological phenotype, is unknown. We show that, unexpectedly, halved SNAP-25 levels at 13–14 DIV not only fail to impair synaptic transmission but instead enhance evoked glutamatergic neurotransmission. This effect is possibly dependent on presynaptic voltage-gated calcium channel activity and is not accompanied by changes in spontaneous quantal events or in the pool of readily releasable synaptic vesicles. Notably, synapses of 13–14 DIV neurons with reduced SNAP-25 expression show paired-pulse depression as opposed to paired-pulse facilitation occurring in their wild-type counterparts. This phenotype disappears with synapse maturation. As alterations in short-term plasticity represent a new mechanism contributing to cognitive impairments in intellectual disabilities, our data provide mechanistic clues for neuronal circuit alterations in psychiatric diseases characterized by reduced expression of SNAP-25.
Keywords: SNAP-25, short-term plasticity, glutamatergic transmission
INTRODUCTION
SNAP-25 (synaptosomal-associated protein of 25 kDa) is a SNARE protein that participates in the regulation of synaptic-vesicle (SV) exocytosis [1–3] and negatively modulates voltage-gated ion channels (VGCCs) [4–7]. Consistently, silencing endogenous SNAP-25 results in increased VGCC activity in glutamatergic neurons [8, 9].
The SNAP-25 gene has been associated with schizophrenia [10, 11], as SNAP-25 protein levels are lower in hippocampi and frontal lobes of schizophrenic patients [12–14]. Also, case control or family-based studies indicated that the SNAP-25 gene is associated with attention deficit hyperactivity disorder [15, 11] and, indeed, reduced SNAP-25 expression has been found to mediate hyperactivity in mice [16, 17]. Furthermore, reduction of SNAP-25 levels is responsible for the massive neurodegeneration in mice genetically devoid of the SV protein cystein spring protein alpha [18].
Despite the involvement of reduced SNAP-25 in psychiatric defects, the underlying cellular mechanisms are at present unknown. We then investigated whether halved SNAP-25 expression results in altered neurotransmission and plasticity in neuronal networks. We demonstrate that in developing hippocampal neurons of SNAP-25 heterozygous mice glutamate release probability is increased, heavily impacting short-term plasticity phenomena.
RESULTS AND DISCUSSION
Glutamatergic currents in developing neurons
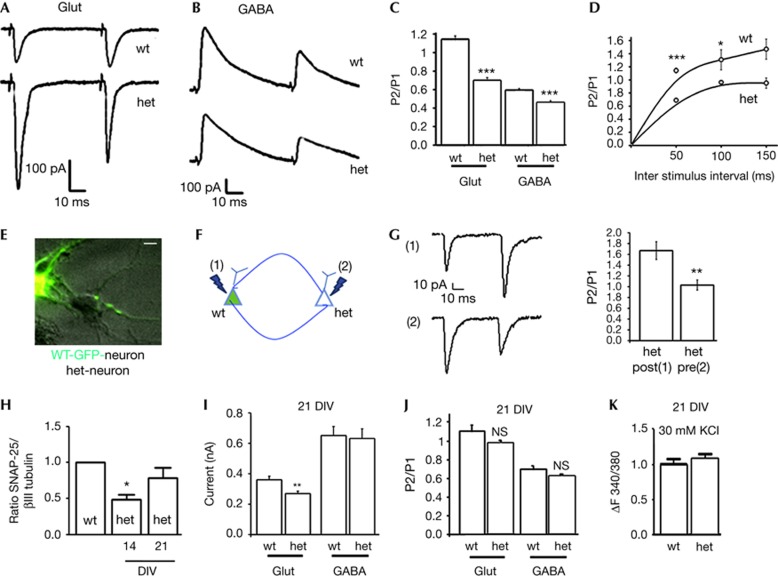
SNAP-25 modulates VGCC current density in glutamatergic neurons [8]. To investigate whether the changes in VGCC current densities in neuronal cell bodies affect neurotransmission, synaptic properties were investigated in SNAP-25+/+ and SNAP-25+/− hippocampal cultures (here in defined as wild type (wt) and het, respectively) at 13–14 days in vitro (DIV). We recorded miniature excitatory (mEPSCs) or inhibitory (mIPSCs) currents (Fig 1A–B), holding neurons at the reversal potential for GABA (γ-aminobutyric acid)- and glutamate-mediated responses (–70 and+5 mV, respectively) in the presence of 1 μM tetrodotoxin. Frequency and amplitudes of both mEPSCs and mIPSCs were not significantly different in het neurons relative to wt, in line with previous reports [19, 3, 18] (mEPSC frequency: 1.41±0.20 versus 1.17±0.16 Hz, wt versus het; mIPSC frequency: 1.30±0.17 versus 1.47±0.24 Hz, wt versus het. mEPSC and mIPSC amplitude distributions were not significantly different).
