Abstract
Objective:
Our objective was to characterize changes in bone resorption in relation to the final menstrual period (FMP), reproductive hormones, body mass index (BMI), and ethnicity.
Methods:
Urinary type I collagen N-telopeptide (NTX), estradiol, and FSH levels were measured annually for up to 8 years spanning the menopause transition in 918 African American, Chinese, Japanese, or Caucasian women.
Results:
Urinary NTX began to increase sharply about 2 years before the FMP, reaching its peak level about 1 to 1.5 years after the FMP. NTX levels declined modestly from 2 to 6 years after the FMP but remained about 20% higher than before the menopause transition. The sharp rise in FSH occurred in conjunction with a sharp decline in estradiol and shortly after FSH levels began increasing rapidly. The mean increase in urinary NTX across the menopause transition was greatest in women with BMI <25 kg/m2 and smallest in women with BMI >30 kg/m2. Increases in NTX were greatest in Japanese women and smallest in African Americans. These differences were attenuated, but not eliminated, when analyses were adjusted for covariates, particularly BMI.
Summary:
During the menopause transition, a decline in ovarian function beginning about 2 years before the FMP is followed by an increase in bone resorption and subsequently by bone loss. The magnitude of the increase in bone resorption is inversely associated with BMI. Ethnic differences in changes in bone resorption are attenuated, but not eliminated, by adjustment for BMI. Ethnic differences in BMI, and corresponding ethnic differences in bone resorption, appear to account for much of the ethnic variation in perimenopausal bone loss.
Accelerated bone loss is a hallmark of the menopause transition. We recently reported that the rate of bone loss accelerates about 1 year before the final menstrual period (FMP) and remains high until about 2 years after the FMP (1). During the last 20 years, techniques have become available to measure a variety of small molecules that are released from the skeleton during bone resorption, including pyridinolines, deoxypyridinolines, and C-terminal and N-terminal telopeptides of type I collagen. With these assays, numerous cross-sectional studies have shown that both urinary and serum type I collagen N-telopeptide (NTX) levels are higher in perimenopausal and postmenopausal women than in premenopausal women (2–6), changes that are generally attributed to diminished estrogen production by perimenopausal and postmenopausal ovaries (2, 7–9). Although cross-sectional studies can assess differences in bone turnover markers between premenopausal and postmenopausal women, they cannot determine when the differences arise, nor can they characterize the temporal interrelationships between changes in reproductive hormones, bone turnover, and bone mineral density (BMD). Longitudinal assessments are needed to delineate those relationships. However, to our knowledge, no studies have reported longitudinal measurements of bone formation or resorption markers beginning while women are still premenopausal and continuing until they have had their FMP. Moreover, little is known about the impact of body mass index (BMI) on changes in bone resorption across the menopause transition, although heavier women do lose bone more slowly (10–14). To address these gaps in our knowledge, we measured urinary NTX, a marker of bone resorption, reproductive hormones, and BMD annually for up to 8 years in a large, multiethnic cohort of women beginning when they were premenopausal or early perimenopausal and continuing until they had their FMP or beyond.
Subjects and Methods
Study population
The Study of Women's Health Across the Nation (SWAN) is a 7-site, longitudinal cohort study from community-based groups of women (15). At baseline, 3302 women between the ages of 42 and 52 years who had menstruated at least once in the previous 3 months and were not taking ovarian steroids were enrolled. The SWAN Bone Density Substudy was conducted in 2407 of these women at 5 of the 7 SWAN clinical sites located in Boston, southeast Michigan, Los Angeles, Oakland, and Pittsburgh. All sites enrolled Caucasian women. Additionally, the Boston, Michigan, and Pittsburgh clinical sites enrolled African American women, whereas the Los Angeles and Oakland sites enrolled Japanese and Chinese women, respectively. This report includes information from all 918 women in the SWAN Bone Density Substudy who experienced a “natural” FMP during the first 8 years of follow-up, ie, they did not use postmenopausal estrogen or progestin therapy or bone-active medications (bisphosphonates, selective estrogen receptor modulators, glucocorticoids, etc,) before their FMP, and did not have a hysterectomy or bilateral oophorectomy before their FMP. The protocol was approved by the Institutional Review Board at each site, and all participants provided written informed consent.
Study protocol
Participants were seen annually for up to 8 years. At each visit, subjects were asked to collect a fasting, non–first-void urine sample before 10:00 am to measure urinary NTX and creatinine (Cr) and a fasting blood sample to measure serum estradiol and FSH levels. Subjects whose menstrual cycles were still regular were asked to provide the urine and blood specimens between days 2 and 5 of their menstrual cycle. Weight and height were measured using calibrated scales and stadiometers.
Biochemical measurements
Urinary NTX and Cr
Urine samples were stored at −80°C until assays were performed. Urine NTX was measured in singlicate using an automated immunoassay (Vitros ECi; Ortho Clinical, Rochester, New York) in the Unipath Laboratories (now SPD Development Company Ltd) using standards and controls of the manufacturer. Urine NTX is expressed in nanomoles of bone collagen equivalents (BCE) and then indexed to urine Cr so that the final values are expressed as nanomoles BCE per millimole Cr. The lower limit of detection of NTX was 10 nmol BCE. Intra- and interassay coefficients of variation were 2.8% and 4.8%, respectively. Unipath Laboratories participated in the United Kingdom External Quality Assessment Scheme (NEQAS). All measurements were within specification and near the median value for all laboratories in the United Kingdom. Urine Cr was measured on a Cobas Mira instrument (Horiba ABX, Montpellier, France) using the Jaffé reaction. The lower limit of detection was 0.014 mM, and the intra- and interassay coefficients of variation were 0.6% and 4.1%, respectively.
