Abstract
Context:
Traditionally, acromegaly is viewed as a disease resulting from GH hypersecretion from an autonomous pituitary somatotropinoma.
Objective:
To test the hypothesis that GH secretion in acromegaly is still subjected to normal hypothalamic control, we studied the daily rhythmicity of GH secretion in normal controls and patients with newly diagnosed, untreated acromegaly.
Design and Setting:
This was an observational inpatient study in the General Clinical Research Center at the University of Michigan.
Patients or Other Participants:
One hundred four normal controls and 67 acromegalic patients were included in the study.
Intervention:
The intervention consisted of frequent blood sampling over 24 hours.
Main Outcome Measure(s):
We hypothesized that acromegalic patients would show rhythmicity, sexual dimorphism, and age-related decline of GH secretion similar to normal controls.
Results:
Both normal controls and the patients exhibited 3 major GH waves with the highest values at 12:00 pm, 5:00 pm, and 1:00 am (P < .001 for all). Both controls and patients exhibited a clear appearance of the nocturnal GH waves, irrespective of the gender (P < .001 for all). The amplitude of the maximal (nocturnal) GH secretory wave (1:00 am) as compared with the nadir GH secretion (9:00 am) was clearly different between the 2 groups, with a significantly smaller magnitude in acromegaly (P < .001). A subsequent subanalysis of both groups was performed separately for both genders. Similar to the entire groups, both controls and patients exhibited a clear appearance of the nocturnal GH waves, irrespective of the gender (P < .001 for all). Patients with clearly elevated GH values have shown an age-related decline of GH secretion (r = −0.35, P < .001), similar to controls.
Conclusions:
The analysis of GH profiles in multiple patients with untreated acromegaly discloses the persistence of the hallmarks of the central control of GH regulation, ie, nictohemeral rhythmicity, sexual dimorphism, and an age-related decline of GH output.
Nictohemeral rhythmicity is a hallmark of the central regulation of pituitary hormonal secretion (1). It is regularly and reliably observed for gonadotropins (2), thyroid-stimulating hormone (3, 4), corticotropin/cortisol (4, 5), and GH (4, 6). Pituitary hormone-secreting adenomas are usually viewed as autonomous entities (7), although some degree of day/nighttime rhythmicity and responsivity to the negative feedback inhibition might be present and is used in the diagnosis of Cushing's disease (8). Similarly, the administration of a GnRH antagonist effectively suppresses elevated FSH concentrations in patients with secretory gonadotropinomas (9), suggesting that even those tumors require GnRH for FSH overproduction.
Similar data in relation of GH secretion in acromegaly are scarce. We have previously shown partial inhibition of GH secretion in the presence of exogenously administered IGF-I (10) as well as during a short-term administration of a specific GHRH antagonist (11). In a small sample of treated and untreated acromegalic patients with relatively homogeneous GH outputs, we have previously suggested the persistence of the nocturnal GH augmentation (12). Subsequently we have shown that the rhythmicity of GH secretion in humans is much more complex than a simple presence of the so-called nocturnal augmentation and consists of 3 distinct major waves of GH output, at 11:00 am/12:00 pm, at 4:00 pm/5:00 pm, and 12:00/2:00 am, with a sexually dimorphic pattern (13).
Thus, we decided to retest our original hypothesis (12) using larger numbers of acromegalic patients and through their direct comparison with a large control group of normal subjects. Furthermore, we aimed to address whether acromegalic patients would show other potential markers of central GH regulation, ie, sexually dimorphic rhythmicity and age-related decline of GH output.
Subjects and Methods
Human data samples were retrospectively collected from prior research protocols approved by the University of Michigan Institutional Review Board and General Clinical Research Center Advisory Committee. Written informed consent was obtained from all subjects (both controls and acromegalic patients) prior to their participation in protocol procedures. All were enrolled in study protocols from March 1996 to January 2002, when the Nichols chemoluminometric assay (Nichols Institute Diagnostics, San Juan Capistrano, California) was used in our laboratory to assess GH concentrations.
We have retrospectively analyzed 24-hour GH profiles (Q10 minutes, Q15 minutes, Q20 minutes, or Q30 minutes) from a total of 180 human subjects: 113 healthy volunteers and 67 acromegalic patients. All patients were newly diagnosed and untreated, and none had renal or hepatic impairment. Neither patients nor normal volunteers were on any medications known to affect GH secretion.
