Abstract
Context:
A reverse J-shaped association between serum 25-hydroxyvitamin D (25[OH]D) concentration and all-cause mortality was suggested in a 9-year follow-up (1991–2000) analysis of the Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994).
Objective:
Our objective was to repeat the analyses with 6 years additional follow-up to evaluate whether the association persists through 15 years of follow-up.
Participants:
The study included 15 099 participants aged ≥20 years with 3784 deaths.
Main Outcome Measure:
Relative risk (RR) of death from all causes was adjusted for age, sex, race/ethnicity, and season using 2 Poisson regression approaches: traditional categorical and cubic splines. Results were given for 9 25(OH)D levels: <20, 20 to 29, 30 to 39, 40 to 49, 50 to 59, 60 to 74, 75 to 99 (reference), 100 to 119, and ≥120 nmol/L.
Results:
The reverse J-shaped association became stronger with longer follow-up and was not affected by excluding deaths within the first 3 years of follow-up. Similar results were found from both statistical approaches for levels <20 through 119 nmol/L. Adjusted RR (95% confidence interval [CI]) estimates for all levels <60 nmol/L were significantly >1 compared with the reference group. The nadir of risk was 81 nmol/L (95% CI, 73–90 nmol/L). For 25(OH)D ≥120 nmol/L, results (RR, 95% CI) were slightly different using traditional categorical (1.5, 1.02–2.3) and cubic splines approaches (1.2, 0.9–1.4). The association appeared in men, women, adults ages 20 to 64 years, and non-Hispanic whites but was weaker in older adults. The study was too small to evaluate the association in non-Hispanic black and Mexican-American adults.
Conclusions:
A reverse J-shaped association between serum 25(OH)D and all-cause mortality appears to be real. It is uncertain whether the association is causal.
Large population and clinical studies have implicated poor vitamin D status as a potential risk factor for a number of chronic and infectious diseases (1, 2). Moreover, several studies have found a nonmonotonic association between vitamin D status (3–8), as measured by circulating levels of serum total 25-hydroxyvitamin D (25[OH]D), and all-cause mortality (1, 2). The shape of this association appears to be asymmetric and in a reverse J-shape, with a clear upturn in the risk of death from all causes at low concentrations of 25(OH)D and possibly a shallow increase in the risk of death with higher serum 25(OH)D levels.
In one study, Melamed et al (5) reported finding a reverse J-shaped association between serum 25(OH)D and all-cause mortality in the approximately 9-year follow-up of the nationally representative Third National Health and Nutrition Examination Survey (NHANES III, 1988–1994). The initial follow-up period included assessment of vital status through December 31, 2000. Since then, the NHANES III mortality follow-up has been extended to include deaths through December 31, 2006.
In this paper, we return to NHANES III with follow-up extended to 15 years to address several important questions about the reverse J-shaped association between 25(OH)D and all-cause mortality. How does the association vary by length of follow-up, age, sex, race/ethnicity, and cause of death? Are the results and their interpretation affected by excluding deaths within the first 3 years of follow-up or by the statistical approach used in the data analyses? Can the nadir of risk for the association and its 95% confidence interval (CI) be estimated? And most importantly, does the reverse J-shaped association persist, suggesting that it may be real?
Subjects and Methods
Study design
NHANES is designed to produce nationally representative data for the civilian noninstitutionalized U.S. population. As with each survey, NHANES III consisted of an initial household interview and a subsequent medical examination in a specially equipped mobile examination center (MEC) (9). The examination for NHANES III took place in the years 1988 through 1994 with a midpoint of 1991. This survey serves as our baseline. Follow-up for vital status and underlying cause of death was conducted passively by periodically matching personal identifying information collected at baseline with the information on death certificates filed with the National Death Index as described below. All procedures in NHANES III were approved by the National Center for Health Statistics Institutional Review Board. Written informed consent was obtained from all participants.
Measurements
Serum 25(OH)D was measured using an RIA kit (DiaSorin, Stillwater, Minnesota) with values reformulated to the kit used in 2004 (10). Reformulated values for serum 25(OH)D in nanomoles per liter (nanograms per milliliter ≈ nanomoles per liter/2.5) are reported in this paper for all analyses (see Supplemental Methods for more details, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Serum creatinine (milligrams per deciliter) was measured by a Roche/Hitachi 737 analyzer (Roche Diagnostics, Indianapolis, Indians) using the kinetic alkaline picrate reaction and calibrated to the Cleveland Clinic Research Laboratory (CCRL) using the equation CCRL creatinine (milligrams per deciliter) = 0.96 × [NHANES III creatinine value (milligrams per deciliter)] − 0.184 (11). Estimated glomerular filtration rate (GFR) was calculated using the Isotope-dilution mass spectrometry-Traceable Modification of Diet in Renal Disease (MDRD) Study equation (12). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kilograms per square meter). Systolic blood pressure (SBP) was the average of up to 6 measurements: up to 3 times during the household interview and up to 3 times during the MEC examination. The season in which the MEC examination took place was defined as winter (November to April) vs summer (May to October). Higher-latitude regions in the northern states tended to be sampled during the summer months, whereas lower-latitude regions in the South tended to be sampled in the winter months. Leisure-time physical activity (LTPA) was assessed by questionnaire that included type and frequency of physical activity; a validated metabolic intensity rating (MET) was applied to each of the 9 activities queried (run/jog, swim, bicycle, aerobics, calisthenics, dancing, yard or garden work, weight lifting, or other physical activity). A total weekly LTPA MET was calculated based on the frequency of reports per month and the conversion of 4.3 wk/mo as recommended in NHANES III documentation. The median weekly LTPA MET was 4.65. Activity was classified as low if below the median because of the high number of zeros that were present. Activity was classified as moderate if above the median and below the third quartile (LTPA MET = 22.5) and high if above 22.5. Participants were asked to show interviewers the containers for all current medications, and the interviewer copied the label. Based on those data from the prescription medication files, medication use was defined as the self-reported use of any of the following within the past month: anticonvulsants, estrogens, glucocorticoids, loop diuretics, or thiazide diuretics. Education was defined as completion of less than high school, graduation from high school, and education past high school. Current cigarette smokers were those who had smoked ≥100 cigarettes over their lifetime and smoked at the time of the interview.