Serum estradiol and FSH
Serum estradiol concentrations were measured in duplicate with a modified, off-line ACS:180 (E2-6) immunoassay using an ACS:180 automated analyzer (Bayer Diagnostics Corporation, Tarrytown, New York). The lower limit of detection was 1.0 pg/mL, and intra- and interassay coefficients of variation averaged 6.4% and 10.6%, respectively. Serum FSH concentrations were measured in singlicate with a 2-site chemiluminometric immunoassay. The lower limit of detection was 1.05 mIU/mL, and intra- and interassay coefficients of variation averaged 6% and 12%, respectively.
Menopause status
At each annual visit, menopause stage was determined based on reports about frequency and regularity of menstrual bleeding. Women were classified as premenopausal if they had experienced at least 1 menstrual period in the last 3 months with no change in the regularity of their menstrual bleeding during the last year. Women were classified as early perimenopausal if they had experienced at least 1 menstrual period in the last 3 months with some change in the regularity of their menstrual bleeding during the last year. Women were classified as late perimenopausal if they had experienced no menstrual bleeding in the last 3 months but some menstrual bleeding during the last 11 months. Women were classified as postmenopausal once they had experienced at least 12 consecutive months of amenorrhea. The FMP was defined retrospectively to the closest month after 12 months of amenorrhea.
Covariates
BMI was calculated from measurements of weight and height [BMI = weight in kilograms/(height in meters)2]. Race/ethnicity was classified by self-report as Caucasian, African-American, Chinese, or Japanese. Baseline physical activity was assessed with a modified Baecke instrument using measures of active living, home, and recreational physical activity (16, 17). Smoking status, menstrually defined menopause transition stage, and prescription medication use were assessed by self-report. The time of urine collection was classified as days 2 to 5 or not days 2 to 5.
Data analyses
A semiparametric stochastic mixed-effects modeling approach was used to incorporate smoothing spline functions for quantifying instantaneous dynamic trajectories of NTX, estradiol, and FSH across time in relation to the number of years before and after the FMP (8, 9, 18, 19). To describe the patterns of change in NTX and serum hormone levels and to estimate the timing of significant changes in these patterns in relation to the FMP, the instantaneous rates of change of the trajectories were estimated with first-order derivatives. The 95% confidence bands were obtained using bootstrapping with 1000 bootstrap samples (20, 21). To estimate the amount of change across the menopause transition from a slope, the data were organized into epochs by setting hinges or turning points, at which the slopes differed significantly and were estimated using Bayesian model averaging methodology (22, 23) and piecewise linear mixed regression models (24). Statistical comparisons of differences in the slopes of 2 consecutive intervals around a turning point were tested using piecewise linear mixed models. If different, the slopes were used as an estimate of the mean amount of change per unit time (annually), and the difference of the 2 slopes was used to identify the change in magnitude of rate. Using this approach, we assessed the timing of the onset of the rise in serum FSH and urine NTX and the fall in serum estradiol in relation to the FMP and the timing of their subsequent slowing in relation to the FMP.
The mean peak NTX value was calculated from each subject's highest NTX value after the FMP. The mean net increase in NTX across the transition was calculated by subtracting the lowest NTX value before the FMP from the highest value after the FMP in each woman and then calculating the average value. For analyses by categories of BMI, subjects were classified as normal (BMI < 25 kg/m2, n = 425), overweight (BMI = 25–30 kg/m2, n = 211), or obese (BMI > 30 kg/m2, n = 282). The net increase in NTX across the transition in each ethnic group was initially determined for all 918 study subjects (African American n = 276; Caucasian, n = 397; Chinese, n = 121; and Japanese, n = 124). Because we previously found that ethnic differences in BMD and rates of bone loss across the menopause transition were largely due to ethnic differences in BMI (10, 25), we then calculated the least squares mean value for the net increase in urine NTX across the transition in each ethnic group in a subset of women whose BMI was less than 29 kg/m2, a value selected because it included substantial numbers of women from all 4 ethnic groups. Within this BMI-restricted subgroup, further adjustments were made for covariates that differed by ethnicity, including BMI. If subjects underwent bilateral oophorectomy or initiated hormone therapy (HT) after their FMP, their data were censored at the time of HT initiation use or at the time of surgery.
Model selection and goodness of fit were evaluated using likelihood ratio tests and the Akaike information criterion. Data management and data analyses were undertaken using Matlab version 7.0 (The MathWorks, Inc, Natick, Massachusetts) and SAS version 9.3 (SAS Institute, Cary, North Carolina) including SAS macro language and SAS/IML.
Results
Baseline characteristics and protocol completion
Table 1 shows baseline characteristics of the 918 women in whom a natural FMP was observed, (who comprise the analytic cohort for this report) and the remaining 1489 women who did not have a natural FMP during the observation period. The groups were similar except for slightly higher mean age and mean baseline FSH level in the women in the analytic dataset, as might be expected because many of the women who were excluded from the analytic dataset had yet to complete the menopause transition.
Table 1.