Figure 1.
Enhanced evoked glutamatergic transmission in het cultures at 14 div. (A–B) Traces of mEPSCs and mIPSCs from wt or het neurons followed by the analysis of frequency and amplitude ((A) frequency, t-test, P=0.399; N=4; amplitude: KS-test not significant; (B) frequency, t-test, P=0.49, N=4; amplitude: KS-test not significant). (C–D) Traces of eEPSCs (C) and eIPSC (D) from wt or het neurons. (E) Analysis of eEPSCs: wt (n=10) versus het (n=10), t-test, P<0.01, N=4; eIPSCs: wt (n=13) versus het (n=8), t-test, P<0.01, N=3). Error bars indicate s.e.m. KS-test, Kolmogorov–Smirnov test; wt, wild type. **P<0.01.
Depolarizations of presynaptic glutamatergic or GABAergic cells in synaptically connected neurons evoked unitary EPSCs or IPSCs, respectively. Notably, evoked EPSCs were significantly larger in het cultures as compared with wt(eEPSCs: 0.26±0.01 versus 0.45±0.04 nA, wt versus het; P<0.05; Fig 1C,E). Conversely, a slight, although significant, reduction of evoked IPSC amplitude was recorded in het neurons relative to wt (0.52±0.03 versus 0.36±0.03 nA, wt versus het, P<0.05; Fig 1D,E).
Glutamate-receptor sensitivity was not affected by reduced SNAP-25, as indicated by unaltered mEPSC amplitudes (Fig 1A) and by similar intracellular Ca2+ transients in response to 30 μM AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) or 100 μM NMDA (N-methyl-D-aspartic acid) stimulation (AMPA: wt=1±0.066, het=0.899±0.078; NMDA: wt=1±0.039, het=0.919±0.046, normalized value; Fig 2A). Conversely, stimulation with 30 mM KCl induced a higher Ca2+ response in het neurons compared with wt (normalized het F340/380: 1.2±0.06, P<0.05).
Figure 2.
Enhanced glutamatergic transmission in het cultures does not depend on synapse number or RRP size. (A) Traces and quantification of calcium responses on: (a) KCl: wt (n=76) versus het (n=63), t-test, P=0.007; (b) AMPA: wt (n=22) versus het (n=22), t-test, P=0.33; (c) NMDA: wt (n=84) versus het (n=52) t-test, P=0.15; N=3. (B) Staining of neurons for SV2A (red) and βIII tubulin (green) (top panels) and for βIII tubulin (green), vGAT (red) and vGlut (blue). Calibration bar=10 μm. (C) SV2 puncta/20 μm: wt (n=24) versus het (n=26), t-test, P=0.82. (D) Measurement of vGAT/vGlut puncta: wt (n=34) versus het (n=54), t-test, P=0.89. (E) Sucrose-evoked responses from wt and het neurons and charge response: wt (n=12) versus het (n=12), t-test, P=0.55, N=3). (F) Charge transfer in calcimycin experiment: wt (n=13) versus het (n=11) t-test, P=0.06, N=3. Error bars indicate s.e.m. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-D-aspartic acid; wt, wild type.
Increased glutamatergic neurotransmission in het neurons did not result from a higher number of synaptic contacts, as indicated by the comparable density of excitatory or inhibitory synapses formed along dendrites (SV2 puncta/20 μm: wt=8.20±0.66; het=7.96±0.82; % of SV2; vGAT/vGlut puncta: wt=0.68±0.14, het=0.70±0.10; Fig 2B–D). Moreover, enhanced EPSC amplitudes were not owing to changes of the readily releasable pool of SVs, as revealed by hypertonic sucrose applications, inducing Ca2+-independent release [20] (charge transfer, wt=1.59±0.23 nC; het=1.71±0.23 nC, Fig 2E). Finally, the charge transfer at glutamatergic synapses induced by 40 μM calcimycin, which causes calcium-dependent exocytosis bypassing activation of VGCCs [21], was lower, although not significantly, in het neurons with respect to wt (wt=0.29±0.38 nC, het=0.22±0.57 nC, Fig 2F), suggesting a requirement of presynaptic calcium channels in the SNAP-25-dependent effects.
