Of all the differences that distinguish people, the most obvious and most intriguing one is their sex. The relationship between males and females has had a profound influence on human civilization, causing wars and societal upheavals, and inspiring countless works of art, music and literature. For as long as humans have both struggled with and enjoyed the existence of the two sexes, they have tried to understand how this crucial difference comes about. Philosophers, naturalists and, more recently, scientists have debated this question for more than 3,000 years. Yet, despite the progress made, the debate remains open.
For as long as humans have both struggled with and enjoyed the existence of the two sexes, they have tried to understand how this crucial difference comes about
To explore the difference between the sexes and our view of them, we start with one of the oldest tales of their origin. The Book of Genesis tells us that Adam, the first human, was a man, and that Eve, the first woman, was created from one of Adam's ribs. Although this story is almost regarded as Gospel truth, it might actually be based on a mistranslation. The Old Testament was originally written in Hebrew, and an old rabbinical tradition dating to pre-Christian times, interprets the Hebrew word zela not as ‘rib’ but as ‘side’, which could well be the correct meaning. In other words, Adam was originally bisexual, part male and part female, and was subsequently bisected [1]. The same ambiguity of meaning is also found in European languages: according to the Oxford English Dictionary, the word ‘coast’ is derived from the Latin word costa, meaning ‘rib’, ‘flank’ or ‘side’. Adam's original bisexuality is supported by both internal and comparative evidence. The verse immediately following the story of the creation of Eve reads: “That is why a man leaves his father and mother and is united to his wife, and the two become one flesh”, Genesis 2.24. This would hardly be intelligible if Adam had merely lost a rib, but it would be logical once he realized that he had lost his other half.
The bisection of Adam links the Biblical account to an incident in Plato's Symposium, in which Aristophanes describes the origin of the sexes. According to Aristophanes, primeval men were bisexual and exceedingly strong. After Zeus cut them in half and Apollo healed their wounds, the two halves came together in mutual embraces. Both stories are modified versions about a primitive bisexual being that was bisected by a deity—an ancient Oriental legend that was also known in Mesopotamia, Persia and India. It is, of course, a more egalitarian version than the Creation of Eve from one of Adam's ribs. Aristophanes actually recognized three sexes: male, female and bisexual, to account for homosexuality.
Yet, the ancient Greeks were anything but egalitarian. Galen, the Greek physician of the second century AD, summed up their views as follows: “Now just as mankind is the most perfect of all animals, so within mankind the man is more perfect than the woman, and the reason for the perfection is his excess of heat, for heat is Nature‘s primary instrument. Hence in those animals that have less of it, her workmanship is necessarily more imperfect, and so it is no wonder that the female is less perfect than the male, by as much as she is colder than he” [2]. The idea that males are hotter than females is attributed to the Greek philosopher Empedocles (circa 490–430 BC). Aristotle thought that males had an abundance of the superior element, fire, whereas females have an abundance of water.
The assumed superiority of the male over the female is matched by the similarly assumed superiority of the right over the left side. The pairs, right and left, male and female, light and darkness are part of the Pythagorean Table of Opposites. Although it is not known which of the Pythagoreans held these views, it is certain that the arrangement of right, male and light on the good side, and of left, female and darkness on the bad side, was in line with the thinking of the earliest Greek writers; the symbolic significance of laterality is not confined to the Ancient Greeks, as is amply illustrated by the meanings of the English words ‘right’ and ‘righteous’, compared with ‘left’ and ‘sinister’.
The combination of right/left with male/female gave rise to the first theory of sex determination. The Greek philosopher Parmenides thought that the embryo's position in the womb determined its sex: those on the right side develop into males, and those on the left into females. According to Anaxagoras, the decision depended on the father's semen: that from the right testis developing into a male child, and from the left testis into a female.
Science & Society Series on Sex and Science.
Sex is the greatest invention of all time: not only has sexual reproduction facilitated the evolution of higher life forms, it has had a profound influence on human history, culture and society. This series explores our attempts to understand the influence of sex in the natural world, and the biological, medical and cultural aspects of sexual reproduction, gender and sexual pleasure.