Baseline Characteristics (Median [Interquartile Range])
| All Women | Caucasian | African American | Chinese | Japanese | P valuea | |
|---|---|---|---|---|---|---|
| Women who experienced a natural FMP | ||||||
| n (%) | 918 (100) | 397 (43.2) | 276 (30.1) | 121 (13.2) | 124 (13.5) | |
| Age, y | 47 (3)b | 47 (4)b | 46 (4)b | 47 (4)b | 47 (3)b | .0001 |
| BMI, kg/m2 | 25.9 (10.0) | 26.3 (9.3) | 30.4 (10.8) | 22.3 (4.0) | 22.1 (3.6) | .2506 |
| Age at FMP, y | 51.3 (3.5) | 51.2 (3.6) | 51.0 (3.5) | 51.4 (3.6) | 51.9 (3.1) | NA |
| Physical activity score | 7.6 (2.3)c | 8.0 (2.3) | 7.2 (2.5) | 7.3 (2.6) | 7.7 (2.0) | .0239 |
| FSH, IU/L | 19 (20)b | 19 (20)b | 18 (22)c | 21 (21)b | 20 (19)b | .0001 |
| Estradiol, pg/mL | 57 (59) | 58 (61) | 62 (61) | 49 (58) | 46 (55) | .5271 |
| NTX, nmol BCE/mmol Cr | 32.1 (18.6) | 33.7 (19.3) | 33.0 (18.3) | 25.7 (15.1) | 28.9 (16.5) | .2510 |
| Women who did not experience a natural FMP | ||||||
| n (%) | 1489 (100) | 796 (53.5) | 407 (27.3) | 129 (8.7) | 157 (10.5) | |
| Age, y | 46 (4) | 46 (4) | 46 (4) | 47 (4) | 47 (4) | |
| BMI, kg/m2 | 26.1 (9.5) | 26.1 (8.6) | 30.5 (10.7) | 22.4 (4.0) | 22.1 (4.2) | |
| Age at FMP, y | NA | NA | NA | NA | NA | |
| Physical activity score | 7.7 (2.5) | 8.1 (2.5) | 7.3 (2.5) | 7.3 (2.5) | 7.8 (2.2) | |
| FSH, IU/L | 16 (15) | 16 (14) | 16 (17) | 16 (17) | 15 (14) | |
| Estradiol, pg/mL | 55 (54) | 56 (54) | 56 (55) | 49 (54) | 51 (55) | |
| NTX, nmol BCE/mmol Cr | 31.9 (17.8) | 33.0 (18.7) | 32.1 (17.6) | 26.3 (14.2) | 30.6 (17.4) |
Abbreviation: NA, not available.
P value for nonparametric Kruskal-Wallis Test of overall comparison of women who experienced a natural FMP vs women who did not experience a natural FMP.
P < .0001 vs women who did not experience a natural FMP.
P < .05 vs women who did not experience a natural FMP.
Table 2 shows the reasons that the 1489 women were excluded from the analytic cohort: 905 women had not had their FMP yet (205 of whom were on HT, making detection of the FMP impossible), 153 women had a surgically induced FMP, 416 women had used HT before their FMP (some of whom also used other exclusionary medications), 13 had other exclusions (eg, medications known to affect bone turnover), and 2 had missing data. Sixty-eight of these women also had used other osteoporosis medications, and 88 also had a history of significant glucocorticoid use. As has been reported previously, African Americans had a disproportionate number of hysterectomies.
Table 2.
Reasons That Subjects Did not Experience a Natural FMP (by Hormone Therapy Use)
| Reasons | Total excluded from analysis | Caucasian | African American | Chinese | Japanese |
|---|---|---|---|---|---|
| Not on HT | 760 | 375 | 239 | 65 | 81 |
| Still menstruating | 700 | 351 | 207 | 64 | 78 |
| Hysterectomy before FMP | 45 | 21 | 21 | 1 | 2 |
| Other | 13 | 1 | 11 | 0 | 1 |
| Missing | 2 | 2 | 0 | 0 | 0 |
| On HT | 729 | 421 | 168 | 64 | 76 |
| Still menstruating | 205 | 117 | 38 | 26 | 24 |
| Hysterectomy before FMP | 108 | 47 | 46 | 5 | 10 |
| Other | 416 | 257 | 84 | 33 | 42 |
| Total | 1489 | 796 | 407 | 129 | 157 |
The 2407 women in the entire cohort completed 16 003 of a possible 17 771 (90%) study visits. In those 16 003 visits, 15 537 (97.1%) of the urine samples for NTX were collected per protocol (before 10:00 am), 440 (2.7%) were collected after 10:00 am, and 26 (0.2%) did not have time of collection recorded. The 918 women who had a natural FMP during the study period completed 6477 of a possible 7204 (90%) study visits. In those 6477 visits, 6284 (97.0%) of the urine samples for NTX were collected per protocol (before 10:00 am), 185 (2.9%) were collected after 10:00 am, and 8 (0.1%) did not have time of collection recorded.
Changes in urine NTX in relation to the FMP
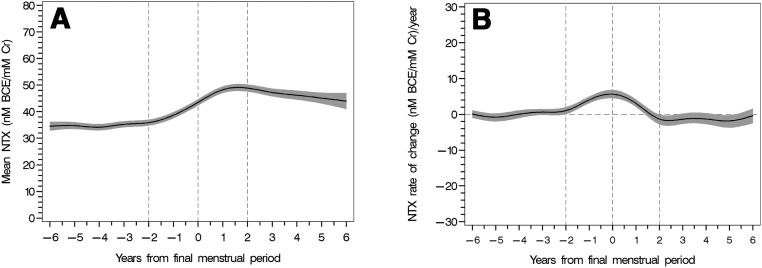
Mean ± SEM urine NTX levels were fairly constant from 6 years before the FMP until 2 years before the FMP at which time they began to rise sharply, peaking about 1.3 years after the FMP (Figure 1). Urine NTX levels increased at a rate of 4.5 ± 0.2 nmol/mmol Cr per year (P < .0001) from a mean level of 35 ± 1 to peak level of 49 ± 1 nmol BCE/mmol Cr during the 4-year interval spanning the FMP (P < .0001). Thereafter, NTX levels declined at a rate of 1.4 ± 0.3 nmol/mol Cr (P < .0001) before stabilizing at a level of 47 ± 1 nmol/mmol Cr approximately 4 to 6 years after the FMP. The peak rate of change in NTX occurred at the time of the FMP (Figure 1B). Changes in NTX were adjusted for race/ethnicity, BMI, smoking status, time of urine collection, physical activity, menopause status, and time to FMP. BMI accounted for only 0.5% of the variation in NTX, and the complete model explained an additional 16.2%.
Figure 1.
Mean urine NTX levels (A) and instantaneous rates of change (B) in relation to years from the FMP in the women who experienced a natural FMP (n = 918). The shaded areas denote the 95% confidence intervals.