Nine participants from the group of normal volunteers had to be excluded from the analysis because of incomplete 24-hour GH profiles, and the final number of GH measurements totaled 23,081. All acromegalic patients had acral enlargement coupled to other signs and symptoms of acromegaly, in addition to high age-adjusted IGF-I levels. They all had pituitary magnetic resonance imaging (MRI) study performed, and all but 1 had MRI-identifiable pituitary adenoma. A GH-secreting pituitary adenoma was histologically and immunochemically identified in all resected cases. In the single patient with a negative MRI study, pathology identified a cluster of adenomatous GH-containing cells and her clinical and biochemical pictures normalized postoperatively. Three patients were not operated: 2 for medical reasons and 1 who refused surgery.
Participants consumed a standard isocaloric hospital diet consisting of 3 meals (7:00 am, 12:00 pm, and 5:00 pm) and a bedtime snack during a 24-hour sample collection. Blood sampling was performed at a frequency of every 10 (n = 130), 15 (n = 8), 20 (n = 32), or 30 minutes (n = 1), according to each specific protocol. Serum GH was measured in duplicate and always by a chemiluminescent assay (Nichols Institute Diagnostics) and with an assay sensitivity of 0.01 μg/L. All samples from a given patient were studied in the same assay, and the coefficient of variation was 6% at GH concentrations above 1 μg/L and below 6% at GH concentrations between 0.01 and 1.0 μg/L.
Statistical analysis
Due to significant heterogeneity in interindividual GH concentrations between both normal volunteers and acromegalic patients, data had to be normalized for GH rhythmicity analyses. The mean GH concentrations in each individual data array were calculated in 1-hour blocks to minimize the influence of spuriously elevated values. The median hourly GH concentration was identified in each data array in every individual. The ratios of mean hourly and the median hourly GH values were calculated and log transformed [natural logarithm (ln) (hourly mean per daily median)]. The rationale for ln-transformation was as follows: 1) to uniformly level out the interindividual variation and 2) to separate cross-sectional analysis of this variable for each hour that was normally distributed and separate cross-sectional analysis for each gender data that were normally distributed (as examined by Q-Q plots). Therefore, a positive ratio shows that the mean hourly GH value for a particular segment of the study is higher than the median hourly GH, whereas a negative ratio shows the opposite and the zero express a ratio equal to unity when mean and median are equal.
Categorical data were compared through the χ2 test followed by Yates correction or Fisher's exact test, as appropriate. Hourly mean composite of ln-transformed GH ratios differences were evaluated within each group (either normals or acromegalic patients) and then according to gender to each of these groups. All 24-hour time points were compared with the specific nadir (9:00 am) through a Student t test (paired) followed by Bonferroni correction. Comparisons between different groups were done through Student's t test (unpaired) after checking for normality distribution supposition.
For the analysis of age dependence of GH secretion, linear regression analysis was performed to evaluate the relationship between 24-hour GH means and age in both controls and patients. Due to the fact that the acromegalic group showed a subgroup of patients with wide age distribution but with uniformly normal/low GH (14), the analysis of age dependence for these patients had to be done after exclusion of patients with 24-hour GH means less than 4.6 μg/L (15). This cutoff was chosen because it was the highest 24-hour GH mean observed in our group of normals, as published elsewhere (14). Linear regression analysis was also used to evaluate the relationship between tumor volume and age of the patients.
Software R of public domain and GraphPad Prism 5.0 (San Diego, California) were used for analysis. All data are shown as means ± SE. P < .05 was taken as significant. For comparisons where Bonferroni correction had to be used (P < .05/24), P < .002 was the cutoff for significance.
Results
Descriptive analysis
The 2 groups (normals and acromegalic patients) did not differ significantly for gender, although both groups had a significantly higher proportion of males (67.3% for controls vs 64.2% for acromegalic patients, P > .05). However, the control group of normal volunteers was significantly younger than the acromegalic group (22.1 ± 2.17 vs 46.8 ± 5.70 years old, respectively, P < .001). In the group of normal patients, 41.20% of women were postmenopausal, whereas this number was 58.33% in the acromegalic arm (P > .05).