The observation that reductions of SNAP-25 levels do not significantly affect neurotransmission in inhibitory neurons (see also Sharma et al [18]) is in line with the finding that het excitatory, but not inhibitory, neurons show enhanced voltage-gated calcium currents [8]. The small reduction in eIPSC amplitude recorded in het neurons could be ascribed to the SNARE properties of SNAP-25. Indeed SNAP-25, although being expressed at very low levels in most GABAergic terminals in situ [22–25], appears to be part of the GABAergic SNARE complex [26, 27].
Short-term plasticity at SNAP25+/− synapses
A simple form of short-term synaptic plasticity associated to presynaptic release properties is the paired-pulse ratio (PPR) of two consecutive synaptic responses. Paired-pulse facilitation (PPF) occurs at low-probability synapses, requiring accumulation of intra-terminal Ca2+ to reliably induce SV fusion. Conversely, paired-pulse depression (PPD) results from a prompt depletion of the readily releasable pool of transmitter at high-probability synapses. Both PPF and PPD rely on Ca2+-dependent mechanisms triggering fusion of docked SVs [5] and indeed neurons might change use-dependent plasticity depending on extracellular calcium concentration, presynaptic calcium accumulations and expression of calcium-binding proteins [28–30].
At 13–14 DIV, when excitatory synapses in wt cultures were stimulated by two consecutive stimuli delivered with an inter-spike interval of 50 ms, PPF prevailed. Conversely, in neurons obtained from het mice, identical protocols induced PPD of EPSCs (PPR: 1.14±0.04 versus 0.68±0.03, wt versus het; Fig 3A,C). Notably, a similar reduction in PPR was obtained in wt glutamatergic neurons following elevation of extracellular calcium concentration to 4 mM (PPR: wt, 4 mM Ca2+=0.82±0.021; N=3 independent experiments; one-way analysis of variance Kruskal–Wallis one-way analysis of variance on Ranks, P<0.01). In contrast, only a slight, although significant, reduction in PPR occurred at het inhibitory synapses, which were characterized, as their wt counterpart, by prevalence of PPD (wt=0.54±0.025, het=0.46±0.017, Fig 3B,C). Plotting PPRs as a function of inter-stimulus interval showed that the inter-spike interval of 50 ms is the most effective in inducing PPD (PPR ISI 100: wt=1.30±0.16, het=0.96±0.02; PPR ISI 150: wt=1.46±0.25, het=0.95±0.08, N=2 independent experiments; Fig 3D).
Figure 3.
Shift from paired-pulse facilitation to paired-pulse depression at 14 DIV hippocampal het synapses. (A–B) Traces of short-term plasticity experiments. (C) PPR at glutamatergic synapses: wt (n=10) versus het (n=10), t-test, P<0.001, N=4; GABAergic synapses: wt (n=13) versus het (n=8), t-test, P<0.001, N=4. (D) PPR plotted against different interpulse intervals. PPR ISI 100: t-test, P=0.048; PPR ISI 150: t-test P=0.619. (E) Merged bright field and fluorescence image of a mixed culture (wt-GFP with het neurons). Calibration bar=10 μm. (F) Short-term plasticity in pairs where either the presynaptic [2] or the postsynaptic [1] neuron was het for SNAP-25. (G) PPR: (pre)-wt-GFP=1.44±0.21, (n=5); (pre)-het=0.85±0.2 (n=5) t-test, P=0.001. (H) Quantitive western blotting for SNAP-25 expression: wt versus het 14 DIV, t-test, P=0.042. (I) eEPSC: wt (n=4) versus het (n=9), t-test, P=0.01, N=3. (J) PPR: glut: wt (n=4) versus het (n=9), t-test, P=0.058; GABA: wt (n=4), het (n=5), t-test, P=0.06, N=2. (K) Calcium responses: wt (n=62) versus het (n=55), t-test, P=0.83, N=3. Error bars indicate s.e.m. DIV, days in vitro; GABA, γ-aminobutyric acid; GFP, green fluorescent protein; NS, not significant; PPR, paired-pulse ratio; SNAP-25, synaptosomal-associated protein of 25 kDa; wt, wild type. *P<0.05; **P<0.01; ***P<0.005.