Assessment of vital status
The details of the matching methodology used to determine vital status through December 31, 2006, have been published (13). Briefly, vital status was assessed based on a probabilistic match between personal identifiers from NHANES III and the death certificate records from the National Death Index. All NHANES III participants who were 17 years and older at the time of the survey were eligible for mortality follow-up.
Causes of death
Analyses were conducted using the public-use NHANES III Linked Mortality file with deaths coded to the Tenth Revision of the International Classification of Diseases (ICD 10) and with underlying cause of death given in 113 cause groupings. Underlying cause of death was then categorized into 5 broad cause groups: 1) cancer; 2) cardiovascular diseases (CVDs); 3) other chronic, infectious, and maternal, i.e., related to childbirth; 4) accidents; and 5) unknown cause of death. The 5 cause groups included the following 113 Recode Groups of ICD 10: 1) cancer, 019–043; 2) CVD, 053–074; 3) other, 001–018, 44–52, and 075–111; and 4) accidents, 112–135.
Analytic sample
A total of 23 258 participants aged 20 years and older were selected to participate in NHANES III, 18 825 were interviewed, and 16 573 received the MEC examination including a blood draw. Excluded from that sample were those missing information on vital status (n = 11); women who were pregnant at baseline (n = 338); those who were missing data for serum total 25(OH)D (n = 765), serum creatinine (n = 344), BMI (n = 34), and SBP (n = 25); and those with no follow-up time from the date of examination (n = 7) for a total analytic sample size of 15 099 participants.
Statistical analysis
All analyses were conducted using SAS version 9.2 or Stata version 11. Survey procedures from both computer packages were used to incorporate the sampling weights and design effects into the data analyses. Poisson regression (14) was used to model the association between serum total 25(OH)D and risk of death using 1) a traditional categorical variable approach (15) and 2) a cubic polynomial via restricted cubic splines (16). The traditional approach was used to evaluate risk in a categorical variable for serum total 25(OH)D with 9 levels of 25(OH)D: <20, 20 to 29, 30 to 39, 40 to 59, 50 to 59, 60 to 74, 75 to 99, 100 to 119, and ≥120 nmol/L. The cubic-splines approach with 5 knots determined at the fifth, 27.5th, 50th, 72.5th, and 95th percentiles was used to estimate the nadir of the mortality curve and its 95% CI (17). Estimates of the mortality rates and mortality rate ratios, or relative risk (RR), were calculated for each of the 9 intervals, defined above in the traditional approach, at the median value of each interval. The complex survey SE and 95% CI were estimated for both approaches using the delta method.
Four different confounder models were used to evaluate the association: model 1, age-adjusted only; model 2, adjusted for age, sex, race/ethnicity, and season; model 3, adjusted for all the variables in model 2 plus self-reported diabetes (yes/no), congestive heart failure (yes/no), stroke (yes/no), myocardial infarction (yes/no), and cancer other than skin cancer (yes/no); and model 4, adjusted for all the variables in model 3 plus GFR, BMI, SBP, current smoking (yes/no), LTPA (low, moderate, or high), education (less than High school, high school, and high school or more), and medication use (yes/no). Model 2 was chosen as the primary model to avoid the effects of overadjustment, multicollinearity, and within-person error. In preliminary analyses, the results were approximately the same regardless of the modeling approach or the confounder model.
Results
The analytic sample included 11 315 individuals presumed to be alive and 3784 presumed to be deceased during the follow-up period (Table 1 and Supplemental Table 1). Decedents were significantly older at baseline with shorter periods of follow-up than those presumed to be alive. Five percent (weighted) of the sample consisted of Mexican-Americans, 10% non-Hispanic blacks, and 77% non-Hispanic whites. However, the proportion of non-Hispanic whites was higher among decedents (83%), whereas it was slightly lower for Mexican-Americans. Decedents were less educated. At baseline, they also had significantly lower mean serum total 25(OH)D levels and calculated GFR as well as higher mean BMIs and SBP levels. A significantly higher percentage of decedents reported using anticonvulsants, estrogens, glucocorticoids, loop diuretics, or thiazide diuretics at baseline. Moreover, they were much more likely to report a baseline medical history of diabetes, congestive heart failure, stroke, myocardial infarction, and cancer. As a result, it appears that participants who eventually died during the follow-up period were in poorer health at baseline.
Table 1.
