Abstract
Context:
The contribution of brown adipose tissue (BAT) to the energy balance in humans exposed to sustainable cold has not been completely established, partially because of measurement limitations of both BAT activity and energy expenditure (EE).
Objective:
The objective of the study was to characterize the role of BAT activation in cold-induced thermogenesis (CIT).
Design:
This study was a single-blind, randomized crossover intervention.
Setting:
The study was conducted at the National Institutes of Health Clinical Center.
Study Participants:
Thirty-one healthy volunteers participated in the study.
Interventions:
The intervention included mild cold exposure.
Main Outcomes:
CIT and BAT activation were the main outcomes in this study.
Methods:
Overnight EE measurement by whole-room indirect calorimeter at 24°C or 19°C was followed by 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (PET) scan. After 36 hours, volunteers crossed over to the alternate study temperature under identical conditions. BAT activity was measured in a 3-dimensional region of interest in the upper torso by comparing the uptake at the two temperatures.
Results:
Twenty-four volunteers (14 males, 10 females) had a complete data set. When compared with 24°C, exposure at 19°C resulted in increased EE (5.3 ± 5.9%, P < .001), indicating CIT response and mean BAT activity (10.5 ± 11.1%, P < .001). Multiple regression analysis indicated that a difference in BAT activity (P < .001), age (P = .01), and gender (P = .037) were independent contributors to individual variability of CIT.
Conclusions:
A small reduction in ambient temperature, within the range of climate-controlled buildings, is sufficient to increase human BAT activity, which correlates with individual CIT response. This study uncovers for the first time a spectrum of BAT activation among healthy adults during mild cold exposure not previously recognized by conventional PET and PET-computed tomography methods. The enhancement of cold-induced BAT stimulation may represent a novel environmental strategy in obesity treatment.
Cold-induced thermogenesis (CIT) can account for approximately 10% increase in energy expenditure (EE) (1–5), and its interindividual variability in humans is substantial (1, 6, 7). The demonstration of brown adipose tissue (BAT) in adults has prompted active research on this tissue as a target for obesity and diabetes treatment. Cold exposure is the natural afferent signal for BAT, and several reports indicate an association between BAT activity and favorable metabolic profile (8–11). The contribution of BAT activity, usually measured by 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET)-computerized tomography (CT), to CIT is unclear. To maximize BAT visualization, most reported PET-CT studies are performed after exposure to intense cold, which in turn can confound the assessment of the contribution of BAT to CIT because of shivering. The independent contribution of BAT to CIT in humans has been difficult to establish because quantitative parameters were seldom used (12, 13), cooling was intense or impractical, and radiation exposure is a concern (9, 13). The applicability of these findings to the CIT response encountered in daily life is therefore uncertain. We demonstrated that exposure to minimal cold, well within the range of climate-controlled buildings, generates variable CIT response in the absence of shivering (1). We thus hypothesized that these conditions are sufficient to induce a measurable activation of BAT.
Here we present a study aimed to characterize the prevalence of BAT activation and its contribution to individual CIT responses after minimal reduction in environmental temperature. To precisely quantify BAT activity, we developed and validated a novel PET-based analytical assay.
Materials and Methods
Study protocol
Details of the protocol and methods are reported in the Supplemental Material, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Volunteers provided informed consent for the participation in this study, which was approved by the National Institute of Diabetes and Digestive and Kidney Diseases-National Institute of Arthritis and Musculoskeletal and Skin Diseases Institutional Review Board. Briefly, healthy volunteers aged 18–60 years, with a body mass index of 20.0–27.0 kg/m2, were recruited. Females were studied in the early follicular phase. This was a randomized, single-blind, crossover study. After a 2-day equilibration diet, volunteers were assigned to a 12-hour overnight stay in a whole-room indirect calorimeter (respiration chamber) at either 24°C or 19°C and received a 15-mCi 18F-FDG dose via the air-tight port. After 1 hour of equilibration in the respiration chamber, the PET scan was performed in a temperature-controlled room. Study subjects wore hospital scrubs and slept in a bed with cotton sheets but no blankets. After 36 hours, volunteers crossed over to the alternate study temperature. This study design allowed attributing changes in EE between 19°C and 24°C solely to CIT response. Throughout the study, volunteers received a caffeine-free diet (50% carbohydrate, 20% protein, 30% fat). Caloric requirements were individually calculated. Each study volunteer underwent continuous recordings of EE and respiratory quotient as previously described (1).