Among the group of normal volunteers, GH measurements were done in 70 (67.3%) every 10 minutes, in 8 (7.7%) every 15 minutes, in 26 (25.0%) every 20 minutes, and none every 30 minutes. For the acromegalic group, GH measurements were 60 (89.6%) every 10 minutes, none every 15 minutes, in 6 (8.9%) every 20 minutes, and 1 (1.5%) every 30 minutes (P < .01 for comparison between groups).
Pattern of GH secretion in controls and acromegalic patients
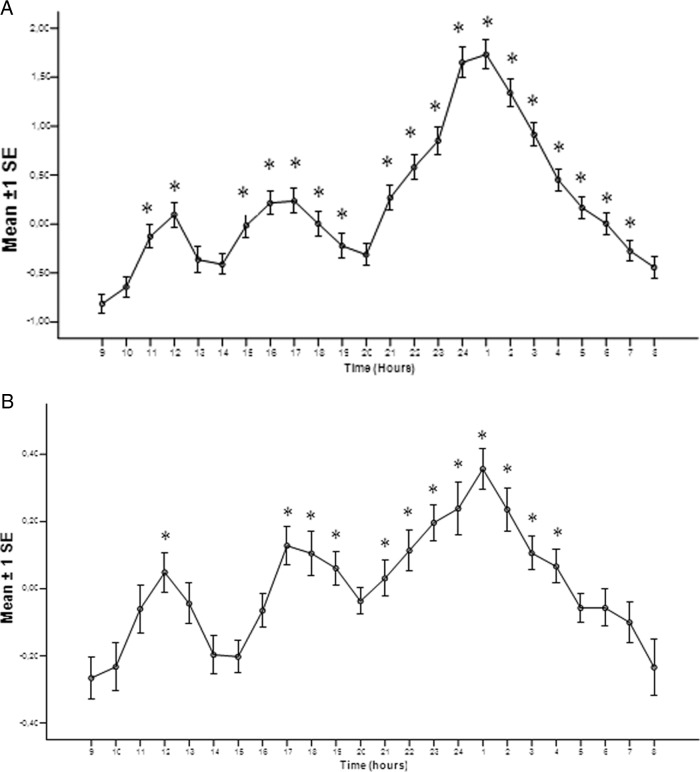
Figure 1A shows the mean composite profile of ln-transformed ratios of the hourly mean over daily median for the control group. The analysis of the daytime GH secretion in this group shows the presence of a statistically significant (P < .001) 3-wave GH secretion pattern, as previously demonstrated elsewhere (13). The maximal ratios occurred at 1200 pm, at 5:00 pm, and, most prominently, at 1:00 am. The same analysis for acromegalic patients is shown in Figure 1B. The statistically significant (P < .001) 3-wave pulsatile GH pattern was again observed, with the maximum peaks identical in timing with the control population.
Figure 1.
Mean composite profile of ln-transformed ratios of the hourly mean over daily median for the controls (A) and acromegalic patients (B). *, P < .05 for comparisons with the rhythm nadir (9:00 am).
The amplitudes of maximal variation of ln-transformed GH concentration ratio (1:00 am) as compared with the nadir (9:00 am) in acromegalic patients were significantly smaller when compared with normal controls, respectively (0.62 ± 0.11 vs 2.55 ± 0.20, P < .001). Thus, the rhythmicity of GH secretion in acromegalic patients is identical in structure and timing but not with the magnitude as compared with the normal subjects.