Unadjusted Weighted Means and Percentages (SE) of All Persons Aged 20 Years and Older by Vital Status: NHANES III Baseline Survey 1988 to 1994 With Follow-up Through 2006a
| Variables | All Persons (n = 15 099) | Alive (n = 11 315) | Deceased (n = 3784) |
|---|---|---|---|
| Age at interview, y | 45 (0.47) | 41 (0.36) | 66 (0.62)b |
| Person-years follow-up, y | 13.8 (0.22) | 15 (0.24) | 8.4 (0.22)b |
| Serum 25(OH)D (nmol/liter)c | 64 (0.73) | 65 (0.81) | 60 (0.77)b |
| Calculated GFR, mL/(min · 1.73m2)d | 92 (0.52) | 95 (0.53) | 78 (0.77)b |
| BMI, kg/m2 | 26.6 (0.10) | 26.4 (0.12) | 27.1 (0.11)b |
| SBP, mm Hg | 123 (0.40) | 119 (0.33) | 138 (0.62)b |
| Men, % | 49 (0.45) | 48 (0.50) | 50 (1.19) |
| Mexican-Americans, % | 5 (0.40) | 5 (0.46) | 3 (0.24)b |
| Non-Hispanic blacks, % | 10 (0.58) | 10 (0.57) | 10 (0.86) |
| Non-Hispanic whites, % | 77 (1.24) | 76 (1.34) | 83 (1.24)b |
| Other race/ethnic group, % | 8 (0.83) | 9 (0.94) | 4 (0.67)b |
| Season (% Winter) | 38 (3.75) | 38 (3.83) | 37 (4.18) |
| Current smokers (yes), % | 28 (0.80) | 29 (0.88) | 27 (1.24) |
| LTPA, % | |||
| Low, <4.65 METS/wk | 39 (1.26) | 37 (1.26) | 47 (1.83)b |
| Moderate, 4.65–22.5 METs/wk | 33 (0.66) | 35 (0.72) | 27 (1.24)b |
| High, >22.5 METS/wk | 28 (1.14) | 29 (1.20) | 26 (1.58) |
| Education, % | |||
| Less than high school | 25 (1.03) | 21 (1.00) | 43 (1.78)b |
| High school | 33 (0.74) | 34 (0.80) | 31 (1.04)b |
| More than high school | 42 (1.22) | 45 (1.21) | 26 (1.49)b |
| Medicine usagee (yes), % | 16 (0.48) | 13 (0.48) | 27 (1.13)b |
| Self-reported history, % | |||
| Diabetes | 5 (0.26) | 3 (0.24) | 15 (0.72)b |
| Congestive heart failure | 2 (0.15) | 1 (0.12) | 8 (0.53)b |
| Stroke | 2 (0.17) | 1 (0.11) | 8 (0.55)b |
| Heart Attack | 4 (0.27) | 1 (0.14) | 13 (0.89)b |
| Cancerf | 4 (0.22) | 2 (0.24) | 10 (0.66)b |
Sources were the Centers for Disease Control and Prevention, National Center for Health Statistics, Third National Health and Nutrition Examination Survey 1988–1994, and the public use version of the NHANES III Linked Mortality File.
The t test for the difference between those assumed alive and assumed deceased is significant with P < .01.
Based on serum 25(OH)D values (nanomoles per liter) calibrated to the DiaSorin assay kit available in 2004 using the following equation: NHANES III 25(OH)D (corrected to 2004 RIA) = 0.8429 × NHANES III 25(OH)D (1988–1994 RIA) + 2.5762 (millimoles per liter). (See Ref. 10 for more details.)
Serum creatinine (milligrams per deciliter) was calibrated to the Cleveland Clinic Research Laboratory (CCRL) using the equation CCRL creatinine = 0.96 (NHANES III creatinine value) − 0.184 mg/dL (11) and GFR was calculated using the IDMS-Traceable Modification of Diet in Renal Disease (MDRD) Study equation (see Ref. 12 for more details).
Self-reported use of any of the following prescription medications: anticonvulsants, glucocorticoids, estrogens, loop diuretics, or thiazide diuretics during the 30 days before the interview.
Self-reported medical history of cancer other than skin cancer.
The additional 6 years of follow-up from 2000 to 2006 led to a 50% increase in the numbers of deaths, i.e., 2257 deaths vs 3784 deaths (Table 2 and Supplemental Table 2). However, through 2006 there were still relatively few deaths for persons with serum total 25(OH)D concentrations at the low end of the range (<20 nmol/L), i.e., n = 79 deaths, and especially at the high end of the range (≥120 nmol/L), ie, n = 36 deaths. In the lowest category (<20 nmol/L), about half the deaths occurred among non-Hispanic blacks. CVD deaths accounted for 44% of the deaths with 29% in the broad other category, and 22% of the deaths were classified as having cancer as the underlying cause. For the 36 deaths that occurred in the highest category (25[OH]D level ≥120 nmol/L), 13 were associated with lung disease. Only 145 of the deaths, or 4% of the total, were due to accidents. Cause of death information was available for 99% of the decedents.
Table 2.