PET studies
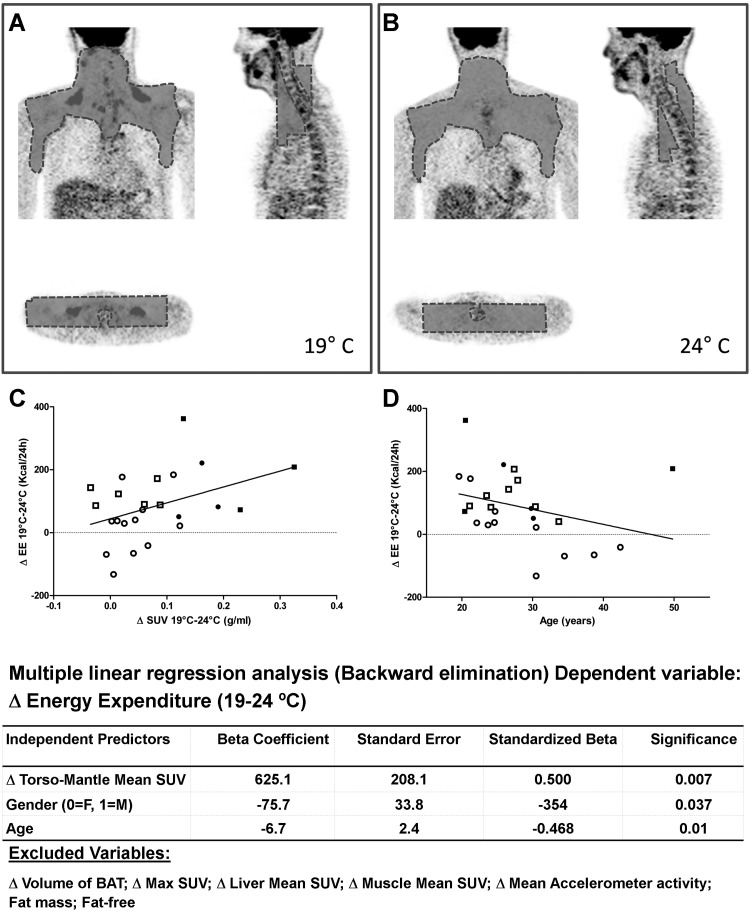
Volunteers were classified as BAT positive or BAT negative by a nuclear medicine physician (C.M.M.) blinded to the study temperature. Using a customized program, a 3-dimensional mantle region of interest of BAT activation was defined in the upper torso region from the skull base to the base of the heart, including cervical, supraclavicular, and superior mediastinal regions (12, 13), and excluding vertebrae and major muscle groups (Figure 1). The mantle was applied into the second PET image and slightly adjusted to fit within the same anatomic criteria. Three outcome parameters were derived to quantify BAT activation: 1) the maximum BAT standardized uptake value, representing the maximum intensity of standardized uptake value (SUV) of a single voxel, 2) the mean SUV of the entire torso-mantle region, representing the overall metabolic activity over the areas recognized to contain BAT in humans and 3) the volume of the activated BAT, defined as the sum of the volume of all voxels with SUV values of 2.0 g/mL or greater (8). Using this modality, we measured 18F-FDG uptake within the region of interest, even in subjects BAT negative (by conventional clinical detection modality). This novel method was validated by comparing BAT parameters derived simultaneously from either PET torso-mantle or conventional PET-CT in 6 volunteers. The mean SUV of the torso-mantle region correlated with BAT volume (R = 0.89, P = .017) and 18F-FDG uptake (R = 0.97, P < .001) (See Supplemental Material). The mean SUV of liver and gluteus maximus regions were also calculated to ascertain the response to mild cold exposure of other metabolically active tissues.
Figure 1.
