Abstract
Context:
Gut-derived serotonin has been proposed as a regulator of bone formation, and inhibition of gut serotonin synthesis increases bone formation in rodents. Carcinoid neuroendocrine tumors can produce very high levels of circulating serotonin and so offer a model of serotonin excess in humans.
Objectives:
The objective of the study was to determine whether patients with carcinoid syndrome have lower bone formation markers, lower bone density, or poor bone structure compared with healthy controls.
Design:
We conducted a cross-sectional study of 25 patients with carcinoid syndrome and 25 healthy controls, individually matched to carcinoid patients by gender, age, height, and body mass index.
Outcome Measures:
We measured circulating serotonin in blood and plasma and 5-hydroxyindoleacetic acid (5HIAA) in plasma and urine. We measured lumbar spine and hip bone mineral density by dual-energy x-ray absorptiometry, the distal radius and tibia with high-resolution peripheral quantitative computed tomography, and bone turnover with serum osteocalcin, amino-terminal propeptide of type I procollagen (PINP) and C-terminal telopeptide of type I collagen (CTX).
Results:
All measures of serotonin and 5HIAA were higher in carcinoid patients than in controls. No measures of bone density or bone structure differed significantly between cases and controls. Osteocalcin was higher in the cases than controls (26.0 vs 21.1 ng/mL, P = .02). PINP and CTX did not differ between cases and controls. In patients with carcinoid syndrome, plasma 5HIAA was positively correlated with osteocalcin. In controls, whole-blood serotonin was positively correlated with osteocalcin, PINP, and CTX (R values = 0.40–0.47, all P < .05.)
Conclusions:
High circulating serotonin in carcinoid syndrome is not associated with clinically significant lower bone density, poorer bone structure, or lower bone formation markers.
Low-density lipoprotein receptor-related protein 5 (Lrp5) is thought to be an important regulator of osteoblast function as a Wnt coreceptor. Activating mutations of Lrp5 cause a high bone mass phenotype (1), and inactivating mutations cause the rare osteoporosis pseudoglioma syndrome (2).
Studies of tissue-specific Lrp5 knockout mice found that osteoblast-specific knockout mice have a normal bone phenotype, but gut-specific knockout mice have low bone mass, suggesting that altered Lrp5 signaling in osteoblasts does not cause the bone phenotype in Lrp5 mutations. Gut-specific knockouts have high circulating serotonin due to loss of inhibition of tryptophan hydroxylase 1 (Tph-1), the rate-limiting enzyme for serotonin synthesis in enterochromaffin cells (3). Reduction of circulating serotonin (by knocking out Tph-1, a low tryptophan diet or treatment with a Tph-1 inhibitor) rescues the low bone mass phenotype in gut-specific Lrp5 knockouts and ovariectomized rodents (3–5), suggesting that alterations in gut synthesis of serotonin are the mechanism through which Lrp5 mutations affect bone mass. However, these findings have not been consistent in all models (6).
Osteoblasts express serotonin receptors, so a role for serotonin in regulation of bone formation is plausible (7). Patients with high bone mass due to activating mutations of Lrp5 have reduced circulating serotonin, and patients with osteoporosis pseudoglioma syndrome have high circulating serotonin (3, 8, 9).
If circulating serotonin is an important regulator of osteoblast function in humans, then Tph-1 inhibitors may have potential as anabolic treatments for osteoporosis.
Carcinoid neuroendocrine tumors can produce serotonin in excess, with symptoms of flushing, sweating, diarrhea, and fibrosis of the mesentery and heart valves (carcinoid syndrome) (10). Therefore, carcinoid syndrome offers a model to study the effects of serotonin excess on the skeleton in humans. A previous study found no differences in biochemical bone turnover markers between patients with carcinoid disease who were hypersecretors of serotonin compared with patients with carcinoid disease who were nonsecretors of serotonin (11). However, this study had some limitations. There was no healthy control group; circulating serotonin was assessed by the urinary metabolite 5-hydroxyindoleacetic acid (5HIAA; not by blood measurement of serotonin), and there were no measurements of bone density or bone structure.
The aim of this study was to determine whether patients with serotonin excess due to carcinoid syndrome have the following: 1) lower biochemical markers of bone formation, 2) lower bone mineral density (BMD), and 3) poor bone microarchitecture compared with healthy controls.
This is the first study to use high-resolution peripheral peripheral quantitative computed tomography (HR-pQCT) to measure bone microarchitecture in carcinoid syndrome.