Adjusted RR of Death From All Causes (95% CI) and Estimated Nadir (95% CI) by Serum Total 25(OH)D Concentration and Statistical Approach: NHANES III, Baseline 1988 to 1994 and With Follow-up Through 2006 (15 Years)a
| Category | Serum Total 25(OH)D, nmol/L |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 | 20–29 | 30–39 | 40–49 | 50–59 | 60–74 | 75–99 | 100–119 | ≥120 | |
| Median, nmol/L | 17.7 | 26.2 | 35.2 | 45.1 | 54.8 | 67.0 | 83.6 | 106.9 | 133.4 |
| Deaths, n | 79 | 297 | 592 | 694 | 668 | 775 | 533 | 110 | 36 |
| Sample size, n | 251 | 1270 | 2340 | 2790 | 2526 | 3046 | 2156 | 518 | 202 |
| Model 1b | |||||||||
| Categorical RR (95% CI) | 2.0 (1.5–2.6) | 1.5 (1.3–1.8) | 1.3 (1.1–1.6) | 1.1 (0.9–1.3) | 1.2 (1.01–1.4) | 1.1 (0.96–1.3) | 1.0 | 1.1 (0.8–1.4) | 1.6 (1.0–2.4) |
| Cubic-splines RR (95% CI) | 1.7 (1.4–2.0) | 1.5 (1.3–1.8) | 1.2 (1.1–1.5) | 1.1 (0.96–1.3) | 1.1 (0.98–1.3) | 1.0 (0.97–1.1) | 1.0 | 1.1 (0.97–1.2) | 1.2 (0.9–1.5) |
| Nadir (95% CI) | 79 (72–87) | ||||||||
| Model 2c | |||||||||
| Categorical RR (95% CI) | 2.2 (1.6–2.9) | 1.6 (1.4–2.0) | 1.5 (1.2–1.7) | 1.2 (1.02–1.4) | 1.2 (1.1–1.4) | 1.1 (1.0–1.3) | 1.0 | 1.1 (0.8–1.4) | 1.5 (1.02–2.3) |
| Cubic-splines RR (95% CI) | 1.9 (1.5–2.2) | 1.7 (1.4–1.9) | 1.4 (1.1–1.6) | 1.2 (1.04–1.4) | 1.2 (1.02–1.3) | 1.1 (0.99–1.1) | 1.0 | 1.1 (0.95–1.2) | 1.2 (0.9–1.4) |
| Nadir (95% CI) | 81 (73–90) | ||||||||
| Model 3d | |||||||||
| Categorical RR (95% CI) | 2.1 (1.6–2.7) | 1.6 (1.4–2.0) | 1.4 (1.2–1.6) | 1.2 (1.0–1.4) | 1.2 (1.03–1.4) | 1.1 (0.96–1.2) | 1.0 | 1.1 (0.8–1.4) | 1.5 (0.96–2.3) |
| Cubic-splines RR (95% CI) | 1.8 (1.5–2.2) | 1.6 (1.4–1.9) | 1.3 (1.1–1.5) | 1.2 (1.01–1.3) | 1.1 (1.0–1.3) | 1.1 (0.99–1.1) | 1.0 | 1.1 (0.96–1.2) | 1.2 (0.9–1.5) |
| Nadir (95% CI) | 80 (72–88) | ||||||||
| Model 4e | |||||||||
| Categorical RR (95% CI) | 1.6 (1.2–2.2) | 1.5 (1.2–1.8) | 1.3 (1.1–1.5) | 1.1 (0.96–1.3) | 1.2 (1.01–1.3) | 1.1 (0.99–1.3) | 1.0 | 1.1 (0.9–1.4) | 1.4 (0.9–2.2) |
| Cubic-splines RR (95% CI) | 1.5 (1.3–1.9) | 1.4 (1.2–1.6) | 1.2 (1.03–1.4) | 1.1 (0.97–1.3) | 1.1 (1.0–1.2) | 1.0 (0.99–1.1) | 1.0 | 1.1 (0.9–1.2) | 1.1 (0.9–1.4) |
| Nadir (95% CI) | 81 (70–92) | ||||||||
Sources were the Centers for Disease Control and Prevention, National Center for Health Statistics, Third National Health and Nutrition Examination Survey 1988–1994, and the public use version of the NHANES III Linked Mortality File.
Adjusted for age.
Minimally Adjusted model: Adjusted for age, sex, race-ethnicity and season.
Adjusted for all the variables in Model 2 plus self-reported, diabetes, congestive heart failure, stroke, heart attack, and cancer other than skin cancer.
Fully adjusted model: Adjusted for all the variables in Model 3 plus calculated MDRD glomerular filtration rate, body mass index, physical activity, current smoking, education and medication use.
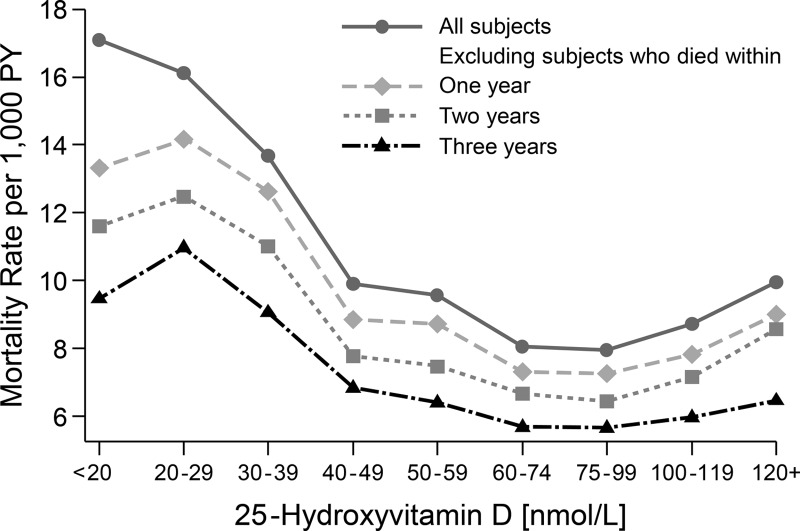
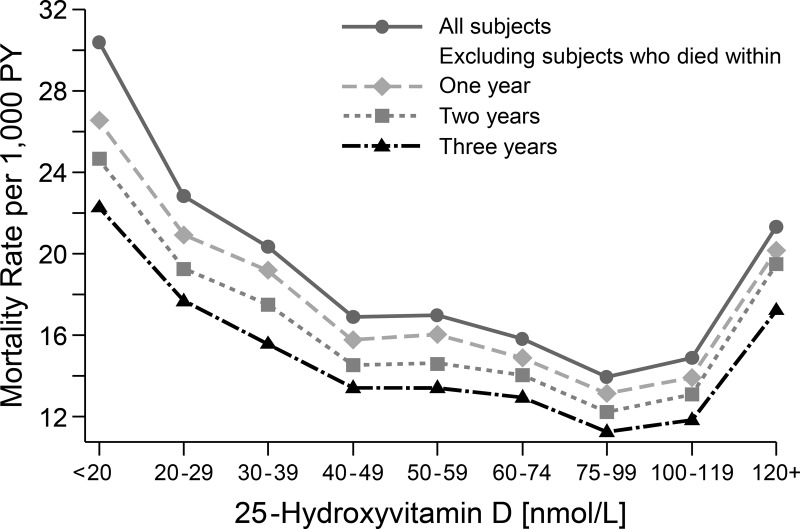
Through the year 2000 there appeared to be a reverse J-shaped pattern between the death rate and serum total 25(OH)D level, after adjusting for age, sex, season, and race/ethnicity (Figure 1). The rise in the mortality rate was much steeper and higher at the low end of the 25(OH)D distribution than at its high end. Sequentially, excluding deaths that occurred within the first, second, and third years of follow-up led to a flattening of the curve across the 25(OH)D concentration range. During the first year of follow-up, 79% (weighted percentage) of the 38 deaths for those with a serum total 25(OH)D concentration <30 nmol/L were due to CVD (data not shown). When the additional deaths between 2000 and 2006 were added to the analysis, the association between serum total 25(OH)D and mortality became stronger (Figure 2). A more pronounced reverse J-shaped association was present, and persisted, when deaths during the first 3 years of follow-up were excluded.
Figure 1.
Mortality rate adjusted for age, sex, race/ethnicity, and season by serum 25(OH)D concentration (nanomoles per liter): 9-year follow-up of NHANES III through 2000 (n = 15 099).