In acute hippocampal slices from adult wt or het mice, 10 EPSCs at 30 Hz evoked in CA1 pyramidal neurons by Schaffer-collateral stimulation revealed the absence of a shift from PPF to PPD. However, at 30 Hz EPSCs facilitated significantly less in het neurons (area of normalized EPSC amplitudes versus stimulus number: 14.6±0.7 and 12.6±0.6 wt and SNAP-25+/−, respectively; supplementary Fig 1 online), going in the same direction of cultured hippocampal neurons.
As a further confirmation that presynaptic reduction ofSNAP-25 is responsible for the shift from PPF to PPD, paired recordings carried out from mixed cultures of het and wtgreen fluorescent protein (GFP) neurons (Fig 3E) revealed that the direction of PPR depended on the genotype of presynaptic neurons (Fig 3E and G). Indeed, wt (GFP+) presynaptic neurons invariably produced facilitating EPSCs, whereas presynaptichet neurons induced depressing glutamatergic responses (Fig 3F(1), respectively; (pre)-wt-GFP-positive=1.44±0.2; (pre)-het=0.85±0.2; Fig 3G).
SNAP-25 expression increases in wt [31] and in SNAP-25 het cultures during maturation in vitro (Fig 3H). Notably, at 21 DIV, when SNAP-25 expression was significantly increased, differences in evoked responses (eEPSC and eIPSC) and in short-term plasticity disappeared (eEPSC (nA): wt=0.360±0.023, het=0.266±0.0157; PPR in EPSCs: wt=1.10±0.06, het=0.97±0.03; PPR of IPSCs: wt=0.70±0.02; het= 0.62±0.01, Fig 3I–J). Accordingly, depolarization-induced Ca2+ responses in het neurons were comparable to those of wt neurons (ΔF340/380: wt=1±0.041, het=1.012±0.042, Fig 3K, compare with Fig 2A), indicating that neurotransmission and short-term plasticity defects can be fully recovered in parallel with increases in protein expression. The milder phenotype observed in het hippocampal slices, as compared with cultured neurons, might be owing therefore to upregulation of protein expression, which occurs also in vivo during postnatal development [32].
Notably, the recovery of the presynaptic altered phenotype in het neurons at 21 DIV is associated with the development of postsynaptic defects as indicated by the significantly lower amplitude of eEPSCs at 21 DIV (Fig 3I), which is accompanied by reduced PSD-95 density and altered spine maturation (Fossati et al, unpublished work). Two processes might therefore take place in neurons developing in the presence of reduced SNAP-25 levels, the first occurring at the presynaptic level at early developmental stages, when the protein is substantially reduced, and a second occurring at later stages of maturation and mainly impacting the postsynapse. Notably, het mice, at the age of 7 weeks, show motor hyperactivity, a phenotype that is rescued in the adult animal [32], while adult SNAP-25 heterozygous mice seem defective in different forms of associative learning [32].