Relationships between changes in NTX, estradiol, and FSH
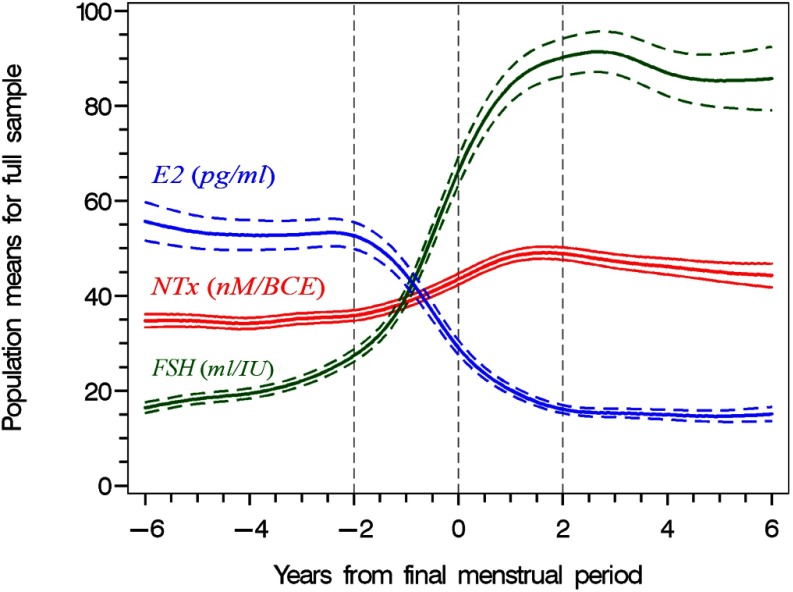
Serum estradiol levels began to fall rapidly about 2 years before the FMP, reached their nadir about 2 years after the FMP, and then remained stable (Figure 2). The rate of change of estradiol was a virtual mirror image of pattern of change in NTX with a maximal rate of decline at the time of the FMP. Serum FSH levels increased slowly in the period from 6 to 2 years before the FMP with a marked acceleration beginning shortly before the rapid rise in NTX and the sharp decline in estradiol levels. FSH levels peaked about 1.5 to 2 years after the FMP, declined slightly 2 to 4 years after the FMP, and then remained stable.
Figure 2.
Population mean urine NTX, serum estradiol, and serum FSH levels in relation to years from FMP in the women who experienced a natural FMP (n = 918). The dashed lines denote the 95% confidence intervals.
Relationships between BMI and changes in urine NTX across the transition
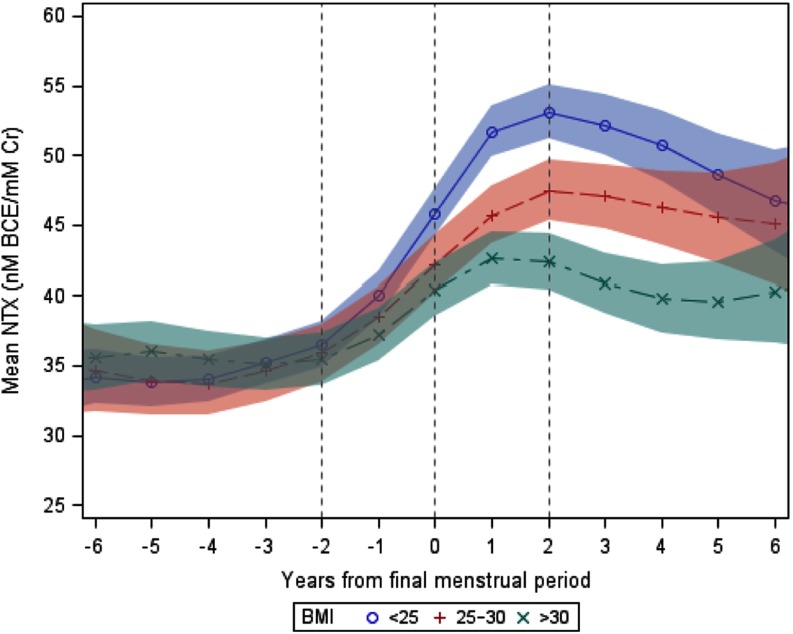
Table 3 shows the baseline characteristics of the women who experienced a natural FMP when grouped according to baseline BMI. Changes in NTX levels were minimal and similar in the 3 BMI categories in the period from 6 to 2 years before the FMP (Figure 3). Urine NTX levels began to increase sharply about 2 years before the FMP in women with BMI <25 or 25 to 30 and about 1.5 years before the FMP in women with BMI >30 kg/m2. Mean unadjusted rates of rise in urine NTX from the onset of the rapid rise (1.5–2 years before the FMP) until NTX levels reached a plateau (1–2 years after the FMP) were fastest in women with BMI <25 kg/m2 (5.7 ± 0.3 nmol BCE/mmol Cr per year, P < .0001), intermediate in women with BMI 25–30 kg/m2 (3.8 ± 0.4 nmol BCE/mmol Cr per year, P < .0001), and slowest in women with BMI >30 kg/m2 (3.3 ± 0.5 nmol BCE/mmol Cr per year, P < .0001). Similarly, peak urine NTX levels and the net increase in NTX levels were greatest in women with BMI <25 kg/m2 and smallest in women with BMI >30 kg/m2 (Table 4).
Table 3.