Figure 2.
Mortality rate adjusted for age, sex, race/ethnicity, and season by serum 25(OH)D concentration (nanomoles per liter): 15-year follow-up of NHANES III through 2006 (n = 15 099).
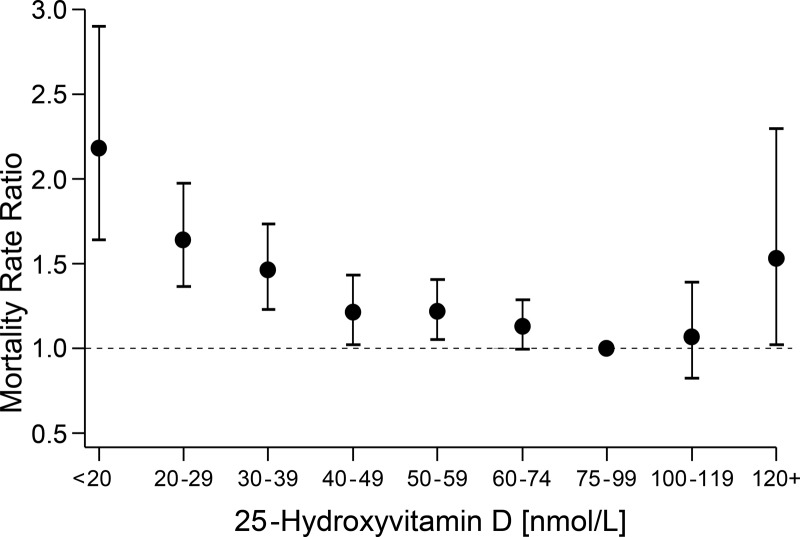
Examining the association for the total analytic sample, adjusted for age, sex, race/ethnicity, and season (model 2), where the y-axis is the RR scale rather than the mortality rate per 1000 person-years scale, and including the 95% CI for the RR provides a somewhat different perspective (Figure 3 and Table 2, model 2). Compared with the reference group, i.e., persons with a serum total 25(OH)D level between 75 and 99 nmol/L, the curve appears to be relatively shallow with risk of death varying little between 40 and 119 nmol/L. For persons with a 25(OH)D at the low end of the scale, risk of death increased sharply below 40 nmol/L, and to a lesser extent, risk of death increased above 119 nmol/L. However, serum 25(OH)D concentrations of 40 to 49 nmol/L and 50 to 59 nmol/L were associated with a statistically significantly increased risk of death when compared with the referent category (75–99 nmol/L). The association of ≥120 nmol/L was statistically significant (RR = 1.5, 95% CI = 1.02–2.3) using model 2 but not with the fully adjusted model (model 4). Risk of death was lowest at serum total 25(OH)D concentrations of about 80 nmol/L (range = 79–82 nmol/L) with 95% CI between approximately 70 and 90 nmol/L regardless of confounder model. Results were very similar using the cubic-splines approach (Table 2); however, the confidence limits tended to be wider, especially in the tails. For example, with 25(OH)D ≥120 nmol/L, results (RR, 95% CI) were slightly different using traditional categorical (1.5, 1.02–2.3) and cubic-splines approaches (1.2, 0.9–1.4).
Figure 3.
Mortality rate ratio or RR adjusted for age, sex, race/ethnicity, and season by serum 25(OH)D concentration (nanomoles per liter) using traditional epidemiological approach of defining categories of risk: 15-year follow-up of NHANES III through 2006 (n = 15 099).
A reverse J-shaped association appeared to be present in both men and women (Table 3). (See Supplement Tables 2 and 3 for the numbers of deaths and sample size by length of follow-up, demographic group, cause of death, and baseline serum concentration of total 25(OH)D in nanomoles per liter.) For women, risk of death increased progressively as serum 25(OH)D declined for those with values less than 75 nmol/L, whereas for men, risk did not appear to increase until values were <40 nmol/L. In general, women were at increased risk of death when 25(OH)D concentrations were between 100 and 119 nmol/L, whereas for men, increased risk occurred with values ≥120 nmol/L.
Table 3.
Adjusted RR of Death (95% CI) From All Causes by Demographic Group or From Three Major Cause Groups by Serum Total 25(OH)D Concentration: NHANES III, Baseline 1988 to 1994 and With Follow-up Through 2006 (15 Years)a
| Category | Serum Total 25(OH)D, nmol/L |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| <20 | 20–29 | 30–39 | 40–49 | 50–59 | 60–74 | 75–99 | 100–119 | ≥120 | |
| Total | 2.2 (1.6–2.9) | 1.6 (1.4–2.0) | 1.5 (1.2–1.7) | 1.2 (1.02–1.4) | 1.2 (1.1–1.4) | 1.1 (1.00–1.3) | 1.0 (Reference) | 1.1 (0.8–1.4) | 1.5 (1.02–2.3) |
| Sex | |||||||||
| Men | 2.3 (1.3–4.1) | 1.5 (1.2–2.0) | 1.3 (1.00–1.6) | 1.1 (0.9–1.4) | 1.0 (0.9–1.3) | 1.1 (0.9–1.3) | 1.0 (Reference) | 0.9 (0.6–1.2) | 1.7 (1.1–2.6) |
| Women | 2.4 (1.7–3.4) | 1.9 (1.5–2.3) | 1.7 (1.3–2.2) | 1.4 (1.0–1.8) | 1.5 (1.2–1.9) | 1.3 (1.0–1.6) | 1.0 (Reference) | 1.5 (1.04–2.1) | 1.1 (0.4–2.8) |
| Age, y | |||||||||
| 20–64 | 2.2 (1.4–3.6) | 1.8 (1.2–2.6) | 1.6 (1.1–2.2) | 1.5 (1.04–2.0) | 1.2 (0.9–1.6) | 1.3 (1.02–1.7) | 1.0 (Reference) | 1.1 (0.6–1.9) | 1.8 (1.05–3.2) |
| ≥65 | 2.1 (1.6–2.8) | 1.5 (1.3–1.8) | 1.4 (1.2–1.7) | 1.1 (0.9–1.2) | 1.2 (1.04–1.4) | 1.0 (0.9–1.3) | 1.0 (Reference) | 1.1 (0.9–1.3) | 1.2 (0.8–1.8) |
| Race/ethnicity | |||||||||
| Mexican-American | 1.9 (0.9–4.0) | 1.4 (0.8–2.3) | 1.3 (0.8–2.0) | 1.1 (0.7–1.7) | 0.9 (0.6–1.5) | 0.9 (0.6–1.4) | 1.0 (Reference) | 0.7 (0.2–2.5) | 1.0 (0.5–1.9) |
| Non-Hispanic black | 2.1 (1.3–3.2) | 1.1 (0.8–1.6) | 1.2 (0.9–1.6) | 1.0 (0.6–1.5) | 1.0 (0.6–1.5) | 1.2 (0.8–1.6) | 1.0 (Reference) | 2.1 (1.1–4.0) | 2.4 (0.8–7.0) |