Analysis of PET images using the torso-mantle method demonstrating 18F-FDG uptake in BAT depots located in the cervical-supraclavicular-thoracic region during exposure to 19°C in a 26-year-old man (A). No FDG uptake was visible when the scan was performed at 24°C (B). Correlations between BAT uptake, age, and cold-induced thermogenesis are shown. C, Correlation between cold-induced thermogenesis (expressed as the difference in EE between 19°C and 24°C) and BAT activation (SUV). D, Correlation between cold-induced thermogenesis and age. Square symbols, females; round symbols, males; filled symbols, BAT positive; empty symbols, BAT negative. Bottom, Stepwise regression analysis for independent contribution to cold-induced thermogenesis.
Physiological and laboratory parameters
Electrocardiography was recorded by a Holter monitor, and heart rate variability was measured using established methods (1). Skin and core temperatures were measured using ingestible capsules and dermal patches, and physical movements were measured using accelerometers (1). Blood samples were drawn immediately before 18F-FDG injection. Twelve-hour urine collections were also performed.
Statistical analysis
Prism 5 (GraphPad, La Jolla, California) and SPSS (IBM, Armonk, New York), were used for statistical analysis. An α error of .05 was considered the threshold for statistical significance. Bonferroni multiple comparison adjustments were made when appropriate.
Results (see also Supplemental Material)
Study volunteers and EE data
Twenty-four (14 males, 10 females, aged 28.1 ± 7.3 years) of 31 study volunteers had a complete data set of PET scans and EE recordings and were included in the analysis for the determinants of CIT. Cold exposure resulted in an increase in EE (5.3% ± 5.9%, P < .001) greater in women than in men (9.1% ± 4.3% vs 2.3% ± 5.3%, P = .002).
Hormonal data
Cold exposure resulted in an increase in urinary norepinephrine (P = .003) and cortisol (P < .001), and in serum TSH (P < .001) without significant changes in serum free T4, T3, or cortisol levels. Morning plasma ACTH levels were significantly lower after 12 hours of cold exposure (P = .004) (Table 1).
Table 1.
Cold-Stimulated BAT Activity, Energy Expenditure, and Laboratory Values (Data Are Reported as Mean ± SD)
| Entire Group (n = 24) |
Males (n = 14) |
Females (n = 10) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 24°C | 19°C | P | 24°C | 19°C | P | 24°C | 19°C | P | |
| 18F-FDG uptake | |||||||||
| Torso mantle mean SUV, g/mL | 0.78 ± 0.05 | 0.85 ± 0.09 | <.001 | 0.75 ± 0.05 | 0.82 ± 0.06 | .002 | 0.80 ± 0.04 | 0.90 ± 0.10 | .02 |
| BAT volume (>2.0 g/mL), cm3 | 28 ± 25 | 63 ± 76 | .009 | 23 ± 20 | 50 ± 55 | .06 | 35 ± 32 | 82 ± 99 | .08 |
| Torso mantle max SUV, g/mL | 4.16 ± 2.06 | 5.29 ± 2.85 | .03 | 3.61 ± 0.5 | 5.13 ± 2.8 | .05 | 4.94 ± 3.1 | 5.51 ± 3.0 | .37 |