Downregulation of SNAP-25 changes PPR
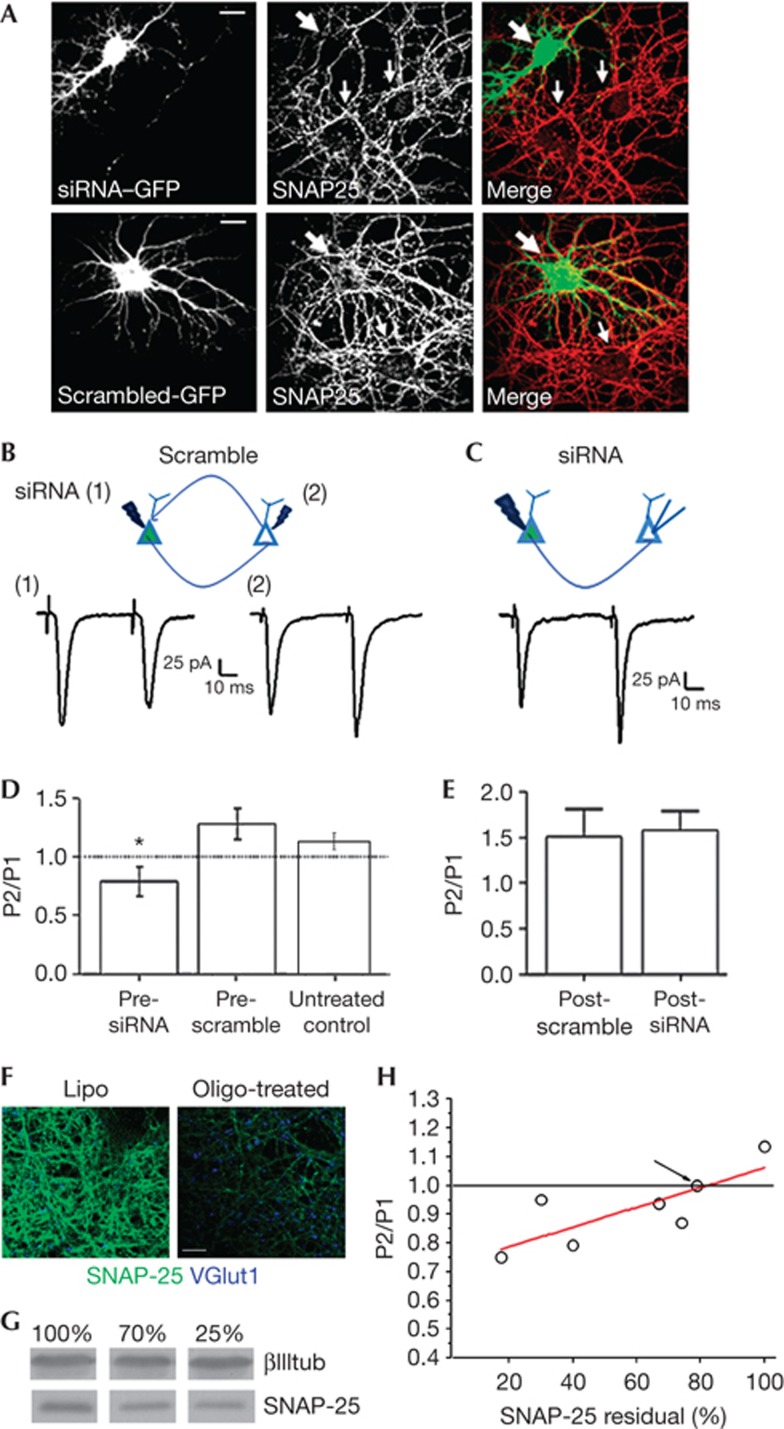
Recovery of normal excitatory neurotransmission and short-term plasticity on SNAP-25 developmental increase prompted us to provide a direct demonstration that the switch from PPF to PPD is a direct consequence of SNAP-25 reduction at the presynaptic level. Acute protein downregulation by double-stranded small interfering RNA oligonucleotides (siRNAs) was carried out and dual whole-cell recordings were performed from connected neurons. siRNA, but not scramble oligonucleotide, application reduced SNAP-25 levels, leaving ∼40% residual (Fig 4A and Grumelli et al [33]). Notably, acute reduction of SNAP-25 by siRNAs in the presynaptic glutamatergic neuron reduced PPR (scramble siRNA, presynaptic=1.28±0.13, Fig 4B (2), SNAP-25 siRNA, presynaptic=0.79±0.12, Fig 4B (1), untreated control= 1.11±0.069, Fig 4B,D). Importantly, no reductions were present when scramble or SNAP-25 siRNA was transfected in the postsynaptic neuron (scramble siRNA, postsynaptic=1.508±0.29, SNAP-25 siRNA, postsynaptic=1.57±0.21, Fig 4C,E). Therefore, even acute reductions of SNAP-25 in wt presynaptic neurons switch PPF to PPD at glutamatergic synapses.
Figure 4.