Materials and Methods
We conducted a single-center, cross-sectional, observational study of patients with carcinoid syndrome and individually matched controls.
Subjects
We recruited 26 patients with active midgut carcinoid disease (neuroendocrine tumors of the jejunum and ilieum) from the Sheffield neuroendocrine clinic and 26 healthy controls through poster advertisements, e-mails to hospital and university staff, and mailings from Sheffield general practices. Controls were individually matched to a case by gender, age (±5 years), height (±5 cm), and body mass index (BMI; ±3 kg/m2). The effects of high serotonin on body fat mass and distribution are not yet well described. Body size and weight affect bone mass [through true physiological actions and effects on technical aspects of bone densitometry by dual-energy x-ray absorptiometry (DXA)] and biochemical markers of bone turnover. Particularly because patients with carcinoid disease might have lost weight due to diarrhea and tumor load, it was important to ensure there were not systematic differences in body size between patients and controls. BMI is an unsophisticated measure of body composition but does correlate with BMD and biochemical markers of bone turnover, and by matching for height and BMI, we tried to eliminate the effects of body size and weight in comparisons of patients and controls. Duration of carcinoid syndrome was derived from clinical history.
Volunteers were excluded if they had any disease or were taking any medication known to affect bone metabolism, if they did not have at least 2 evaluable vertebrae on DXA, had previously fractured both radii or tibiae, if they were pregnant or trying to conceive, or if they were unable to give informed consent.
We excluded 1 pair from the final analysis because all of the control subject's bone turnover markers were extreme high outliers. This subject was subsequently investigated and found to have vitamin D deficiency.
The study was approved by the South Yorkshire Research Ethics Committee, and all participants gave written informed consent.
Imaging
Bone density at the lumbar spine and proximal femur in grams per square centimeter was measured by DXA (Hologic Discovery, Bedford, Massachusetts). The short-term precision for BMD on this machine is 1.0% for the lumbar spine, 1.1% for the total hip, and 1.4% for the femoral neck.
HR-pQCT images of the distal radius and distal tibia for measurement of bone geometry, density, and microarchitecture were obtained with the XtremeCT device (Scanco Medical AG, Zurich, Switzerland) using standard protocols. The short-term precision for this machine at the distal radius is 0.9%–5.5% for BMD measurements and 1.4%–6.8% for microarchitecture measurements. The short-term precision at the distal tibia is 0.2%–1.7% for BMD measurements and 0.2%–5.3% for microarchitecture measurements.
Biochemical markers of bone turnover
Bone formation was evaluated with amino-terminal propeptide of type I procollagen (PINP) and osteocalcin (OC). Bone resorption was evaluated with β-C-terminal telopeptide of type I collagen (CTX). All 3 markers were measured on timed, fasting morning samples with the Cobas e411 analyzer (Roche Diagnostics, Pensberg, Germany); PINP coefficient of variation (CV) of 2.1%, OC CV of 1.6%, and CTX CV of 6.8%.
Serotonin and metabolites
Subjects followed dietary restriction (of high-serotonin foods: avocados, bananas, tomatoes, plums, walnuts, eggplants, pineapples) and were asked not to take acetaminophen (paracetamol) or cough medicines for 48 hours before and during urine collection and blood sampling. Blood samples were fasting morning samples.
Serotonin was measured in 10 μL of whole blood by liquid chromatography tandem mass spectrometry. Blood was lysed using 90 μL of water, and then 20 μL of dueterated (D4) 5-hydroxytryptamine was added as an internal standard and 200 μL of acetonitrile was added to precipitate the proteins. The samples were vortexed and centrifuged at 2500 rpm for 5 minutes. The liquid chromatography tandem mass spectrometry was performed on a Waters Acquity UPLC system coupled to a Waters Quattro Premier mass spectrometer (Milford, Massachusetts). The sample was injected onto a Phenomenex SecurityGuard strong cation exchange 4- × 3-mm guard column and the eluant from this column directed to waste for 0.7 minutes. After this time, the eluant was directed through a C18 Onyx monolithic 4.8- × 25-mm column and into the mass spectrometer. The assay is linear to at least 100 μmol/L, and the lower limit of quantification is 40 nmol/L. The results are reported as nanomoles per 109 platelets.