Pattern of GH secretion, according to gender, in controls and acromegalic patients
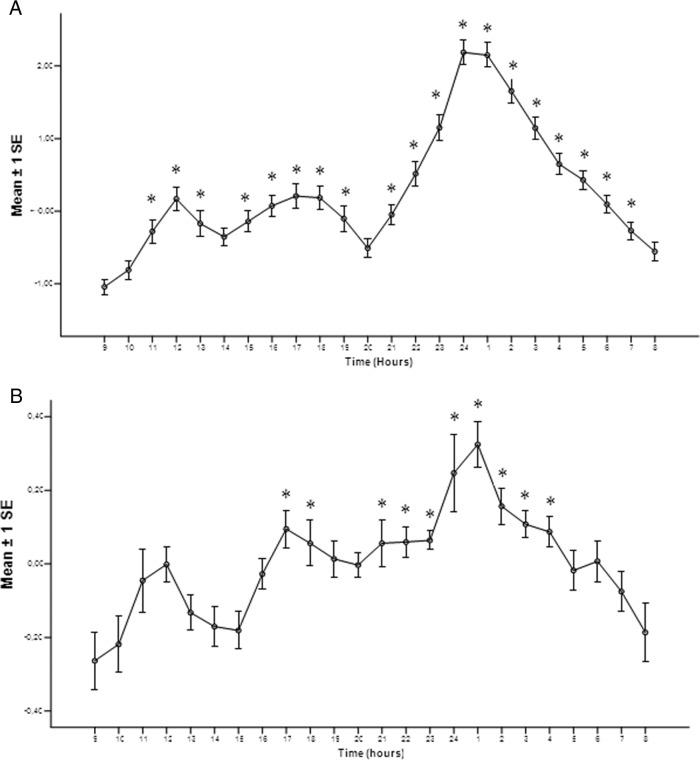
Figure 2A shows the mean composite profiles of ln-transformed ratios of the hourly mean to median GH concentrations in control men, with the first 2 small waves peaking at 12:00 pm and 5:00 pm, followed by a large wave peaking at midnight. This secretion pattern confirms the relative apulsatility of GH secretion during waking hours in men with a prominent augmentation of GH secretion at midnight, as shown earlier (13, 16). Interestingly, the acromegalic male patients (Figure 2B) also showed a 3-wave pattern GH secretion, although statistical significances were reached in only 2 of the 3 prominent waves, peaking at 5:00 pm and 1:00 am.
Figure 2.
Mean composite profile of ln-transformed ratios of the hourly mean over daily median for the control men (A) and male acromegalic patients (B). *, P < .05 for comparisons with the rhythm nadir (9:00 am).
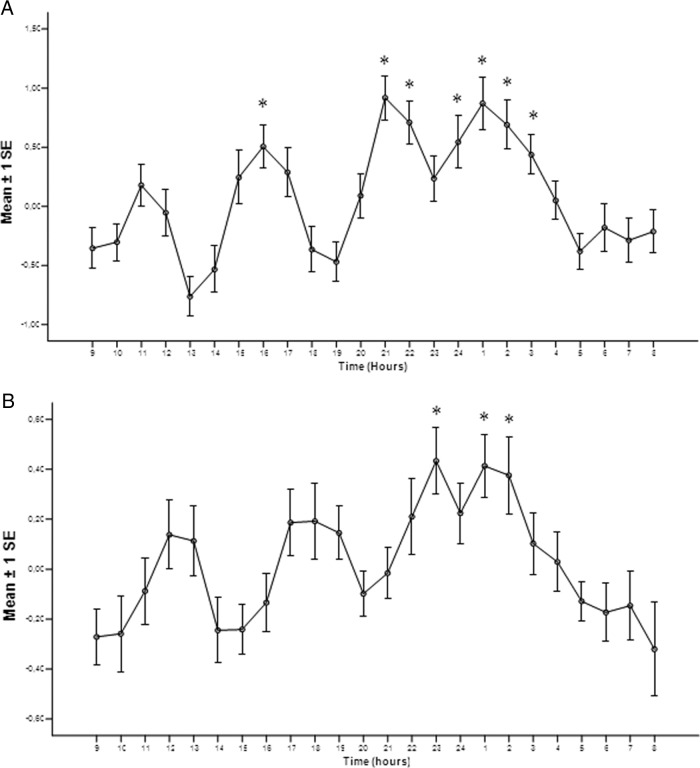
In control women (Figure 3A), the composite profiles of ln-transformed ratios of the hourly mean to daily median GH concentrations exhibited maximal values at 4:00 pm, 9:00 pm, and 1:00 am (P < .05). However, the comparison of control women (Figure 3A) and control men (Figure 2A) shows that the variation of ln-transformed GH ratios between the night peak and the nadir (9:00 am) between groups was much smaller in women than in men, respectively (1.23 ± 0.32 vs 3.21 ± 0.21, P < .001). This is also fully compatible with our earlier data (13, 16), showing relative stability of GH output throughout the 24-hour period in women. Likewise, female acromegalic patients (Figure 3B) elicited preserved significant augmentation of GH secretion after sleep, at 11:00 pm, 1:00 am, and 2:00 am (P < .001 for all).