Baseline Clinical Characteristics of Subjects According to Baseline BMI
| BMI, kg/m2 |
|||
|---|---|---|---|
| <25 | 25–30 | >30 | |
| n | 418 | 208 | 282 |
| Race, n (%) | |||
| Caucasian | 169 (40.4) | 101 (48.6%) | 122 (43.3%) |
| African American | 55 (13.2) | 74 (35.6) | 146 (51.8) |
| Chinese | 94 (22.5) | 20 (9.6) | 6 (2.1) |
| Japanese | 100 (23.9) | 13 (6.3) | 8 (2.8) |
| Smoking, n (%) | |||
| Never | 270 (64.6) | 118 (56.7) | 147 (52.1) |
| Past | 89 (21.3) | 53 (25.5) | 80 (28.4) |
| Current | 59 (14.1) | 37 (17.8) | 55 (19.5) |
| Age, ya | 47.0 (3.7) | 47.2 (4.1) | 47.0 (4.0) |
| Age at FMP, ya | 51.3 (3.5) | 51.3 (3.0) | 51.4 (3.6) |
| Physical activity scorea | 8.0 (2.4) | 7.8 (2.2) | 7.1 (2.4) |
| Estradiol, pg/mLa | 62 (66) | 53 (59) | 53 (52) |
| FSH, IU/La | 20.4 (23.3) | 19.7 (23.2) | 16.6 (15.5) |
| NTX, nmola BCE/mmol Cra | 31.9 (19.6) | 32.0 (17.6) | 32.7 (17.8) |
Median (interquartile range).
Figure 3.
Mean urine NTX levels and 95% confidence limits (shaded areas) in relation to years from the FMP in women with BMI <25 kg/m2 (blue circles and shading), 25–30 kg/m2 (red + and shading), or >30 kg/m2 (green x and shading).
Table 4.
Mean ± SE Pre-FMP Trough, Post-FMP Peak, and Net Change in NTX by BMI Category
| BMI Category, kg/m2 | Pre-FMP Trough Valuea | Post-FMP Peak Valueb | Net Changec |
|---|---|---|---|
| <25 | 27.1 ± 0.7 | 62.7 ± 1.1d | 35.4 ± 1.0e |
| 25 < BMI < 30 | 27.3 ± 0.9 | 57.5 ± 1.7d | 29.8 ± 1.5e |
| >30 | 26.1 ± 0.8 | 51.3 ± 1.4d | 23.9 ± 1.3e |
Mean trough values calculated using the subject-specific lowest value before the FMP.
Mean peak values calculated using the subject-specific highest value after the FMP.
Mean net change calculated using the subject-specific difference of peak minus trough value.
P < .01 vs peak value in other BMI categories.
P < .005 vs net change in other BMI categories.
Ethnic patterns of NTX across the menopause transition
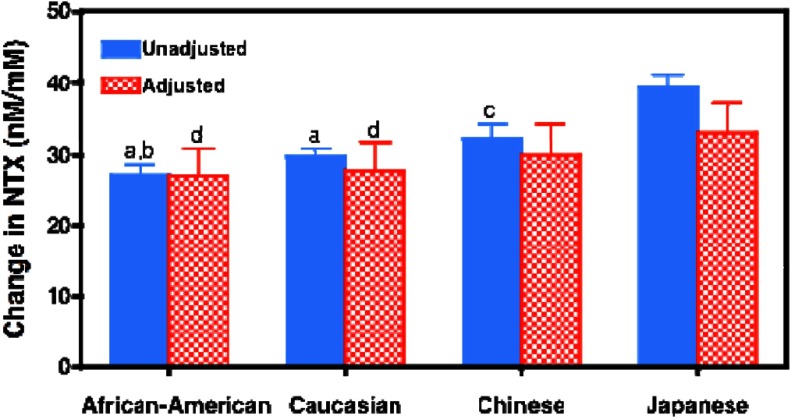
Figure 4 shows the average net increase in urinary NTX across the menopause transition in African American, Caucasian, Chinese, and Japanese women with and without stratification and adjustment for BMI. The time of onset of the sharp rise in NTX was similar in all 4 race/ethnic groups (data not shown). Before stratification based on BMI and adjustment for covariates, increases in urinary NTX levels were largest in Japanese women, followed by Chinese, Caucasian, and African American women (P < .01 for Japanese vs each of the other 3 groups and P < .05 for Chinese vs Caucasian women). Restricting the analysis to women with BMI <29 kg/m2 and adjusting for BMI reduced the difference in the average net increase in urine NTX between Japanese and African American women by nearly 50%. Additional adjustment for other covariates that differed by ethnicity (physical activity, age at FMP, date of collection [within days 1–5 or not within days 1–5], smoking, SWAN site, and BMI did not further reduce the difference between Japanese and African American women. The average net increase in urinary NTX across the menopause transition remained significantly greater in Japanese women than in the Caucasian or African American women in the fully adjusted, BMI-stratified model (P < .001 for both comparisons).
Figure 4.
Unadjusted (blue solid bars) and adjusted (red hatched bars) mean ± SEM net changes in urine NTX levels across the entire menopause transition in African American, Caucasian, Chinese, or Japanese women. For each woman, the net increase in NTX across the transition was calculated as the difference between the lowest NTX value from any of her visits before the FMP and the highest value from any of her visits after the FMP. Because the values in each woman represent the maximal net change in NTX across the menopause transition, these changes are greater than the difference between the overall mean NTX values before and after the FMP. Unadjusted mean net changes in urine NTX are the average net increases for all women in each of the 4 race/ethnic groups. The adjusted values were generated by restricting the cohort to women with BMI <29 and then adjusting for covariates that differed by ethnicity including study site, BMI, smoking, alcohol intake, physical activity, age at FMP, and the date of the blood draw. a, P < .0001 vs unadjusted values of Japanese women; b, P = .031 vs unadjusted values of Chinese women; c, P < .01 vs unadjusted values of Japanese women; d, P < .01 vs adjusted values of Japanese women.
Discussion
In this study, we demonstrated that bone resorption begins to increase above premenopausal levels about 2 years before the FMP, continues to increase for about 1 to 1.5 years after the FMP, declines slightly for a short time, and then remains relatively stable from 4 to 6 years after the FMP. Increases in urine NTX occurred in conjunction with decreases in serum estradiol levels, both of which began about 2 to 3 months after serum FSH levels began to rise steeply. As we reported previously for BMD (10, 25), body size was an important determinant of bone resorption, and adjustment for ethnic differences in body size attenuated apparent ethnic differences in bone resorption during the transition.