5HIAA was measured in plasma by liquid chromatography and tandem mass spectrometry on a Waters Acquity UPLC system coupled to a Waters Quattro Premier mass spectrometer. The sample preparation uses 50 μL of plasma, 10 μL of D2 5HIAA as internal standard, and 200 μL of acetonitrile to precipitate the proteins. The samples were vortexed and centrifuged at 2500 rpm for 5 minutes. Liquid chromatography was carried out using a Phenomenex SecurityGuard Gemini c18 4- × 2-mm guard column coupled to a Sielc Primesep B 3.2- × 50-mm column. The assay is linear to 10 000 nmol/L (12).
Urinary 5HIAA was measured on a 24-hour collection by reverse-phase HPLC, using a Thermo Scientific ChromQuest system (Loughborough, United Kingdom).
Statistics
Paired-samples Student t tests were used to compare cases and controls. Variables for which the differences between pairs were not normally distributed were log transformed for analysis.
To make a power calculation, the SD of the paired differences could not be estimated because there are no existing data on BMD in carcinoid patients against controls. Therefore, a model of paired differences of obese and lean subjects in another study was used to make an estimate of paired difference variability. The clinically significant difference in BMD was estimated as 8%, equivalent to about 0.5 SD scores [1 decrease in SD score is associated with about a 2-fold increase in fracture risk (13)]. This calculation found that 26 pairs would have 85% power to detect an 8% difference in BMD. An interim power calculation was performed when 16 pairs had been recruited, which found that 26 pairs would have 80% power to detect an 8% difference in hip BMD.
Spearman's correlation was used to assess correlations between measurements of circulating serotonin and bone turnover markers.
P < .05 was accepted as statistically significant.
Results
Cases and controls were well matched (Table 1). The mean duration of symptomatic carcinoid syndrome was 4.7 years (range 0.5–17.0 years). Twelve patients were currently receiving treatment with somatostatin analogs. No patients or controls were receiving treatment with selective serotonin reuptake inhibitors.
Table 1.
Participant Characteristics, Serotonin, and 5HIAA
| Controls (n = 25) | Carcinoid (n = 25) | |
|---|---|---|
| Gender, male:female | 16:9 | 16:9 |
| Age, y | 63.9 (7.7) | 64.4 (7.0) |
| Height, cm | 173.1 (9.7) | 172.8 (9.7) |
| BMI, kg/m2 | 26.5 (3.5) | 26.4 (3.5) |
| Blood serotonin, nmol/109 platelets | 0.35 (0.16–0.80) | 8.37 (1.00–47.19)a |
| Plasma 5HIAA, nmol/L | 56.0 (25.0–105.0) | 396.7 (42.0–9592.0)a |
| 24-h urine 5HIAA, μmol per 24 h | 24.0 (15.0–49.0) | 122.8 (27.0–1411.0)a |
Results for age, height, and BMI are given as arithmetic mean (SD). Results for serotonin and 5HIAA given as geometric mean (range). Serotonin and 5HIAA were log transformed for analysis.
Paired-samples t test, P < .001.
Blood serotonin, plasma 5HIAA, and urine 5HIAA were all significantly higher in cases than in controls (Table 1).
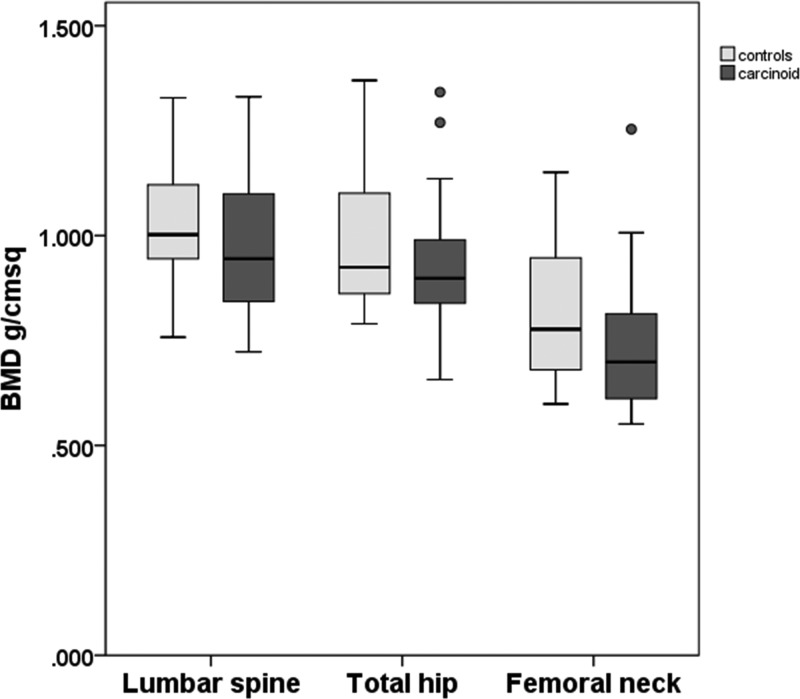
Bone mineral density by DXA did not differ between cases and controls at the lumbar spine [mean paired difference 0.046 g/cm2, 95% confidence interval (CI) −0.038 to 0.13], total hip (mean paired difference 0.063 g/cm2, 95% CI −0.003 to 0.129), or femoral neck (mean paired difference 0.053 g/cm2, 95% CI −0.023 to 0.130) (Figure 1).