During the course of millennia, the left/right theory of sex determination was used as a basis for sex prediction and selection, both for domestic animals and in humans. Fig 1 illustrates a statement in an Alexandrian manuscript from the first century AD. It states that, if a bull dismounts from the right side of the cow after mating, as he does in Fig 1, the conception will result in a bull calf, whilst dismounting from the left side results in a heifer calf. For humans who want to be sure of fathering a son, a sixteenth century Venetian tract lists eight requirements: (i) warmth; (ii) quantity of seed, because the male is the more perfect being; (iii) complete purity of the woman regarding her monthly purification; (iv) origin of the semen from the right testis; (v) ejaculation of the semen into the right half of the uterus; (vi) warm condition of the female ‘semen’; (vii) northern winds, as these, by an apparent counteraction, warm and strengthen the body; and (viii) optimum ages of the parents. Whilst it is remarkable that the theory associating male sex with the right side, and female sex with the left side has lasted for so long, it is equally remarkable that when the theory is divested of its more extravagant trimmings, it can be found to contain a core element of truth.
Figure 1.
A sixteenth century woodcut illustrating a statement from an Alexandrian manuscript of the first century AD, stating that if a bull dismounts from the right side of a cow conception will result in a bull calf, whilst descending from the left side will result in a heifer calf. Redrawn by A.J. Lee, adapted from reference [25].
Throughout most of history, any theories about sex determination had to be formulated in the absence of knowledge of the component parts: eggs and sperm cells. The problem with cells is that they are extremely small and require compound microscopes and specialized techniques to observe them, which became available only in the seventeenth century. The Dutch scientist of the seventeenth century, Antonie van Leeuwenhoek, examined semen from men and dogs, and found it to be populated by a multitude of tiny, eel-like, little animals. He described his discovery of the spermatic animalcules in a letter to the Royal Society in London in 1676. But where did they come from, and what was their function? The animalcules became a subject of controversy, which lasted for more than a century and a half.
van Leeuwenhoek thought that the animalcules provided the substance of the embryo, while the egg provided the nourishment; but this seemed to run counter to the view that all living things originate from an egg. This latter view was popularized by the seventeenth century English physician William Harvey, famed for his discovery of the blood circulation, who was also a pioneer in the study of reproduction. Although Harvey failed to detect any connection between the ovary and the product of reproduction, he maintained in his book Exercitationes de Generatione Anmalium that “all animals whatsoever, even viviparous ones, nay man himself, are all engendered from an egg”. This publication contained a frontispiece of Jupiter holding an egg, from which all manner of animals emerged: on its shell, the words ex ovo omnia were engraved and subsequently much misquoted. Although the meaning of ‘egg’ then was not exactly the same as it is today, and despite the absence of evidence of any connection between ‘egg’ and embryo, it served to popularize an important idea that eventually proved its worth.
More information about the mammalian egg was provided by the Dutch physician and anatomist, Reinier de Graaf, who dissected female rabbits at various intervals after mating, and partly succeeded in tracing the ‘eggs’ from the ovary, through the oviduct, to the uterus. His publication on the female organs of generation appeared in 1672. Although de Graaf was aware that the egg, or ovum, in the oviduct was smaller than the ovarian follicle that now bears his name, he did not see the egg himself; but his conclusion that mammalian eggs and ovaries are directly comparable to those of birds has withstood the test of time. It also encouraged the seventeenth century idea that embryos are formed from eggs rather than spermatic animalcules. Indeed, it took another century and a half until Russian naturalist Carl von Baer described and illustrated the mammalian egg inside the Graafian follicle; but he regarded the spermatic animalcules as parasites, and perpetuated this idea by naming them ‘spermatozoa’. The riddle of the spermatozoon was eventually solved by the nineteenth century Swiss anatomist Albert von Kölliker, who concluded that spermatozoa are products of the testes that need to come into contact with the egg to produce an embryo (Sidebar A).
Sidebar A | Further reading.
Early Theories of Sexual Generation by F.J. Cole (1930) and The Birth of the Cell by H. Harris (1999) are two fascinating books describing the research that led to the discovery of spermatozoa and mammalian eggs. Early work on the origins of mitochondria is covered well in The Biogenesis of Mitochondria by D.B. Roodyn and D. Wilkie (1967), whilst the presence of mitochondrial DNA has since been amply confirmed (see reference [21] for example, or Böhm R, Herdegen T (2010) Mitogen-activated protein kinases and mitochondrial morphology and bioenergetics. Am J Neuroprot Neuroregen 10: 1–5).