Only a few previous studies have examined longitudinal changes in BMD and/or bone turnover across the menopause transition beginning while women were premenopausal or early perimenopausal and continuing until or beyond their FMP. In contrast to our data, one group reported that BMD did not change significantly before the FMP in a group of 156 women between the ages of 48 and 64 but then decreased linearly for about 6 years after the FMP (26). However, in these women, BMD was measured in the forearm using single-photon absorptiometry, a technique that is much less sensitive for detecting bone loss than is dual-energy x-ray absorptiometry (DXA) (27). Thus, earlier changes in BMD might have gone undetected. In 75 premenopausal women over age 46 monitored for nearly 10 years, spine and hip BMD loss accelerated about 2 to 3 years before the FMP and then began to slow about 3 to 4 years after the FMP (28). Because initial measurements were made using dual-photon absorptiometry and follow-up measurements were made with 2 different DXA machines, these data had to be transformed twice, which could have introduced a systematic error into the estimates of BMD change across the transition. Additionally, the small sample size may have limited the ability to determine precisely when bone loss accelerates and declines. Finally, in a cohort of 629 women age 24 to 44 at baseline, spine BMD fell at a rate of 1.7% per year in the 3 years before the FMP, 3.3% per year in the 2 years after the FMP, and then slowed to 1.1% per year for the next 5 years (29). Only 183 of these women (29%) were followed until their FMP, however, raising the question of whether the reported timing and magnitude of changes in the rates of bone loss in this subset of the study subjects were representative of the entire cohort. In an earlier report from SWAN, before most women had experienced their FMP, we assessed rates of bone loss in relation to menstrually defined menopause stages and reported that bone loss was minimal in premenopausal and early perimenopausal women, accelerated markedly in the late perimenopause, and continued at a rapid rate in the first 1 to 2 years after the FMP (10). Subsequently, once most of the SWAN women had experienced their FMP, we reported that BMD begins to decline steeply about 1 year before the FMP and that the rate of bone loss begins to slow 2 years after the FMP (1). Another recent report from SWAN demonstrated that higher premenopausal NTX levels and larger increases in NTX across the transition are associated with a greater fracture risk, irrespective of BMD (30). When coupled with the current findings, the data demonstrate that a decline in ovarian function is followed quickly by an increase in bone resorption that, after a lag of about 1 year, leads to measurable bone loss. Bone loss continues at a rapid rate until about 2 years after the FMP and then slows (11) in conjunction with a mild decrease in bone resorption. Variation in bone resorption not only leads to differing rates of bone loss but it is also an independent predictor or fracture risk.
Longitudinal data describing changes in bone turnover starting when women are still premenopausal or early perimenopausal and continuing for 5 or more years after the FMP are scarce but may have important clinical implications. First, this information may play a vital role in decisions related to screening for osteoporosis. The optimal time to repeat bone density testing in women whose initial screening results do not suggest that treatment should be started is currently one of the most contentious issues in osteoporosis management. Although some investigators have argued, based on data in older women, that the optimal time to repeat bone density screening can be determined solely from the initial DXA T-scores (31), others have argued that rates of bone loss should also be considered to determine when screening should be repeated (32). Additionally, knowledge of expected rates of bone loss in postmenopausal women may inform decisions related to medical therapy for osteoporosis. In light of the multiple safety concerns related to long-term antiresorptive therapy that currently confront patients and healthcare providers (33–36), it is particularly important to characterize the natural history of changes in BMD and the determinants of those changes to help patients and healthcare providers to predict the risks associated with decisions to withhold medical therapy for osteoporosis. Although the current data characterize rates of bone resorption quite thoroughly for the first several years after the FMP, relatively few women in SWAN have been followed for more than 5 years after the FMP so that our estimates of changes in bone resorption are quite uncertain beyond that time. Additional data are needed to characterize rates of bone resorption and rates of bone loss as women progress further into their postmenopausal years so that patients, healthcare providers, and policy makers will continue to have the information needed to help make rational decisions regarding evaluation and treatment of osteoporosis as women progress further into their postmenopausal years.
Several previous studies have examined cross-sectional relationships between bone turnover and/or BMD and reproductive hormones in women. In elderly postmenopausal women, the rate of bone loss is inversely related to serum estradiol levels (37). Fracture rates and rates of bone loss are higher in elderly women with estradiol levels <5 pg/mL than in women with estradiol levels >5 pg/mL (38, 39). A negative association between postmenopausal estradiol levels and rates of bone loss has also been reported in women aged 46 to 56 followed for just over 3 years (40). In contrast, most studies have failed to identify significant relationships between estradiol levels and bone turnover markers in elderly postmenopausal women (41–43). In SWAN, the association between NTX and fracture rate was not altered when serum estradiol and FSH levels were included in the statistical models (30). The European Vertebral Osteoporosis Study (EVOS) reported a negative association between serum estradiol levels and bone turnover markers in women under the age of 65 years, although this study did not examine women during the menopause transition (44). Cross-sectional studies have consistently demonstrated that various biochemical indices of bone formation and bone resorption are higher in postmenopausal than in premenopausal women, with increases typically ranging from 25% to 150% (2–5, 45–47). One of these cross-sectional studies included perimenopausal women and found that urine NTX levels were 20% higher than in premenopausal women (2). In 2375 women participating in SWAN, we found no differences in baseline serum osteocalcin or urine NTX levels between women who were premenopausal versus early perimenopausal (14). Higher serum FSH levels were significantly, although weakly, associated with higher urine NTX levels, whereas no significant associations were observed between urine NTX and serum estradiol or testosterone levels (14). The current data demonstrate that changes in both estradiol and FSH occur in close temporal proximity to changes in bone resorption during the years immediately surrounding the FMP. However, during the time interval beginning 6 years before the FMP until 2 years before the FMP, FSH levels are changing, whereas NTX and estradiol levels are quite stable so that during this time interval, the pattern of change in NTX mimics that of estradiol more closely than it mimics the pattern of FSH. Whether or not FSH is directly involved in the pathogenesis of postmenopausal bone loss, as has been hypothesized (48), cannot be determined from these data.