| Liver mean SUV, g/mL | 1.79 ± 0.20 | 1.85 ± 0.18 | .07 | 1.83 ± 0.20 | 1.88 ± 0.19 | .28 | 1.71 ± 0.16 | 1.78 ± 0.14 | .11 |
| Muscle mean SUV, g/mL | 0.64 ± 0.11 | 0.65 ± 0.08 | .66 | 0.65 ± 0.13 | 0.65 ± 0.10 | .92 | 0.62 ± 0.08 | 0.63 ± 0.08 | .34 |
| Metabolic chamber | |||||||||
| Energy expenditure, kcal/h | 70.60 ± 12.34 | 74.27 ± 11.58 | .001 | 78.51 ± 10.47 | 80.31 ± 11.38 | .19 | 60.53 ± 4.84 | 66.57 ± 6.08 | .001 |
| Respiratory quotient | 0.834 ± 0.028 | 0.830 ± 0.038 | .36 | 0.831 ± 0.028 | 0.828 ± 0.031 | .67 | 0.839 ± 0.027 | 0.833 ± 0.046 | .46 |
| Laboratory data | |||||||||
| Urine norepinephrine, μg per 12 hours | 14.3 ± 7.0 | 18.5 ± 8.8 | .003 | 14.7 ± 5.7 | 17.6 ± 7.5 | .08 | 12.7 ± 8.6 | 20.3 ± 11.0 | .007 |
| TSH, μIU/mL | 2.43 ± 1.59 | 3.12 ± 1.80 | <.001 | 2.6 ± 1.6 | 3.3 ± 1.8 | .006 | 2.1 ± 1.5 | 2.7 ± 1.7 | .002 |
| Free T4, ng/dL | 0.98 ± 0.14 | 0.99 ± 0.14 | .448 | 1.00 ± 0.14 | 0.98 ± 0.13 | .36 | 0.94 ± 0.15 | 1.00 ± 0.14 | .03 |
| T3, ng/dL | 100.1 ± 17.3 | 102.5 ± 20.0 | .377 | 102.3 ± 15.5 | 104.7 ± 18.5 | .54 | 96.1 ± 20.3 | 98.6 ± 22.7 | .46 |
| ACTH, pg/mL | 53.3 ± 41.1 | 38.1 ± 27.7 | .004 | 56.6 ± 43.9 | 40.9 ± 31.1 | .04 | 47.4 ± 36.5 | 33.1 ± 20.6 | .05 |
| Cortisol, μg/dL | 14.5 ± 4.0 | 15.8 ± 4.3 | .197 | 15.0 ± 3.9 | 15.6 ± 4.4 | .64 | 13.6 ± 4.2 | 16.1 ± 4.4 | .09 |
| Urine cortisol, μg/g creatinine | 8.0 ± 8.5 | 15.4 ± 16.5 | <.001 | 6.7 ± 3.8 | 13.1 ± 7.9 | .002 | 10.6 ± 13.8 | 20.0 ± 26.7 | .0001 |
| Glucose, mg/dL | 84.7 ± 7.2 | 84.8 ± 7.0 | .544 | 87.8 ± 5.3 | 86.0 ± 5.4 | .07 | 78.9 ± 6.8 | 82.7 ± 9.2 | .25 |
| Insulin, mIU/mL | 3.1 ± 1.5 | 3.4 ± 2.0 | .466 | 3.1 ± 1.3 | 3.4 ± 2.2 | .42 | 3.2 ± 1.9 | 3.3 ± 1.6 | .80 |
| HOMA | 0.66 ± 0.33 | 0.71 ± 0.46 | .746 | 0.68 ± 0.31 | 0.73 ± 0.53 | .52 | 0.62 ± 0.38 | 0.67 ± 0.31 | .43 |
| Free fatty acids, μEq/L | 10.22 ± 4.46 | 11.33 ± 5.60 | .030 | 375.0 ± 173.1 | 418.5 ± 211.0 | .37 | 340.5 ± 132.6 | 372.5 ± 180.6 | .66 |
| Creatine kinase, U/L | 117.1 ± 74.0 | 156.1 ± 148.0 | .15 | 135.3 ± 81.10 | 180.70 ± 166.2 | .15 | 85.82 ± 44.48 | 116.0 ± 107.1 | .23 |
Abbreviation: HOMA, homeostasis model assessment. Statistical significance after Bonferroni correction for multiple comparisons is reported in bold. No correction was applied to the laboratory and hormonal data.
Seven subjects (4 males, 3 females) showed visually detectable 18F-FDG uptake at 19°C but none at 24°C. At 19°C, compared with 24°C, mean SUV, BAT volume, and maximum SUV within torso-mantle increased by 10.5% ± 11.1% (P < .001), 153.4% ± 192.2% (P = .001), and 31.7% ± 60.5% (P = .017), respectively. 18F-FDG uptake in liver and skeletal muscle did not show significant changes. The relative increase in mean SUV was similar in women and men (11.3% ± 13.9% vs 8.8% ± 8.6%, P = .60). During cold exposure core temperature was unchanged, whereas skin temperature dropped significantly.