The actual coming together was witnessed by the late nineteenth century German zoologist Oscar Hertwig in the sea urchin Toxopleustes lividus. He discovered that during fertilization two nuclei unite, one derived from the egg and the other from the spermatozoon. The last quarter of this century also witnessed the discovery of chromosomes and their naming by the German anatomist Wilhelm Waldeyer. The stage was set for the century of genetics.
Throughout most of history, any theories about sex determination had to be formulated in the absence of knowledge of the component parts: eggs and sperm cells
The year 1900 is widely acknowledged as the year of the ‘rediscovery’ of Gregor Mendel’s laws. The turn of the century was also accompanied by a sea change in the scientific explanation of sex determination from environmental to hereditary causes. In the first edition of his book, The Cell in Development and Inheritance (1896), the American scientist Edmund Wilson wrote: “the determination of sex is not by inheritance, but by the combined effect of external conditions”. In 1909, however, he wrote that in all probability sex was controlled by internal factors of the germ cells and not as a response to corresponding external conditions [3]. Wilson himself was one of the pioneers who brought about this change in outlook.
The first sex differences were found in insects and were based on chromosomes, rather than on the entities that Mendel called ‘factors’ and subsequently became known as genes. Wilson discovered two chromosomal differences in the spermatocytes (early sperm cells) of insects: either a chromosome was present in one class and absent in the other, or both classes contained two chromosomes that differed in size. The American geneticist Netty Stevens investigated the chromosomes in both sexes of the common meal worm Tenebrio molitor, and found that in males, but not in females, one chromosome was smaller than the others [4]. She concluded that this chromosome must be responsible for the development of the male sex. The larger chromosome of the pair became known as the ‘X’, the smaller one as the ‘Y’ and both as ‘sex chromosomes’.
The existence of the correct sex chromosome constitution in humans was not established until 45 years later, following the development of new cytological techniques that counteracted the natural tendency of mammalian chromosomes to clump together. In 1956, Joe Hin Tjio and Albert Levan announced the correct chromosome number in human cells to be 46 [5], and Charles Ford and John Hamerton showed that, in males, this number included an X and a Y chromosome [6].
In the years that followed, sex-chromosome abnormalities were discovered in quick succession. In 1959, the publication of three seminal papers shed light on the function of human sex chromosomes in sexual development. The publications were of a patient with Klinefelter syndrome, a male phenotype with an XXY chromosome set [7]; and two patients with Turner syndrome, an anomaly also known as ‘gonadal dysgenesis’, with a female phenotype and just a single X chromosome [8,9]. These findings demonstrated that the human Y chromosome, and it soon turned out, the Y chromosome of other mammalian species, determines male sex, and that male development does not depend on the number of X chromosomes; nor does female development depend on the presence of two X chromosomes.
Now that it was known that the mammalian Y chromosome had a male-determining function, scientists searched for the male-determining gene, named TDF (testis-determining factor). The choice of name was based on the idea that the fetal testis, by its hormonal secretion, has a pivotal role in switching the reproductive tract from a bipotential, able to develop in either the male or female direction, into the male pathway [10]. In a later publication, Alfred Jost and colleagues, who had originated this idea, announced that the Sertoli cell is the first detectable cell in the testis, which pinpointed the function of TDF/switching uncommitted somatic gonadal cells into Sertoli cells [11]. Previous evidence had indicated that the male-determining gene would be on the short arm of the Y chromosome [12].
Carl von Baer described and illustrated the mammalian egg inside the Graafian follicle; but he regarded the spermatic animalcules as parasites, and [named] them ‘spermatozoa’
In the years that followed, several candidate genes were proposed and later dropped, when further research argued against them. The search ended with the isolation of SRY from a 35 kb region of the Y chromosome [13], and a corresponding Sry gene from the Y chromosome of the mouse. The injection of Sry on a 15 kb genomic fragment into female embryos caused some of them to develop into sterile males [14]. Further evidence for the male-determining function of SRY in humans was obtained from sex-reversed XY females (this condition is now known as 46, XY complete gonadal dysgenesis), some of whom had mutations in SRY, which could explain their unusual sexual development [15]. It is widely believed that SRY/Sry is the only male-determining gene on the mammalian Y chromosome, although other Y-chromosomal genes are required for males to be fertile.