In addition to reproductive hormones, body size was an important determinant of the pattern of change of NTX across the menopause transition. The increase in NTX levels was more than twice as great in nonobese than in obese women. We previously reported that the rate of BMD loss during the late perimenopausal and early postmenopausal years was 35% to 55% lower in women in the top versus the bottom tertile of body weight (10) and that the rate of bone loss during the period of time spanning 5 years before until 5 years after the FMP was inversely related to BMI (1). Other groups have also reported that rates of bone loss during the menopause transition are slower in heavier women (12, 29). The current finding of an inverse relationship between body size and bone resorption provides a plausible biochemically based explanation for the reported relationships between body size and rates of bone loss. Specifically, the concordance of the relationship between body size with changes in BMD loss and with changes in NTX suggests that the slower rate of bone loss across the transition in heavier women is due to an attenuation in bone resorption and thus is truly a physiologic effect of obesity on bone loss and is not merely the result of an artifact of DXA related to obesity (49–51). The mechanism whereby obesity may affect bone turnover is unknown.
Our study has limitations. First, hormone levels were determined on the basis of a single annual sample. Those levels may not provide a robust estimate of hormone levels, particularly during the perimenopausal years when cycles are irregular and the timing of blood sampling could have a large impact on hormone levels. Second, because there is geographic variation in bone turnover (52) and the Chinese and Japanese women each come from a single SWAN site, it is difficult to determine whether differences in bone resorption in these Asian women are due to ethnicity or location. Additionally, the use of BMI as a measure of obesity does not indicate whether the inverse relationship between BMI and bone resorption is due to relative increases in fat mass, lean mass, or both in women with high BMI. Finally, although temporal associations between changes in hormones and changes in bone turnover may be consistent with causative relationships, causation cannot be proven in observational studies.
In summary, bone resorption, as indicated by urine NTX levels, begins to increase about 2 years before the FMP and continues to increase for about 1 to 1.5 years after the FMP, after which it begins to decline. Increases in bone turnover were greater in nonobese women, and differences in BMI accounted for much of the ethnic variation in the magnitude of NTX increases across the transition. The concordance between the relationships of body size with changes in bone resorption and with changes in BMD suggests that the previously reported reduction in bone loss in obese women is not merely due to an artifact of DXA in overweight subjects. Finally, these findings suggest that clinicians should be aware of the risk of rapid bone loss in the 1 to 2 years before and after the FMP, particularly in nonobese women.
Acknowledgments
Clinical centers were University of Michigan, Ann Arbor—Siobán Harlow, principal investigator [PI] 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
National Institutes of Health (NIH) Program Office: National Institute on Aging, Bethesda, MD—Winifred Rossi 2012–present; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1994–2001.
Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair.
We thank the study staff at each site and all the women who participated in SWAN.
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), U.S. Department of Health and Human Services, through the National Institute of Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Disclosure Summary: None of the authors report any conflicts of interest.
Footnotes
- BCE
- bone collagen equivalent
- BMD
- bone mineral density
- BMI
- body mass index
- Cr
- creatinine
- DXA
- dual-energy x-ray absorptiometry
- FMP
- final menstrual period
- HT
- hormone therapy
- NTX
- type I collagen N-telopeptide
- SWAN
- Study of Women's Health Across the Nation.
References
- 1. Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multietnhic cohort: results from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res. 2012;27:111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ebeling PR, Atley LM, Guthrie JR, B, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81:3366–3371 [DOI] [PubMed] [Google Scholar]
- 3. Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994;79:1693–1700 [DOI] [PubMed] [Google Scholar]
- 4. Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11:337–349 [DOI] [PubMed] [Google Scholar]
- 5. Gorai I, Taguchi Y, Chaki O, Nakayama M, Minaguchi H. Specific changes of urinary excretion of cross-linked N-telopeptides of type I collagen in pre- and postmenopausal women: correlation with other markers of bone turnover. Calcif Tissue Int. 1997;60:317–322 [DOI] [PubMed] [Google Scholar]
- 6. Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–1258 [DOI] [PubMed] [Google Scholar]
- 7. de Papp AE, Bone HG, Caulfield MP, et al. A cross-sectional study of bone turnover markers in healthy premenopausal women. Bone. 2007;40:1222–1230 [DOI] [PubMed] [Google Scholar]
- 8. Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF., Jr Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93:3958–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sowers MR, Zheng H, McConnell D, Nan B, Harlow SD, Randolph JF., Jr Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J Clin Endocrinol Metab. 2008;93:3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density çhanges during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ravn P, Cizza G, Bjarnason NH, et al. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–1627 [DOI] [PubMed] [Google Scholar]
- 12. Macdonald HM, New SA, Campbell MK, Reid DM. Influence of weight and weight change on bone loss in perimenopausal and early postmenopausal Scottish women. Osteoporos Int. 2005;6:163–171 [DOI] [PubMed] [Google Scholar]
- 13. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113 [DOI] [PubMed] [Google Scholar]
- 14. Sowers MR, Finkelstein JS, Ettinger B, et al. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14:44–52 [DOI] [PubMed] [Google Scholar]