Figure 3.
Mean composite profile of ln-transformed ratios of the hourly mean over daily median for the control women (A) and female acromegalic patients (B). *, P < .05 for comparisons with the nadir (9:00 am).
Age dependence of daily GH output in controls and acromegalic patients
The control group (Figure 4A) consisted of 104 subjects ranging in age between 13 and 77 years. Their mean 24-hour GH concentrations varied greater than 100-fold, ranging between 0.03 and 4.6 μg/L. Linear regression analysis revealed that 24-hour GH means correlated inversely and significantly to age (n = 104, r = −0.36, P < .001).
Figure 4.
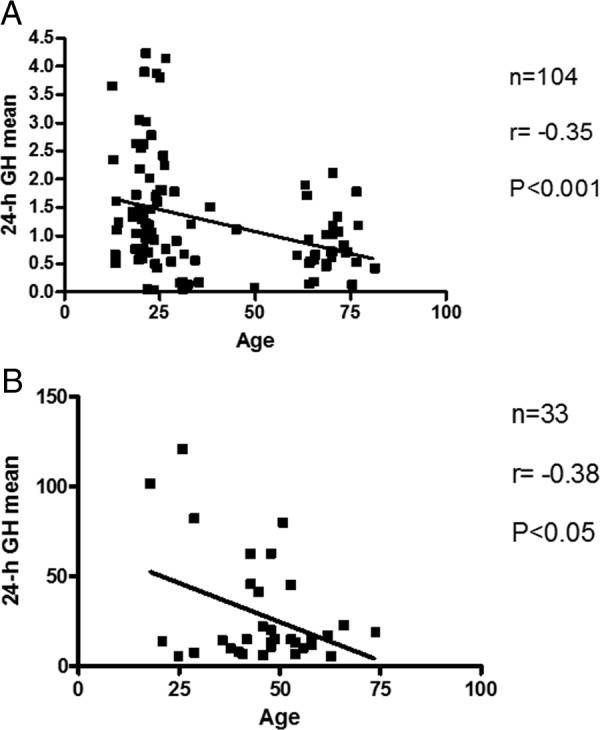
Linear regression analysis of daily GH output vs age for the controls (A) and acromegalic patients (B).
Our database of acromegalic patients contained a disproportionally high number of newly diagnosed patients with mean 24-hour GH concentrations within the normal range, below 4.6 μg/L (n = 34 of 67). Thus, the entire data set of acromegalic patients was dominated by the patients of widely different ages (23–74 years of age) exhibiting very narrow, only 6.5-fold range of mean 24-hour GH concentrations (0.7–4.6 μg/L). Thus, only the patients exhibiting daily GH output above the normal range and a degree of intersubject GH variability (between 4.7 and 121 μg/L, ∼25-fold difference), comparable with normal controls, were included in the final analysis (n = 33). In this group (Figure 4B), a similar negative correlation between daily GH output and the age was observed (n = 33, r = −0.38, P < .05), whereas the average age of this subgroup was not different from the entire group of acromegalic patients (P > .05).
The linear regression analysis of tumor volumes vs age did not show a significant correlation of tumor volumes and age in acromegalic patients (r = 0.14, P > .05).
Discussion
GH rhythmicity has been shown to be an important regulator of multiple physiological phenomena in males and females, including differences in metabolism in humans (17, 18), somatic growth in rats (19, 20), GH binding protein, and IGF-I mRNA in peripheral tissues in both rats and humans (19, 20, 21). The differential parameters of GH secretion, ie, rhythmicity, may thus carry specific messages that are tissue specific and possess major biological significance. Our data show, for the first time, that the neuroendocrine GH rhythmicity is preserved in a large cohort of acromegalic patients. Moreover, it shows that both sexual dimorphism of GH rhythm and age-related decline of GH secretion in acromegalic patients with high GH values are maintained in acromegaly.