Paired-pulse depression after acute downregulation of SNAP-25 expression in the presynaptic neuron. (A) SNAP-25 labelling of rat neurons co-transfected with either SNAP-25 siRNA plus GFP or scramble siRNA plus GFP. Large arrows point to neurons transfected with either siRNA (top panels) or scramble (bottom panels) constructs. Small arrows point to non-transfected neurons. Calibration bar=20 μm. (B–C) top: cartoons of recording configurations. (B) (1) stimulation of presynaptic siRNA-treated neuron; (2) stimulation of presynaptic scramble neuron. Bottom: traces of experiments described above; (C) bottom: traces of recordings experiments in scramble siRNA-treated postsynaptic neuron. (D) PPR in scramble siRNA presynaptic (n=6) versus SNAP-25 siRNA presynaptic (n=7), t-test, P=0.018. (E) PPR in SNAP-25 siRNA postsynaptic (n=3) versus scramble siRNA postsynaptic (n=5), t-test, P=0.87, N=4. (F) Examples of 14 DIV rat hippocampal cultures immunostained for SNAP-25 (green) and vGlut1 (blue) on reduction of SNAP-25 expression through stealth oligonucleotides. Calibration bar=10 μm. (G) Western blotting showing varied reduction of SNAP-25 levels following stealth oligonucleotide treatment. (H) Plot of PPR versus residual levels of SNAP-25 in rat hippocampal cultures treated with stealth oligonucleotides. The value of PPR relative to SNAP-25 levels recorded in 21 DIV het neurons is pointed by the arrow. Error bars indicate s.e.m. DIV, days in vitro; GFP, green fluorescent protein; PPR, paired-pulse ratio; siRNA, small interfering RNA; SNAP-25, synaptosomal-associated protein of 25 kDa. *P<0.05.
To directly assess whether varied levels of SNAP-25 expression correlate with different PPR ratio, we used three different siRNA oligonucleotides to achieve controlled SNAP-25 downregulation (Fig 4F,G). Different concentrations of oligonucleotides were transfected in rat hippocampal neurons at DIV 10 and the extent of protein reduction was assessed by quantitative western blotting, after electrophysiological recording at DIV 14. A correlation between PPR values and levels of residual SNAP-25 was present, with 20–25% reductions of SNAP-25 being sufficient to shift PPF to PPD (Fig 4H).
CONCLUSIONS
Activity-dependent presynaptic processes producing various forms of short-term plasticity are believed to control several essential neural functions, such as information processing, working memory and decision making. Presynaptic abnormalities were reported at excitatory hippocampal synapses in a mouse model of Fragile X syndrome, leading to defects in short-term plasticity and information processing [34, 35]. These changes were associated with exaggerated calcium influx in presynaptic neurons during high-frequency stimulation [34]. Reduced SNAP-25 levels, leading to abnormal presynaptic short-term plasticity at glutamatergic terminals, at least during early developmental stages, might therefore contribute to cognitive impairments in intellectual disability syndromes. Interestingly, analysis of allele frequencies of two genetic variants in SNAP-25 indicated that SNAP-25 might be directly involved in intellectual disability syndromes [36].
METHODS
Animals. All the experimental procedures followed the guidelines established by the Italian Council on Animal Care and were approved by the Italian Government decree No. 27/2010 (supplementary Information online).
Cell cultures. Hippocampal neurons were established from E18 fetal het or wt littermates C57BL/6 mice or from E18 fetal rats as described by Bartlett [37] with slight modifications (supplementary Information online). Primary hippocampal GFP-positive neuronal cultures were prepared from the hippocampi of C57BL/6 GFP transgenic mice at embryonic day 18.
Acute downregulation of SNAP-25 expression. Silencing of SNAP-25 was achieved via transfection of a pSUPER construct [8, 25]. A nonspecific siRNA duplex of the same nucleotides but in an irregular sequence (scrambled iSNAP-25 siRNA) was prepared (supplementary Information online). In a different set of experiments, three different double-stranded siRNA oligonucleotides were used to achieve controlled SNAP-25 downregulation.
Immunocytochemical staining. Immunofluorescence staining was carried out using the following antibodies: rabbit anti-SV2A, rabbit anti-vGAT guinea pig anti-vGLUT1, mouse anti-SNAP-25, mouse anti-βIIItubulin. Secondary antibodies were conjugated with Alexa-488, Alexa-555 or Alexa-633 fluorophores.