Determinants of EE and CIT
As expected, individual EE at 24°C was closely associated with fat-free mass (adjusted r2 = 0.742, P < .001); no additional contributions from age, sex, fat mass, body surface area to volume ratio, or body movements were observed. CIT correlated negatively with age (unadjusted r = −0.425, P = .03). The interindividual CIT response correlated with mean SUV (r = 0.392, P = .05), BAT volume (r = 0.460, P = .02), and marginally with maximum SUV (r = 0.350, P = .09). No significant correlations were found between CIT and changes in SUV in liver and muscle or between changes in BAT parameters and changes in catecholamines, TSH, or cortisol. A multiple regression analysis was performed to determine factors affecting the individual CIT; the model included changes (difference 19–24°C) in the following: torso-mantle mean SUV, BAT volume, maximum SUV, muscle SUV, liver SUV, and movements. Age, gender, fat-free mass, and fat mass were also included. The difference in torso-mantle mean SUV (P = .007), age (P = .01), and gender (P = .037) were independent contributors to individual CIT (adjusted R2 = 0.441) (Figure 1).
Discussion
This study was designed to characterize the relationship between BAT and CIT response after minimal changes in environmental temperature. BAT activation, measured as uptake of 18F-FDG in the region in which BAT is commonly observed in adults, strongly correlates with individual CIT responses. This correlation, albeit not statistically significant, persists in individuals characterized as BAT negative by conventional diagnostic criteria. BAT activity, age, and gender were independent predictors of the individual's CIT variability.
This intervention allowed us to capture the range of BAT activation rather than its maximal stimulation, overcoming the ceiling effect of drastic interventions. The comparison of scans and EE recordings at 19°C and 24°C allowed assessing 18F-FDG uptake as a continuous variable, uncovering an independent causal relationship between BAT and CIT. Our demonstration of BAT activation by mild cold provides novel insight on nonshivering thermogenesis in free-living conditions, indicating that BAT is a physiologically relevant determinant in energy balance in humans.
The magnitude of CIT response in this study is similar to our previous daytime observation (1) but smaller than those reported by others (2, 13, 14). The reasons are two-fold. First, the marginal reduction in CIT observed during these nighttime studies is attributable to the decrease in EE during sleep (15). Second, we administered a prolonged and tolerable cold, avoiding spurious EE increase from muscle fasciculation.
The lack of CT scans questions whether all 18F-FDG uptake was located within anatomically defined fat depots; however, our approach was validated against conventional PET-CT. Furthermore, the recording of the average SUV of the 3-dimensional torso-mantle in which BAT is commonly observed has increased the sensitivity of our analysis because the PET-CT analysis of BAT based on a specific SUV threshold fails to capture low-diffuse activity. This is of particular relevance in view of the potential contribution of beige adipocytes within the fat depots (16), which cannot be identified by threshold-based PET-CT analysis. Finally, we included liver and gluteus as control reference points. The absence of significant SUV changes in these two tissues after cold exposure rules out the inadvertent inclusion in our calculation of spurious 18F-FDG uptake. Nonetheless, because the 18F-FDG uptake was somewhat increased (albeit not significantly) in the liver and gluteus, it is possible that these tissues partially contributed to the CIT response. We cannot exclude that our experimental conditions were insufficient to stimulate a robust CIT in males and that thermoneutrality may be higher in females (17), as also indicated by the relative increase in urine norepinephrine.
Consistent with our observations, cold exposure resulted in an increase in urinary catecholamine (1), indicating an increase in sympathetic nervous system tone, the canonical pathway of BAT stimulation, as confirmed by blood pressure, and heart rate variability data. Interestingly, BAT activity did not correlate with an increase in catecholamine levels or changes in heart rate. The dissociation between BAT and sympathetic activation suggests direct BAT stimulation rather than nonspecific global sympathetic nervous system up-regulation. The increase in urinary cortisol excretion during cold exposure suggests a minimal but significant degree of stress; the decrease in morning ACTH indicates a blunted peak secondary to the rise in nocturnal cortisol secretion. The rise in TSH is consistent with compensatory activation of the hypothalamus-pituitary-thyroid axis. The lack of increase thyroid hormones could be due to limited sensitivity of a single measurement; a change in TSH biorhythm cannot be ruled out.