We have previously demonstrated the complexity of GH rhythmicity in humans, including a multiwave pattern of GH secretion in a small cohort of healthy men and women (13). Our current results further confirm the 3-wave pattern in an expanded group of normal individuals in addition to revealing the same pattern in a large cohort of acromegalic patients. These data show that GH rhythm in acromegaly is similar to that observed in normals, although possibly regulated at a higher hypothalamic set point, similarly to what has been described for glucocorticoid regulation in Cushing's disease (8). However, it is noteworthy that our GH rhythm patterns of both normals and patients shown in Figure 1 resemble more what is observed in males due to the preponderance of this gender enrolled to this study.
GH secretory patterns in humans are sexually dimorphic in terms of pulse regularity, amplitude of the diurnal rhythm, and intensity of basal secretion (13, 16). The exact neuroendocrine mechanisms of gender-specific GH regulation in humans are debatable. However, male and female GH profiles are easily distinguished at a glance, with women having more uniform GH pulses throughout the day and men having a large nocturnal pulse and relatively low GH output over the rest of the day, as shown in our gender subsets of controls. Interestingly, when our data from acromegalic patients were studied according to gender, the observed GH secretion patterns in these patients were quite similar to controls. Male patients showed relative apulsatility of GH secretion during waking hours, with a prominent augmentation of GH secretion around midnight, whereas female patients showed relative stability of GH output throughout the 24-hour period, similar to their healthy control counterparts, further corroborating the hypothesis of persistence of hypothalamic GH regulation in acromegaly (10–12). The absence of significance in some daily GH waves in normal and acromegalic women might have been in part due to the sharply curtailed number of female subjects in our database (n = 34 for controls and 24 for acromegalic subjects).
Sexual dimorphism pattern of GH regulation has been extensively studied (13, 16, 20–22), and the 2 main central regulators of GH secretion [GHRH and somatostatin (SRIH)] play a major role. Administration of GHRH antiserum reliably inhibited GH pulses in rats of both sexes (23–25), indicating the crucial role of endogenous GHRH for GH pulse generation. Somatostatin antiserum, in contrast, did not alter GH pulse occurrence but increased interpulse GH levels, indicating that SRIH is important for the maintenance of basal GH secretion in both sexes (25). Further data with a GHRH receptor antagonist in normal humans indicated that GHRH is tonically secreted during the daytime in women but not in men (26). The similarity of our data in acromegalic patients of either gender to their respective controls suggests that in female patients GHRH secretion might be tonically secreted during the daytime with relatively blunted nocturnal GHRH release. Conversely, it is possible that the GH secretion in male patients may depend on acute periodic GHRH bursts arising from a near-zero GHRH background and on massive nocturnal GHRH output (13).
The neuroendocrine mechanisms of somatopause are uncertain. Although the 2 key hypothalamic peptides, GHRH and SRIH, are possibly involved in age-dependent decline of GH secretion (27, 28), data in humans are still scarce. In rodents, aging-associated GH decline has been ascribed to hypothalamic SRIH excess, GHRH deficiency, or both (27, 28). In humans, relative GHRH deficiency was suggested as a possible mechanism of aging-associated decline in GH (29, 30). We have observed here an age dependence of GH secretion in acromegalic patients with obviously high GH values, similar to controls, suggesting that age dependence of the hypothalamic GHRH output might also be present in acromegalic patients. Our data are further supported by the recent study of Tanimoto et al (31), who found a gradual decline of mean GH concentrations in young, middle-aged, and elderly acromegalic patients from 18.5 to 8.8 and 6.7 μg/L, respectively.
However, to address the presence of age dependence in acromegaly, we had to exclude the disproportionate number of patients with mean 24-hour GH concentrations within the normal range, below 4.6 μg/L. This was due to the referral bias whereby community endocrinologists referred their patients with equivocal data (elevated IGF-I but ostensibly normal GH) to our institution for the final diagnosis. The exclusion of these patients with an extremely narrow range of 24-hour GH means made it possible to compare acromegalic patients and normals, thus disclosing the age dependence of GH secretion in acromegalic patients with high GH.
This retrospective study carries some limitations. We do not have the body mass index of each single patient, which could potentially interfere with GH decline in the elderly. In the assessment of GH rhythmicity, we cannot discard a possible interference of food intake upon GH wave peaks as described by others (32), although it does not seem to be the case because none of the groups showed a significant augmentation of GH after breakfast, and the overall pattern of GH secretion remained stable during both normal feeding or fasting conditions (33).