It also became evident that the development of a normal male phenotype requires the correct functioning of many autosomal (non-sex-chromosomal) genes. A review lists 12 non-Y-chromosomal genes that are involved in the development of testes, compared with five genes involved in ovary development [16]. Of special interest is the SRY-related gene SOX9, which needs to be upregulated by SRY to allow testis development. Mutations of SOX9 cause campomelic dysplasia, a severe disease of bone development, which is often associated with female development in patients with XY chromosomes, whereas duplication of SOX9 can lead to male development in the absence of SRY.
SRY is clearly important for the development of male sex, although in rare instances a male phenotype can develop in its absence; but what is the genetic pathway by which SRY creates hormonally competent testes? This proved to be difficult to disentangle, despite the expectation that once the Y-chromosomal male-determining gene was discovered, the elucidation of other genes following in the cascade from the bipotential gonadal rudiment to testis formation would quickly follow. Even after the discovery of further sex-determining genes, their relationship to SRY remains unknown. When a pathogenic mutation has been identified, the phenotypes can also be variable, even within the same family. It has been suggested that new genomic techniques might be required for better diagnoses of patients with disorders of sexual development. But might there be a simpler alternative pathway?
The twentieth century witnessed rapid progress in genetic research, which climaxed with the human genome project. However, in the field of sex determination there was also an unexpected discovery: temperature-dependent sex determination in reptiles [17]. That vertebrates as highly evolved as reptiles could develop into males or females without sex chromosomes and perhaps sex-determining genes was a surprise to many scientists, and indicated that there was, after all, no simple answer to the question of whether sex is determined by internal or external factors.
Temperature-dependent sex determination (TSD) is not an option for mammals and birds, as they control their own internal temperature within a narrow range. Although the evolution of sex chromosomes in our pre-mammalian ancestor is not known for certain, we could imagine that whilst a change from environmental to internal control of sex determination was taking place, it was necessary to replace the effects of temperature by genes.
It is also known that in various mammalian species, developing male gonads grow faster than ovaries at the same age; and as SRY/Sry, the mammalian testis-determining gene, has been slow to reveal its mode of function. Jenna Schmahl and colleagues investigated whether Sry might be involved in the increased growth of XY gonads in mice. Indeed, they reported that an increase in cell proliferation was the first detectable effect of Sry activity [18].
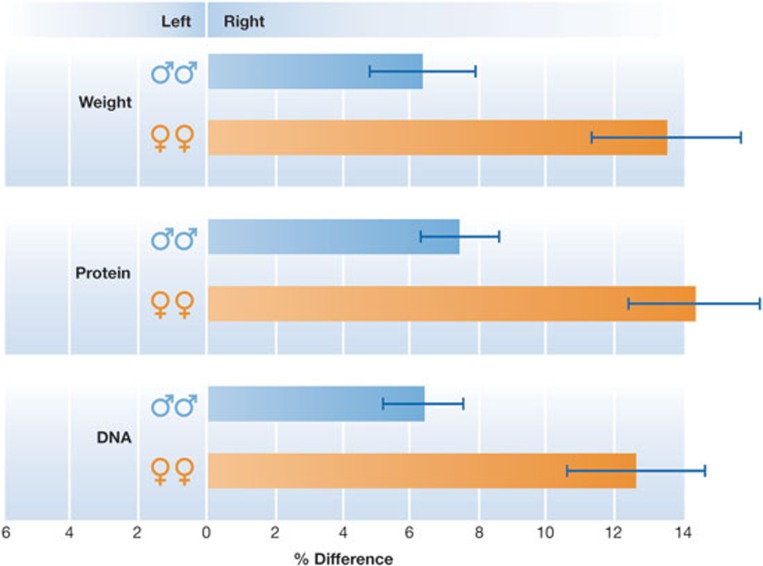
It therefore seems probable that sex-determining genes control aspects of cell growth that, at an earlier stage of evolution, were determined by differences in the environment. In addition to a lower rate of cell proliferation in the developing ovary, cell migration and vascularization are also either absent or occur at a lower rate, indicating that the difference in metabolic rate between male and females—which is so characteristic of mammals in later life—is already manifest in the fetus. Another strange fact about developing human gonads is that not only do testes develop faster than ovaries, but that fetal gonads that are situated on the right are ahead of their counterparts on the left (Fig 2). It looks almost as if the ancient Pythagoreans knew something that today's scientists still need to discover.
Figure 2.
Bilateral asymmetry of human fetal gonads with respect to weight, protein and DNA content (quality measured equals right minus left, expressed as percentage of the mean; mean ± standard error). Adapted from reference [26].