Quantitative western blotting. Homogenates from hippocampal cultures were separated by electrophoresis, blotted on nitrocellulose membrane and analysed by western blot by using monoclonal antibodies against SNAP-25 and beta-III-tubulin. Membranes were washed and incubated for 1 h at room temperature with the secondary antibody IRDye 680-conjugated goat anti-mouse (1:10,000). Blots were scanned using an Odyssey Infrared Imaging System (LI-COR Biosciences).
Calcium imaging. Hippocampal cultures of 13 or 21 DIV were loaded with 5 μM Fura-2 pentacetoxymethylester in KRH for 45 min at 37 °C, washed in the same solution and transferred to the recording chamber of an inverted microscope (Axiovert 100; Zeiss, Oberkochen, Germany) equipped with a calcium imaging unit. After a period for baseline acquisition, neurons were stimulated with different drugs (supplementary Information online).
Cell culture electrophysiology. Whole-cell patch-clamp recordings of EPSCs and IPSCs were obtained from 13–14-day-old neurons using a Multiclamp 700A amplifier (Molecular Devices) and pClamp-10 software (Axon Instruments, Foster City, CA). Currents were sampled at 5 kHz and filtered at 2–5 kHz. mEPSCs or mIPSCs were recorded in presence of tetrodotoxin (l μM). Evoked currents were recorded in isolated pairs of neurons in low-density cultures. Neurons were held at −70 mV, and eEPSC or eIPSC evoked by a 100-mV depolarization pulse in the presynaptic cell lasting 1 ms. Readily releasable pool size was evaluated exposing neurons for 4 s to hypertonic solution of sucrose. The ionophore calcimycine was applied for 90 s.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
This research has received funding from the European Union Seventh Framework Programme under grant agreement n° HEALTH-F2-2009-241498 (‘EUROSPIN’ project) to M.M.; by the Italian Ministry of Health (RF-2009-1545998), by PRIN 2010–2011 and Telethon GGP12115 to M.M.; the Giovanni Armenise–Harvard Foundation: Career Development Award (A.B.); European Research Council (ERC) under the European Community’s 7th Framework Programme (FP7/2007-2013)/ERC grant agreement No 200808 (A.B.). F.A. is supported by the Italian Ministry of Research and Education program ‘FIRB giovani’ 2010, protocol number: RBFR10ZBYZ.
Author contributions: F.A. designed and performed experiments, analysed data and helped writing the paper; R.M. performed experiments and analysed data; I.C., G.F. and S.P. performed experiments; E.M., D.P. and C.V. discussed data; A.B. discussed data and wrote the paper; M.M. designed the study, discussed data and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Jahn RSR (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7: 631–643 [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323: 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P et al. (2002) Genetic ablation of the t-SNARESNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: 19–26 [DOI] [PubMed] [Google Scholar]
- Atlas DWO, Trus M (2001) The voltage-gated Ca2+ channel is the Ca2+ sensor of fast neurotransmitter release. Cell Mol Neurobiol 21: 717–731 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP (2008) Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901 [DOI] [PubMed] [Google Scholar]
- Zamponi GW (2003) The L-type calcium channel C-terminus: sparking interest beyond its role in calcium-dependent inactivation. J Physiol 552: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi DCS, Bozzi Y, Chikhladze M, Grumelli C, Proux-Gillardeaux V, Takahashi M, Franceschetti S, Verderio C, Matteoli M (2008) Activity-dependent phosphorylation of Ser187 is required for SNAP-25-negative modulation of neuronal voltage-gated calcium channels. Proc Natl Acad Sci USA 105: 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe SB, Corradini I, Pozzi D, Verderio C, Matteoli M (2010) Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J Biol Chem 285: 24968–24976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe SBMM (2011) Inactivation kinetics of voltage-gated calcium channels in glutamatergic neurons are influenced by SNAP-25. Channels 5: 304–307 [DOI] [PubMed] [Google Scholar]
- Lewis CM et al. (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet 73: 34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M (2009) SNAP-25 in neuropsychiatric disorders. Ann N Y Acad Sci 1152: 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG (1998) SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb Cortex 8: 261–268 [DOI] [PubMed] [Google Scholar]
- Thompson PM, Kelley M, Yao J, Tsai G, van Kammen DP (2003) Elevated cerebrospinal fluid SNAP-25 in schizophrenia. Biol Psychiatry 53: 1132–1137 [DOI] [PubMed] [Google Scholar]