The present study was strengthened by the inclusion of large numbers of GH profiles in both normal controls and in patients with acromegaly, in addition to the use of a uniform GH assay. Smoothing of GH profiles obtained through the calculation of mean hourly GH concentrations from multiple samples minimized the disproportionate influence of serendipitously high or low values. Moreover, the normalization of mean hourly GH values to the subject's own median hourly GH and logarithm transformation of the ratios eliminated the interindividual variation of GH output without blunting the variations observed at higher levels in acromegaly. These strategies revealed the presence of GH rhythmicity and sexual dimorphism, along with the age dependence of GH output, all markers of the persistent central regulation of GH secretion in patients with pituitary somatotropinomas.
Our data do not challenge the established theory of acromegaly as a primarily pituitary disease. However, they demonstrate that GH secretion from pituitary somatotropinomas is not entirely autonomous but is still subject to the normal hypothalamic regulation, potentially at a different set point. The implications of these findings to diagnosis and treatment strategies should be further explored in the future.
Acknowledgments
A.R.-O. was supported by the Fundação de Amparo à Pesquisa do estado de Minas Gerais and Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Disclosure Summary: The authors have no conflict of interest to disclose.
Footnotes
- MRI
- magnetic resonance imaging
- SRIH
- somatostatin.
References
- 1. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu FC, Butler GE, Kelnar CJ, Huhtaniemi I, Veldhuis JD. Ontogeny of pulsatile gonadotropin releasing hormone secretion from midchildhood, through puberty, to adulthood in the human male: a study using deconvolution analysis and an ultrasensitive immunofluorometric assay. J Clin Endocrinol Metab. 1996;81:1798–1805 [DOI] [PubMed] [Google Scholar]
- 3. Dericks-Tan JS, Merz PG, Schrödter A, Taubert HD. Differences in the nyctohemeral secretion of TSH and PRL in healthy euthyroid men. Exp Clin Endocrinol Diabetes. 1996;104:145–150 [DOI] [PubMed] [Google Scholar]
- 4. Mazzoccoli G, Giuliani F, Sothern RB. A method to evaluate dynamics and periodicity of hormone secretion. J Biol Regul Homeost Agents. 2011;25:231–238 [PubMed] [Google Scholar]
- 5. Veldhuis JD, Iranmanesh A, Naftolowitz D, Tatham N, Cassidy F, Carroll BJ. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86:5554–5563 [DOI] [PubMed] [Google Scholar]
- 6. Shah N, Evans WS, Bowers CY, Veldhuis JD. Tripartite neuroendocrine activation of the human growth hormone (GH) axis in women by continuous 24-hour GH-releasing peptide infusion: pulsatile, entropic, and nyctohemeral mechanisms. J Clin Endocrinol Metab. 1999;84:2140–2150 [DOI] [PubMed] [Google Scholar]
- 7. van Staveren WC, Solís DW, Delys L, et al. Gene expression in human thyrocytes and autonomous adenomas reveals suppression of negative feedbacks in tumorigenesis. Proc Natl Acad Sci USA. 2006;103:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGrath GA, Goncalves RJ, Udupa JK, et al. New technique for quantitation of pituitary adenoma size: use in evaluating treatment of gonadotroph adenomas with a gonadotropin-releasing hormone antagonist. J Clin Endocrinol Metab. 1993;76:1363–1368 [DOI] [PubMed] [Google Scholar]
- 10. Jaffe CA, Pan W, Brown MB, DeMott-Friberg R, Barkan AL. Regulation of GH secretion in acromegaly: reproducibility of daily GH profiles and attenuated negative feedback by IGF-I. J Clin Endocrinol Metab. 2001;86:4364–4370 [DOI] [PubMed] [Google Scholar]
- 11. Dimaraki EV, Chandler WF, Brown MB, et al. The role of endogenous growth hormone-releasing hormone in acromegaly. J Clin Endocrinol Metab. 2006;91:2185–2190 [DOI] [PubMed] [Google Scholar]
- 12. Barkan AL, Stred SE, Reno K, et al. Increased growth hormone pulse frequency in acromegaly. J Clin Endocrinol Metab. 1989;69:1225–1233 [DOI] [PubMed] [Google Scholar]