- 15. Sowers MF, Crawford S, Sternfeld B, et al. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus M, eds. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2003:175–188 [Google Scholar]
- 16. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 17. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323 [DOI] [PubMed] [Google Scholar]
- 18. Zhang D, Lin X, Sowers M. Semiparametric stochastic mixed models for longitudinal hormone data. J Am Stat Assoc. 1998;93:710–719 [Google Scholar]
- 19. Zheng H, Sowers MR, Randolph JF, Jr, Hawlow SD. An integrated quantitative methodology to longitudinally characterize complex dynamic processes associated with ovarian aging and the menopausal transition. J System Cybernet Informat. 2011;9:15–23 [PMC free article] [PubMed] [Google Scholar]
- 20. Claeskens G, Van Keilegom I. Bootstrap confidence bands for regression curves and their derivatives. Ann Stat. 2003;31:1852–1884 [Google Scholar]
- 21. Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77 [Google Scholar]
- 22. Hoeting JA, Madigan D, Raftery AE, Vollinsky CT. Model averaging: A tutorial. Stat Sci. 1999;14:382–401 [Google Scholar]
- 23. Raftery AE. Bayesian model selection in social research. Sociol Methodol. 1995;25 [Google Scholar]
- 24. Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models. 2nd ed Homewood, IL: Irwin; 1985 [Google Scholar]
- 25. Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057–3067 [DOI] [PubMed] [Google Scholar]
- 26. Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28:327–331 [DOI] [PubMed] [Google Scholar]
- 27. Riggs BL, Wahner HW, Melton LJ, 3rd, Richelson LS, Judd HL, Offord KP. Rates of bone loss in the appendicular and axial skeletons of women. Evidence of substantial vertebral bone loss before menopause. J Clin Invest. 1986;77:1487–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Recker R, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15:1965–1973 [DOI] [PubMed] [Google Scholar]
- 29. Sowers MR, Zheng H, Jannausch ML, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95:2155–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cauley JA, Danielson ME, Greendale GA, et al. Bone resorption and fracture across the menopausal transition: the Study of Women's Health Across the Nation. Menopause. 2012;19:1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gourlay ML, Fine JP, Preisser JS, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu EW, Finkelstein JS. Bone density screening intervals for osteoporosis: one size does not fit all. JAMA. 2012;307:2591–2592 [DOI] [PubMed] [Google Scholar]
- 33. Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362:1761–1771 [DOI] [PubMed] [Google Scholar]
- 34. Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res. 2011;26:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shane E, Burr D, Ebeling PR, et al. Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010;25:2267–2294 [DOI] [PubMed] [Google Scholar]
- 36. Woo SB, Hellstein JW, Kalmar JR. Narrative review: bisphosphonates and osteonecrosis of the jaws [published correction appears in Ann Intern Med. 2006;145:235]. Ann Intern Med. 2006;144:753–761 [DOI] [PubMed] [Google Scholar]
- 37. Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349:327–334 [DOI] [PubMed] [Google Scholar]
- 38. Cummings SR, Browner WS, Bauer D, et al. Endogenous hormones and the risk of hip and vertebral fractures among older women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1998;339:733–738 [DOI] [PubMed] [Google Scholar]
- 39. Ettinger B, Pressman A, Sklarin P, Bauer DC, Cauley JA, Cummings SR. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83:2239–2243 [DOI] [PubMed] [Google Scholar]
- 40. Guthrie JR, Ebeling PR, Hopper JL, et al. A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int. 1998;8:282–290 [DOI] [PubMed] [Google Scholar]
- 41. Chapurlat RD, Bauer DC, Cummings SR. Association between endogenous hormones and sex hormone-binding globulin and bone turnover in older women: study of osteoporotic fractures. Bone. 2001;29:381–387 [DOI] [PubMed] [Google Scholar]
- 42. Chapurlat RD, Garnero P, Bréart G, Meunier PJ, Delmas PD. Serum estradiol and sex hormone-binding globulin and the risk of hip fracture in elderly women: the EPIDOS study. J Bone Miner Res. 2000;15:1835–1841 [DOI] [PubMed] [Google Scholar]
- 43. Garnero P, Sornay-Rendu E, Claustrat B, Delmas PD. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: the OFELY study. J Bone Miner Res. 2000;15:1526–1536 [DOI] [PubMed] [Google Scholar]
- 44. Rogers A, Saleh G, Hannon RA, Greenfield D, Eastell R. Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J Clin Endocrinol Metab. 2002;87:4470–4475 [DOI] [PubMed] [Google Scholar]
- 45. Hassager C, Colwell A, Assiri AM, Eastell R, Russell RG, Christiansen C. Effect of menopause and hormone replacement therapy on urinary excretion of pyridinium cross-links: a longitudinal and cross-sectional study. Clin Endocrinol (Oxf). 1992;37:45–50 [DOI] [PubMed] [Google Scholar]
- 46. Kelly PJ, Pocock NA, Sambrook PN, Eisman JA. Age and menopause-related changes in indices of bone turnover. J Clin Endocrinol Metab. 1989;69:1160–1165 [DOI] [PubMed] [Google Scholar]
- 47. Kushida K, Takahashi M, Kawana K, Inoue T. Comparison of markers for bone formation and resorption in premenopausal and postmenopausal subjects, and osteoporosis patients. J Clin Endocrinol Metab. 1995;80:2447–2450 [DOI] [PubMed] [Google Scholar]
- 48. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260 [DOI] [PubMed] [Google Scholar]
- 49. Bolotin HH. Inaccuracies inherent in dual-energy x-ray absorptiometry in vivo bone mineral densitometry may flaw osteopenic/osteoporotic interpretations and mislead assessment of antiresorptive therapy effectiveness. Bone. 2001;28:548–555 [DOI] [PubMed] [Google Scholar]
- 50. Bolotin HH, Sievänen H, Grashuis JL. Patient-specific DXA bone mineral density inaccuracies: quantitative effects of nonuniform extraosseous fat distributions. J Bone Miner Res. 2003;18:1020–1027 [DOI] [PubMed] [Google Scholar]
- 51. Bolotin HH, Sievänen H, Grashuis JL, Kuiper JW, Järvinen TL. Inaccuracies inherent in patient-specific dual-energy X-ray absorptiometry bone mineral density measurements: comprehensive phantom-based evaluation. J Bone Miner Res. 2001;16:417–426 [DOI] [PubMed] [Google Scholar]
- 52. Finkelstein JS, Sowers M, Greendale GA, Lee ML, Neer RM, Cauley JA, Ettinger B. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3051–3056 [DOI] [PubMed] [Google Scholar]