| Non-Hispanic white | 2.1 (1.4–3.1) | 1.9 (1.5–2.3) | 1.5 (1.2–1.8) | 1.3 (1.04–1.5) | 1.2 (1.07–1.5) | 1.1 (1.00–1.3) | 1.0 (Reference) | 1.1 (0.8–1.4) | 1.6 (1.05–2.4) |
| Causes of death | |||||||||
| Cancer | 0.6 (0.3–1.4) | 1.1 (0.7–1.7) | 1.3 (0.9–1.9) | 1.0 (0.7–1.4) | 1.3 (0.9–1.8) | 1.1 (0.7–1.7) | 1.0 (Reference) | 0.9 (0.5–1.8) | 2.1 (0.9–4.8) |
| CVD | 2.0 (1.2–3.5) | 1.7 1.4–2.1) | 1.4 (1.1–1.7) | 1.2 (1.01–1.5) | 1.0 (0.8–1.3) | 1.0 (0.8–1.2) | 1.0 (Reference) | 1.1 (0.7–1.6) | 0.6 (0.2–1.7) |
| Other | 3.8 (2.3–6.3) | 2.0 (1.4–2.8) | 1.7 (1.1–2.5) | 1.3 (0.9–1.8) | 1.4 (1.06–1.9) | 1.2 (0.9–1.6) | 1.0 (Reference) | 1.1 (0.7–1.7) | 2.0 (0.96–4.0) |
Sources were the Centers for Disease Control and Prevention, National Center for Health Statistics, Third National Health and Nutrition Examination Survey 1988–1994, and the public use version of the NHANES III Linked Mortality File. RR was adjusted for age, sex, race/ethnicity, and season. Estimates were based on a Poisson regression model where 25(OH)D was modeled as a categorical variable with 9 levels. Serum total 25(OH)D concentrations (nanomoles per liter) were coded as a series of dummy variables with category 75–99 nmol/L used as the reference category: <20, 20–29, 30–39, 40–49, 50–59, 60–74, 75–99 (reference), 100–119, and ≥120.
The reverse J-shaped association appeared to be stronger for persons aged 20 to 64 years than for those aged 65 years and older. The risk curve for the older participants appeared to be shallower. There was a statistically significant reverse J-shaped association for non-Hispanic whites, which is not surprising because 83% of the deaths occurred among non-Hispanic whites. However, the relatively small numbers of deaths within the Mexican-American and non-Hispanic black race/ethnic participants, especially among those with serum 25(OH)D ≥100 nmol/L, made it very difficult to interpret the relationship between serum 25(OH)D level and risk of death. Clearly, risk of death increased in non-Hispanic blacks when serum total 25(OH)D concentrations were below 20 nmol/L, but the CIs were quite large, again, making the association difficult to interpret.
There was no association between cancer death and 25(OH)D concentrations; however, there was a nonsignificant increase at levels above 120 nmol/L that contributed to the overall reverse J-shaped pattern (Table 3). There was an inverse association between risk of CVD death and serum total 25(OH)D levels at concentrations below 50 nmol/L. As for the very broad category of other causes of death, there appeared to be a reverse J-shaped relationship, but as with cancer, the association above 25(OH)D levels of 75 to 99 nmol/L was not statistically significant.
Discussion
The reverse J-shaped association in the general U.S. population between serum 25(OH)D and risk of death from all causes appears to be real, although that conclusion was based on sparse data, especially at 25(OH)D concentrations above 120 nmol/L. There were, however, consistencies within the NHANES results and with results from other studies that lead to that conclusion: 1) the strengthening of the association with longer follow-up; 2) the persistence of the relationship after eliminating persons with preexisting disease that was fatal within the first 3 years of follow-up; 3) it was present using different statistical modeling approaches; and 4) a reverse J-shaped association was found in men and women and young and old. Those findings as well as reports of a U- or J-shaped association in other studies (3–8) all support the conclusion. The more difficult challenge is to understand what the results mean and what their implications are for clinical and public health practice; i.e., the fact that a reverse J-shaped association exists should not and does not necessarily imply that it is causal.
At 15 years of follow-up there appeared to be a wide, shallow trough of low risk between the areas of increased risk in the tails. By our traditional analysis, the trough was 60 to 99 nmol/L for both sexes combined. Cubic-splines analyses gave similar results, with the nadir at 80 nmol/L and the 95% CI between 70 and 90 nmol/L. A pooled analysis of results from 14 prospective cohort studies involving 5562 deaths supports the conclusion that a reverse J-shaped relationship exits (18). That meta-analysis placed the trough at 75 to 87.5 nmol/L (18) or slightly higher at the lower boundary than our findings.