- 13. Surya S, Symons K, Rothman E, Barkan AL. Complex rhythmicity of growth hormone secretion in humans. Pituitary. 2006;9:121–125 [DOI] [PubMed] [Google Scholar]
- 14. Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL. Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab. 2002;87:3537–3542 [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro-Oliveira A, Jr, Faje AT, Barkan AL. Limited utility of oral glucose tolerance test in biochemically active acromegaly. Eur J Endocrinol. 2011;164:17–22 [DOI] [PubMed] [Google Scholar]
- 16. Jaffe CA, Ocampo-Lim B, Guo W, et al. Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest. 1998;102:153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nørrelund H. The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res. 2005;15:95–122 [DOI] [PubMed] [Google Scholar]
- 18. Pombo M, Pombo CM, Astorga R, Garcia-Mayor RV, et al. Regulation of growth hormone secretion by signals produced by the adipose tissue. J Endocrinol Invest. 1999;22:22–26 [PubMed] [Google Scholar]
- 19. Isgaard J, Carlsson L, Isaksson OG, Jansson JO. Pulsatile intravenous growth hormone (GH) infusion to hypophysectomized rats increases insulin-like growth factor I messenger ribonucleic acid in skeletal tissues more effectively than continuous GH infusion. Endocrinology. 1988;123:2605–2610 [DOI] [PubMed] [Google Scholar]
- 20. Legraverend C, Mode A, Wells T, Robinson I, Gustafsson JA. Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretion in normal and dwarf rats. FASEB J. 1992;6:711–718 [DOI] [PubMed] [Google Scholar]
- 21. Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins PB. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab. 2002;283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- 22. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Painson JC, Tannenbaum GS. Sexual dimorphism of somatostatin and growth hormone-releasing factor signaling in the control of pulsatile growth hormone secretion in the rat. Endocrinology. 1991;128:2858–2866 [DOI] [PubMed] [Google Scholar]
- 24. Ono M, Miki N, Demura H. Effect of antiserum to rat growth hormone (GH)-releasing factor on physiological GH secretion in the female rat. Endocrinology. 1991;129:1791–1796 [DOI] [PubMed] [Google Scholar]
- 25. Plotsky PM, Vale W. Patterns of growth hormone-releasing factor and somatostatin secretion into the hypophysial-portal circulation of the rat. Science. 1985;230:461–463 [DOI] [PubMed] [Google Scholar]
- 26. Jessup SK, Dimaraki EV, Symons KV, Barkan AL. Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J Clin Endocrinol Metab. 2003;88:4776–4780 [DOI] [PubMed] [Google Scholar]
- 27. Müller EE, Cella SG, Parenti M, et al. Somatotropic dysregulation in old mammals. Horm Res. 1995;43:39–45 [DOI] [PubMed] [Google Scholar]
- 28. Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39 [DOI] [PubMed] [Google Scholar]
- 29. Russell-Aulet M, Jaffe CA, Demott-Friberg R, Barkan AL. In vivo semiquantification of hypothalamic growth hormone-releasing hormone (GHRH) output in humans: evidence for relative GHRH deficiency in aging. J Clin Endocrinol Metab. 1999;84:3490–3497 [DOI] [PubMed] [Google Scholar]
- 30. Russell-Aulet M, Dimaraki EV, Jaffe CA, DeMott-Friberg R, Barkan AL. Aging-related growth hormone (GH) decrease is a selective hypothalamic GH-releasing hormone pulse amplitude mediated phenomenon. J Gerontol A Biol Sci Med Sci. 2001;56:M124–M129 [DOI] [PubMed] [Google Scholar]
- 31. Tanimoto K, Hizuka N, Fukuda I, Takano K, Hanafusa T. The influence of age on the GH-IGF1 axis in patients with acromegaly. Eur J Endocrinol. 2008;159:375–379 [DOI] [PubMed] [Google Scholar]
- 32. Canosa LF, Unniappan S, Peter RE. Periprandial changes in growth hormone release in goldfish: role of somatostatin, ghrelin, and gastrin-releasing peptide. Am J Physiol Regul Integr Comp Physiol. 2005;289:R125–R133 [DOI] [PubMed] [Google Scholar]
- 33. Nass R, Farhy LS, Liu J, et al. Evidence for acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]