It is also important to remember that many non-mammalian vertebrates do not follow the same pattern of cellular activity seen during the determination of sex in mammals. In reptiles with temperature-dependent sex determination, the sex that develops depends on the species. In lizards and alligators, low temperatures give rise to females and high temperatures to males; in many turtles, high temperatures give rise to females and low temperatures to males. Horacio Merchant-Larios and Véronica Díaz-Hernández have proposed an update [19] to Emil Witschi's classical hypothesis [20] that two opposing substances, cortexin and medullerin—produced by the cortex and medulla of the genital ridge, respectively—give rise to either an ovary or a testis. They cite evidence that in turtles with TSD, epithelial cells of the central medullary cords express the important testis-determining gene SOX9, whereas cells of the surface epithelium do not, as long as the two epithelial lineages remain in contact. Merchant-Larios and Díaz-Hernández suggest that the regulatory network of each of the two epithelial lineages might be differentially influenced by temperature. Whichever cells sense the effect of temperature first, the subsequent process of differentiation into testis or ovary depends on accelerated growth of one of the two epithelial compartments [19].
The topic of energy metabolism has thus far not been important in genetics, but this might be about to change. A review in Nature by Luke O’Neill and Grahame Hardie concluded that, “Metabolism has recently returned with a vengeance to become a hot topic in biomedical research” [21]. Their insights should be equally relevant to the process of development of two sexual phenotypes from originally undifferentiated rudiments—a process that is also dependent on the availability of cellular energy.
As we have seen, the process of mammalian sex determination is accompanied by a higher rate of cell division in males than in females; in addition, male embryos have mesonephric cell migration, which is absent in female embryos. However, extra activity by an organism requires the consumption of extra energy, which is funnelled through the cellular bioenergetic systems, either by oxidative phosphorylation affected by mitochondria or by glycolysis, to the cells that require it.
The past two decades have witnessed massive progress in our understanding of cellular energy balance and the role of mitochondria in controlling this process. The number, volume and position of mitochondria within cells need to be regulated to satisfy local energy demands. However, damaged and malfunctioning mitochondria have been implicated in the causation of a growing number of diseases, particularly those of the central nervous system and of cancer [22].
That vertebrates as highly evolved as reptiles could develop into males or females without sex chromosomes and perhaps sex-determining genes was a surprise to many scientists…
It might be surprising that there seems to have been little attempt to study the role of mitochondria—often described as the powerhouses of cells—in sex determination, either during normal development, or to discover possible causative agents for disorders of sexual development. Studying energy metabolism in sex determination would seem to be particularly relevant, considering that differences in height, muscular strength and daily food requirements are some of the best-known distinctions between the sexes in our species. Moreover, there is evidence suggesting that, in some disorders of sexual development, faulty energy metabolism is in fact a probable cause.
Mitogen-activated protein kinases (MAPKs) have been shown to be major regulators of cellular proliferation and differentiation, as well as of mitochondrial structure, bioenergetics and mass (Sidebar A). Debora Bogani and colleagues found that in mice, a mutation of MAP3K4, when present in the homozygous form on a particular genetic background, causes sex reversal of XY gonads [23]. In embryos, the gonads show growth deficit and absence of mesonephric cell migration, in contrast with normal XY gonads at this stage. The embryos also showed reduced levels of Sry, as well as of Sox9, at the transcript and protein levels, suggesting that normal functioning of Sry requires an environment with sufficient energy availability. Mutations in MAPK can also lead to abnormal male development in human XY patients. Alexander Pearlman and colleagues have described two families with mutations in MAP3K1, the members of which included both males with genital abnormalities and XY females [24]. The authors also describe MAP3K1 mutations in mice, in which they gave rise to various degrees of sex reversal in XY gonads. It would be of great interest to compare the morphology and activity of mitochondria in these sex-reversed mouse gonads with those in normal ones. Moreover, new techniques have become available that promise to distinguish between research in the twenty-first and twentieth centuries for an increasing number of human diseases and abnormalities and, we might hope, will also help to illuminate questions about normal variants.
The study of sex determination has been made more complex by the discovery of further genes that are involved in the process. Combining sex determination with energy metabolism would almost certainly simplify the subject and make it more interesting and intelligible to more scientists. It would also be an advantage to reunite the two major components of the cell, nucleus and cytoplasm, and to understand their working as an integrated whole. It should perhaps be admitted that during the years of intensive research into genes and genomes, the cytoplasm has been unduly neglected.