The upswing in risk in all-cause mortality at lower 25(OH)D levels has been widely and consistently documented (3–8, 19–31). In our analysis, the upswing became quite steep as 25(OH)D dropped below 40 nmol/L. The upswing in risk at higher 25(OH)D levels, >120 nmol/L, was based on sparse data. Comparatively few investigators have identified an upswing at higher 25(OH)D levels, most likely because they did not use analytic techniques that address this possibility. For example, most examined their data by tertiles or quartiles of total 25(OH)D concentration, thus grouping their highest levels together with levels as low as 60 to 75 nmol/L (19–31), with the result that the reverse J-shaped association was lost. Studies that used a different analytic approach have identified an upswing, however, such as the pooled analysis cited above (18). In a different study of a large cohort of adult patients followed for 3 years in general medical practices in Copenhagen, Denmark, investigators found that the hazard ratio for all-cause mortality increased linearly from 1.0 to 1.5 at 25(OH)D levels between 80 and 150 nmol/L (8). Although those results support the general conclusion that a reverse J-shaped association exists, data from a cohort based entirely on clinic patients may not reflect the association in the general population.
With regard to specific causes of death, a reverse J-shaped association was apparent for the very broad group of other causes of death, whereas a negative association was found for CVD deaths. Deaths at the lower end of serum total 25(OH)D, therefore, appeared to be of 2 general classes, CVD and other causes. At the upper end of the 25(OH)D concentration distribution, the causes contributing to the increase in risk appeared to be other and cancer. The association between risk of cancer death and 25(OH)D concentration was not statistically significant perhaps because of the small sample size and numbers of deaths, which is consistent with results reported by Freedman et al (32); however, the higher number of cancer deaths among those with a serum 25(OH)D concentration at or above 120 nmol/L was a major contributor to the upswing at the higher end of the 25(OH)D distribution, leading us to interpret this nonsignificant finding as potentially important and worthy of further investigation in larger studies.
There are several possible limitations to this study. Serum total 25(OH)D was measured only once at baseline, and we cannot evaluate the impact any changes over time might have had on the association. Additionally, we have not evaluated the impact of within-person error. However, there does not appear to be a large amount of within-person error for 25(OH)D measured within a season (33). Moreover, because 25(OH)D was the only variable with within-person error in models 1 to 3, the RR estimates, if affected at all by within-person error, would be biased toward the null (34). Finally, death from all causes is a rather imprecise measure of disease risk or incidence, which is the more fundamental endpoint for clinical medicine and public health. Other investigators have proposed other relationships between serum total 25(OH)D and the incidence of disease (35–37). Incidence includes an initial event that can be either fatal or nonfatal. However, cause-specific mortality data from NHANES include a mixture of both initial and secondary events and multiple competing risks. To a certain extent then, analyses of incidence data and all-cause mortality data using NHANES data may be examining 2 separate questions. A comparison of the relationship of 25(OH)D to incidence and to all-cause and cause-specific mortality within the same data cohort may help in understanding its relationship to all-cause mortality in the NHANES and other cohorts.
A final cautionary note is that it must be remembered that different 25(OH)D assays may give quantitatively different results regarding the association between 25(OH)D and mortality (38). The results from NHANES III were based on the DiaSorin RIA platform (10). In particular, to understand the association between 25(OH)D and mortality, it is essential, therefore, that the results from different studies be standardized to the same reference measurement procedure. Moreover, in general, standardization of results from clinical and epidemiological research is essential to the development of clinical and public health for vitamin D. Leading that effort is the international Vitamin D Standardization Program (VDSP) (39).
The physiological basis for the reverse J-shaped association of 25(OH)D with mortality risk is not clear. Serum 25(OH)D levels may influence disease risk, but the converse, that disease states may influence 25(OH)D levels, may also be true. Given what we know about the effects of vitamin D deficiency on falls and fractures, it is easy to accept that risk of death would increase at low concentrations (1). However, there is increasing observational evidence consistent with the assertion that 25(OH)D levels may decrease substantially (by up to 40%) in states of systemic inflammation (40–42). These inflammatory states may even affect long-term risk of death, i.e., deaths that did not occur within the first 3 years of follow-up. Therefore, some portion of the deaths in the lower tail may reflect a situation where a disease process has altered the 25(OH)D level. A similar point could be made that people who are terminally ill are apt to spend less time in the sun and on that basis to have lower 25(OH)D levels. At the upper end of the 25(OH)D concentration range, the flattening of the curve when persons with preexisting disease were excluded from the analyses of the follow-up data through the year 2000 (Figure 1) along with the dampening of the association in the older participants, as susceptible individuals pass away, implies that some portion of the deaths in the upper tail may reflect a situation in which disease processes have altered serum 25(OH)D concentrations (8). The basis for this is not at all apparent at this time, but it could conceivably involve genetics, which are known to influence the 25(OH)D increment in response to supplementation with vitamin D3 as well as risk of many serious diseases (43, 44). The upswing at higher 25(OH)D levels could in some cases be attributable to vitamin D supplementation of ill patients.
Vitamin D appears to have a number of functions that are essential to the health of cells, tissues, organs, and organisms. In disease those mechanisms may fail or be altered; or the mechanisms may fail and cause disease. Knowledge is always imperfect, but until the reverse J-shaped association between 25(OH)D and all-cause mortality is better understood, caution needs to be used when developing clinical and public health guidelines. The guidelines need to be developed so as to meet the needs of those with compromised values, however that may be defined, while not producing population shifts in serum total 25(OH)D that would cause some people to achieve concentrations above 100 nmol/L, unless and until these are deemed to be safe, a very difficult task.
Acknowledgments
This work was supported by the National Institute on Aging, National Institutes of Health (NIH) (Grant AG10353) and by an Office of Dietary Supplements administrative supplement to NIH Grant 5R37 HL045508-17.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the NIH, Centers for Disease Control and Prevention, or the U.S. Department of Health and Human Services.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- CVD
- cardiovascular disease
- 25(OH)D
- 25-hydroxyvitamin D
- GFR
- glomerular filtration rate
- LTPA
- leisure-time physical activity
- MEC
- mobile examination center
- MET
- metabolic intensity rating
- NHANES III
- Third National Health and Nutrition Examination Survey
- RR
- relative risk
- SBP
- systolic blood pressure.