- Thompson PM, Sower AC, Perrone-Bizzozero NI (1998) Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia. Biol Psychiatry 43: 239–243 [DOI] [PubMed] [Google Scholar]
- Barr CLFY, Wigg K, Bloom S, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL (2000) Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder. Mol Psychiatry 5: 405–409 [DOI] [PubMed] [Google Scholar]
- Hess EJ, Rogan PK, Domoto M, Tinker DE, Ladda RL, Ramer JC (1995) Absence of linkage of apparently single gene mediated ADHD with the human syntenic region of the mouse mutant Coloboma. Am J Med Genet 60: 573–579 [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 1313–1323 [DOI] [PubMed] [Google Scholar]
- Sharma M, Burre J, Sudhof TC (2011) CSPalpha promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat Cell Biol 13: 30–39 [DOI] [PubMed] [Google Scholar]
- Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET (2007) Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol 98: 794–806 [DOI] [PubMed] [Google Scholar]
- Rosenmund CSC (1996) Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16: 1197–1207 [DOI] [PubMed] [Google Scholar]
- Tokuoka HGY (2006) Myosin light chain kinase is not a regulator of synaptic vesicle trafficking during repetitive exocytosis in cultured hippocampal neurons. J Neurosci 26: 11606–11614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano VLL, Flace P, Girolamo F, Rizzi A, Bosco L, Cagiano R, Nico B, Ribatti D, Ambrosi G (2011) VAMP-2, SNAP-25A/B and syntaxin-1 in glutamatergic and GABAergic synapses of the rat cerebellar cortex. BMC Neurosci 12: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina L, Melone M, Fattorini G, Conti F (2007) Clozapine upregulates the expression of the vesicular GABA transporter (VGAT) in rat frontal cortex. Mol Psychiatry 12: 612–613 [DOI] [PubMed] [Google Scholar]
- Mandolesi G, Vanni V, Cesa R, Grasselli G, Puglisi F, Cesare P, Strata P (2009) Distribution of the SNAP25 and SNAP23 synaptosomal-associated protein isoforms in rat cerebellar cortex. Neuroscience 164: 1084–1096 [DOI] [PubMed] [Google Scholar]
- Verderio C et al. (2004) SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41: 599–610 [DOI] [PubMed] [Google Scholar]
- Tafoya LCMM, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC (2006) Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J Neurosci 26: 7826–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyken JGM, Riedel D, Urlaub H, Jahn R, Chua JJ (2013) Molecular profiling of synaptic vesicle docking sites reveals novel proteins but few differences between glutamatergic and GABAergic synapses. Neuron 78: 285–297 [DOI] [PubMed] [Google Scholar]
- Saviane CSL, Raffaelli G, Voronin LL, Cherubini E (2002) Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3-CA3 synapses in the rat hippocampus. J Physiol 544: 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA (1996) Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci USA 93: 7363–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy TC-MA, Jeromin A, Schweizer FE (2003) Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor-1. Nat Neurosci 6: 1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark CBF, Kaushal A, Mathews JR, Partridge LD, Wilson MC (2004) Developmentally regulated switch in alternatively spliced SNAP-25 isoforms alters facilitation of synaptic transmission. J Neurosci 24: 8796–8805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini IDA et al. (2012) Epileptiform Activity and cognitive deficits in SNAP-25+/− mice are normalized by antiepileptic drugs. Cereb Cortex 12: [Epub ahead of print] doi:; DOI: 10.1093/cercor/bhs316 [DOI] [PubMed] [Google Scholar]
- Grumelli C, Corradini I, Matteoli M, Verderio C (2010) Intrinsic calcium dynamics control botulinum toxin A susceptibility in distinct neuronal populations. Cell Calcium 47: 419–424 [DOI] [PubMed] [Google Scholar]
- Deng PY, Sojka D, Klyachko VA (2011) Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci 31: 10971–10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Neuwirth LS, L'Amoreaux W (2010) Taurine regulation of short term synaptic plasticity in fragile X mice. J Biomed Sci 17(Suppl 1): S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi TS et al. (2012) Supporting the generalist genes hypothesis for intellectual ability/disability: the case of SNAP25. Genes Brain Behav 11: 767–771 [DOI] [PubMed] [Google Scholar]
- Bartlett WPBG (1984) An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J Neurosci 4: 1944–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.