Last, but not least, the realization that the difference between human males and females is based on an interaction between nuclear genes and cytoplasmically controlled energy requirements will, hopefully, increase our understanding of the difference between the sexes by placing it on a wider biological base.

Ursula Mittwoch
Footnotes
The author declares that she has no conflict of interest.
References
- Krappe AH (1936) The birth of Eve. In Gaster Anniversary Memorial Volume (eds Schindler B, Marmorstein A), pp 312–322. London, UK: Taylor's Foreign Press [Google Scholar]
- Galen (1968) On The Usefulness of Parts of the Body Vol 2, Book 14 (trans May MT). Ithaca, New York, USA: Cornell University Press [Google Scholar]
- Wilson EB (1909) Recent researches on the determination and heredity of sex. Science 29: 53–70 [DOI] [PubMed] [Google Scholar]
- Stevens NM (1905) Studies in spermatogenesis, with special reference to the accessory chromosome. Carnegie Institute Washington Publications 36: 1–32 [Google Scholar]
- Tjio JH, Levan A (1956) The chromosome number of man. Heriditas 42: 1–6 [Google Scholar]
- Ford CE, Hamerton JL (1956) The chromosome of man. Nature 178: 1020–1023 [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Strong JA (1959) A case of human intersexuality having a possible XXY sex-determining mechanism. Nature 183: 302–303 [DOI] [PubMed] [Google Scholar]
- Ford CE, Jones KW, Polani PE, de Almeida JC, Briggs JH (1959) A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner's syndrome). Lancet 1: 711–713 [DOI] [PubMed] [Google Scholar]
- Fraccaro M, Kaijser K, Lindsten J (1959) Chromosome complement in gonadal dysgenesis. Lancet 273: 886. [DOI] [PubMed] [Google Scholar]
- Jost A, Vigier B, Prépin J, Perchellet JP (1973) Studies in sex differentiation in mammals. Recent Prog Horm Res 29: 1–41 [DOI] [PubMed] [Google Scholar]
- Jost A, Magre S, Agelopoulou R (1981) Early stages of testicular differentiation. Hum Genet 58: 59–63 [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Ross A (1966) Structural abnormalities of the Y chromosome in man. Nature 210: 352–354 [DOI] [PubMed] [Google Scholar]
- Sinclair AH et al. (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved human binding motif. Nature 346: 240–244 [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgeneic for Sry. Nature 351: 115–121 [DOI] [PubMed] [Google Scholar]
- Affara NA, Chalmers IJ, Ferguson-Smith MA (1993) Analysis of the SRY gene in 21 sex-reversed XY females identifies four new point mutations in the conserved DNA binding domain. Hum Mol Genet 2: 785–789 [DOI] [PubMed] [Google Scholar]
- Eggers S, Sinclair A (2012) Mammalian sex determination – insights from humans and mice. Chromosome Res 20: 215–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieau C, Dorizzi M, Richard-Mercier N (1999) Temperature-dependent sex determination and gonadal differentiation in reptiles. Cell Mol Life Sci 55: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B (2000) Sry induces cell proliferation in the mouse gonad. Development 127: 65–73 [DOI] [PubMed] [Google Scholar]
- Merchant-Larios H, Díaz-Hernández V (2013) Environmental sex determination mechanisms in reptiles. Sex Dev 7: 95–103 [DOI] [PubMed] [Google Scholar]
- Witschi E (1967) Biochemistry of sex differentiation. In Biochemistry of Animal Development (ed Weber R), pp 195–225. New York, USA: Academic [Google Scholar]
- O'Neill LAJ, Hardie DG (2013) Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493: 346–355 [DOI] [PubMed] [Google Scholar]
- Duchen MR, Szabadkai G (2010) Roles of mitochondria in human disease. Essays Biochem 47: 115–137 [DOI] [PubMed] [Google Scholar]
- Bogani D et al. (2009) Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination. PLoS Biol 7: e1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman A et al. (2010) Mutations in MAP3K1 cause 46, XY disorders of sex development and implicate a common signal transduction pathway in human testis determination. Am J Hum Genet 87: 898–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittwoch U (1985) Erroneous theories of sex determination. J Med Genet 22: 164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittwoch U (1985) Males, females and hermaphrodites. Ann Hum Genet 50: 103–121 [DOI] [PubMed] [Google Scholar]