References
- 1. Rosen CJ, Adams JS, Bikle DD, et al. The nonskeletal effects of vitamin D: an Endocrine Society Scientific Statement. Endocr Rev. 2012;33:456–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute of Medicine Dietary Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011 [Google Scholar]
- 3. Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr. 2006;84:616–622; quiz 671–672 [DOI] [PubMed] [Google Scholar]
- 4. Jia X, Aucott LS, McNeill G. Nutritional status and subsequent all-cause mortality in men and women aged 75 years or over living in the community. Br J Nutr. 2007;98:593–599 [DOI] [PubMed] [Google Scholar]
- 5. Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michaëlsson K, Baron JA, Snellman G, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr. 2010;92:841–848 [DOI] [PubMed] [Google Scholar]
- 7. Johansson H, Kanis AO, McClosky E, et al. Low serum vitamin D is associated with increased mortality in elderly men: MrOS Sweden. Osteoporosis Int. 2012;23:991–999 [DOI] [PubMed] [Google Scholar]
- 8. Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD Study. J Clin Endocrinol Metab. 2012;97:2644–2652 [DOI] [PubMed] [Google Scholar]
- 9. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994;32:1–407 [PubMed] [Google Scholar]
- 10. Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the national health and nutrition examination surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis. 2007;50:918–926 [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention National Centers for Health Statistics. NHANES III Linked Mortality File. http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes3_linkage.htm Accessed October 14, 2009
- 14. Long AS, Freese J. Regression Models for Categorical Dependent Variables Using Stata. 2nd ed College Station, TX: Stata Press; 2005:370–372 [Google Scholar]
- 15. Kahn HA, Sempos CT. Statistical Methods in Epidemiology. New York, NY: Oxford University Press; 1989 [Google Scholar]
- 16. Royston P, Altman DG. Regression using fractional polynomials of continuous covariates. Parsimonious parametric modelling. J Royal Stat Soc Series C (Appl Stat). 1994;43:429–467 [Google Scholar]
- 17. Harrell FE., Jr Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer; 2001 [Google Scholar]
- 18. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012;95:91–100 [DOI] [PubMed] [Google Scholar]
- 19. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1439 [DOI] [PubMed] [Google Scholar]
- 20. Ginde AA, Scragg R, Schwartz RS, Camargo CA. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality and all-cause mortality in older adults. J Am Geriatr Soc. 2009;57:1595–1603 [DOI] [PubMed] [Google Scholar]
- 21. Pilz S, Dobnig H, Nijpelst G, et al. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf). 2009;666–672 [DOI] [PubMed] [Google Scholar]
- 22. Cawthon PM, Parimi N, Barrett-Connor E, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab. 2010;95:4625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Semba RD, Houston DK, Bandinelli S, et al. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr. 2010;64:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R. Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromsø study. Eur J Endocrinol. 2010;162:935–942 [DOI] [PubMed] [Google Scholar]
- 25. Eaton CB, Young A, Allison MA, et al. Prospective association of vitamin D concentrations with mortality in postmenopausal women: results from the Women's Health Initiative (WHI). Am J Clin Nutr. 2011;94:1471–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ford ES, Zhao G, Tsai J, Li C. Vitamin D and all-cause mortality among adults in USA: findings from the National Health and Nutrition Examination Survey Linked Mortality Study. Int J Epidemiol. 2011;40:998–1005 [DOI] [PubMed] [Google Scholar]
- 27. Virtanen JK, Murmi T, Voutilainen S, Mursu J, Toumainen TP. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. Eur J Nutr. 2011;50:305–312 [DOI] [PubMed] [Google Scholar]
- 28. Pilz S, Dobnig H, Tomaschitz A, et al. Low 25-hydroxyvitamin D is associated with increased mortality in female nursing home residents. J Clin Endocrinol Metab. 2012;97;E653–E657 [DOI] [PubMed] [Google Scholar]
- 29. Saliba W, Barnett O, Rennert HS, Rennert G. The risk of all-cause mortality is inversely related to serum 25(OH)D levels. J Clin Endocrinol Metab. 2012;97:2792–2798 [DOI] [PubMed] [Google Scholar]
- 30. Schöttker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality: a systematic review of meta-analysis of prospective cohort studies [published online February 17, 2012]. Ageing Res Rev. doi:10.1016/j.arr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 31. Smit E, Crespo CJ, Michael Y, et al. The effect of vitamin D and frailty on mortality among non-institutionalized US older adults. Eur J Clin Nutr. 2012;66:1024–1028 [DOI] [PubMed] [Google Scholar]
- 32. Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III Study (1988–2006). Cancer Res. 2010;70:8587–8597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr. 2013;97:72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu K. Measurement error and its impact on partial correlation and multiple linear regression analyses. Am J Epidemiol. 1988;127:864–874 [DOI] [PubMed] [Google Scholar]
- 35. Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763 [DOI] [PubMed] [Google Scholar]
- 37. Tuohimaa P, Tenkanen L, Ahonen M, et al. Both high and low levels of blood vitamin D are associated with higher prostate cancer risk: A longitudinal, nested case-control study in Nordic countries. Int J Cancer. 2004;108:104–108 [DOI] [PubMed] [Google Scholar]
- 38. Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Current Drug Targets. 2011;12:19–28 [DOI] [PubMed] [Google Scholar]
- 39. Sempos CT, Vesper HW, Phinney KW, Thienpont LM, Coates PM; Vitamin D Standardization Program (VDSP) Vitamin D status as an international issue: national surveys and the problem of standardization. Scand J Clin Lab Invest Suppl. 2012;243:32–40 [DOI] [PubMed] [Google Scholar]
- 40. Duncan A, Talwar D, McMillan DC, Stefanowicz F, O'Reilly DS. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am J Clin Nutr. 2012;95:64–71 [DOI] [PubMed] [Google Scholar]
- 41. Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–1011 [DOI] [PubMed] [Google Scholar]
- 42. Thurnham DJ. Plasma 25-hydroxy-cholecalciferol (vitamin D) is depressed by inflammation: implications and parallels with other micronutrients. Sight Life. 2011;25:38–47 [Google Scholar]
- 43. Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–1177 [DOI] [PubMed] [Google Scholar]
- 44. Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]