The increase in EE is potentially clinically relevant, and BAT activity variability may contribute to overall energy balance, suggesting a protective role of BAT against obesity (9, 11, 18). Thus, BAT activation could represent a novel modality in obesity treatment (19) or weight maintenance. Over a 24-hour period, the CIT response observed in our study represents approximately 20% of the energy deficit commonly prescribed in dietary interventions (20). Projected over a 12-month period, the increase in EE resulting from our experimental conditions would be equivalent to approximately 20 days of fasting. This is obviously an extrapolation because it does not take into account behavioral and metabolic compensatory responses to negative energy balance.
In conclusion, this study demonstrates that a small reduction in temperature, well within the range of climate-controlled buildings, is sufficient to increase BAT activity in humans. This study uncovers for the first time a spectrum of BAT activation not limited to individuals with visible BAT on PET imaging during mild cold exposure, not previously recognized by conventional PET-CT analyzing methods.
Acknowledgments
We gratefully acknowledge the help and professionalism of the nursing, laboratory, and ancillary personnel of the National Institutes of Health Clinical Center. The critical revision of the data from Xiongce Zhao, PhD [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)] Statistical Core, is gratefully acknowledged. This research could have not been accomplished without the selfless participation of the study volunteers. The authors are grateful to Drs Lynnette Nieman (Eunice Kennedy Shriver National Institute of Child Health and Human Development) and Phillip Gorden (NIDDK) for their invaluable encouragement and suggestions. This study was registered (clinicaltrials.gov) with the identifier number NCT00521729.
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, Programs Z01-DK047057-06 and Z01-DK075001-09 (to F.S.C.), and Program Z01 DK071014 (to K.Y.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- CT
- computerized tomography
- CIT
- cold-induced thermogenesis
- EE
- energy expenditure
- 18F-FDG
- 2-[18F]-fluoro-2-deoxy-D-glucose
- PET
- positron emission tomography
- SUV
- standardized uptake value.
References
- 1. Celi FS, Brychta RJ, Linderman JD, et al. Minimal changes in environmental temperature result in a significant increase in energy expenditure and changes in the hormonal homeostasis in healthy adults. Eur J Endocrinol. 2010;163:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoneshiro T, Aita S, Matsushita M, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011;19:1755–1760 [DOI] [PubMed] [Google Scholar]
- 3. Claessens-van Ooijen AM, Westerterp KR, et al. Heat production and body temperature during cooling and rewarming in overweight and lean men. Obesity (Silver Spring). 2006;14:1914–1920 [DOI] [PubMed] [Google Scholar]
- 4. Dauncey MJ. Influence of mild cold on 24 h energy expenditure, resting metabolism and diet-induced thermogenesis. Br J Nutr. 1981;45:257–267 [DOI] [PubMed] [Google Scholar]
- 5. DeGroot DW, Havenith G, Kenney WL. Responses to mild cold stress are predicted by different individual characteristics in young and older subjects. J Appl Physiol. 2006;101:1607–1615 [DOI] [PubMed] [Google Scholar]
- 6. van Marken Lichtenbelt WD, Schrauwen P, van De Kerckhove S, Westerterp-Plantenga MS. Individual variation in body temperature and energy expenditure in response to mild cold. Am J Physiol Endocrinol Metab. 2002;282:E1077–E1083 [DOI] [PubMed] [Google Scholar]
- 7. Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr. 2002;56:288–296 [DOI] [PubMed] [Google Scholar]
- 8. Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–E606 [DOI] [PubMed] [Google Scholar]
- 11. Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96:192–199 [DOI] [PubMed] [Google Scholar]
- 12. Yoneshiro T, Aita S, Matsushita M, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011;19:13–16 [DOI] [PubMed] [Google Scholar]
- 13. Ouellet V, Labbe SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14:272–279 [DOI] [PubMed] [Google Scholar]
- 15. White DP, Weil JV, Zwillich CW. Metabolic rate and breathing during sleep. J Appl Physiol. 1985;59:384–391 [DOI] [PubMed] [Google Scholar]
- 16. Bostrom P, Sparks LM, Ye I, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253 [DOI] [PubMed] [Google Scholar]
- 18. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508 [DOI] [PubMed] [Google Scholar]
- 19. Celi FS. Brown adipose tissue—when it pays to be inefficient. N Engl J Med. 2009;360:1553–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Heart, Lung, and Blood Institute The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Bethesda, MD: National Heart, Lung, and Blood Institute; 2000 [Google Scholar]