Figure 1.
BMD by DXA. Boxes indicate the 25th and 75th centiles, central lines indicate the median, whiskers indicate the minimum and maximum values that are not outliers, and circles represent outliers. Lumbar spine paired differences were not normally distributed, so values were log transformed for analysis (paired samples t test; no significant differences between controls and carcinoid).
There were no differences between cases and controls in any of the measurements of BMD, bone geometry, or microarchitecture at the radius or the tibia (Table 2).
Table 2.
HR-pQCT of the Radius and Tibia
| Controls | Carcinoid | |
|---|---|---|
| Radius area, mm2a | 396.4 (106.0) | 386.6 (102.6) |
| Radius BMD, mg HA/mm3 | 294.0 (58.5) | 290.9 (59.3) |
| Radius cortical BMD, mg HA/mm3 | 819.9 (87.0) | 820.3 (59.1) |
| Radius cortical thickness, mm | 0.672 (0.227) | 0.671 (0.196) |
| Radius trabecular BMD, mg HA/mm3 | 176.2 (32.4) | 174.6 (44.9) |
| Radius trabecular BV/TV | 0.147 (0.027) | 0.146 (0.038) |
| Radius trabecular number, mm−1 | 2.14 (0.26) | 2.12 (0.25) |
| Radius trabecular thickness, mm | 0.069 (0.011) | 0.068 (0.012) |
| Tibia area, mm2a | 858.2 (153.6) | 847.7 (191.7) |
| Tibia BMD, mg HA/mm3 | 298.2 (47.3) | 285.3 (65.7) |
| Tibia cortical BMD, mg HA/mm3 | 851.2 (59.7) | 832.2 (67.1) |
| Tibia cortical thickness, mm | 1.13 (0.29) | 1.03 (0.35) |
| Tibia trabecular BMD, mg HA/mm3 | 190.8 (35.9) | 184.8 (45.7) |
| Tibia trabecular BV/TV | 0.159 (0.030) | 0.154 (0.038) |
| Tibia trabecular number, mm−1 | 2.01 (0.28) | 1.91 (0.34) |
| Tibia trabecular thickness, mm | 0.080 (0.012) | 0.081 (0.013) |
Abbreviation: BV/TV, bone volume fraction; HA, hydroxyapatite. Results given as mean (SD).
Paired differences are not normally distributed, and data are log transformed for analysis (paired samples t test; no significant differences between controls and carcinoid).
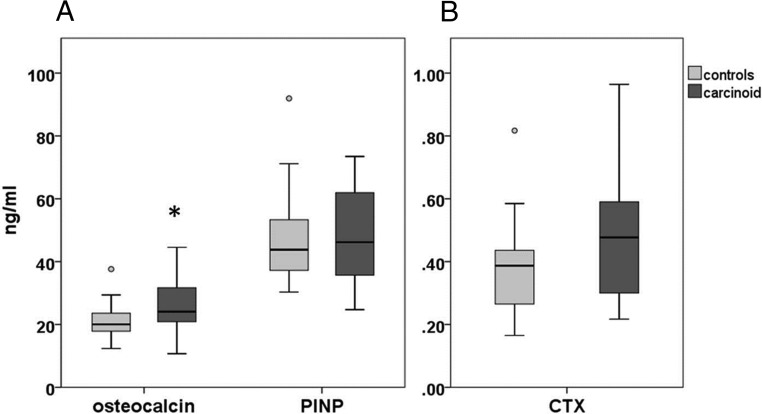
PINP (mean paired difference 1.5 ng/mL, 95% CI −7.0 to 10.1) and CTX (mean paired difference 0.086 ng/mL, 95% CI −0.021 to 0.192) did not differ between cases and controls. OC was higher in cases than controls (mean paired difference 4.9 ng/mL, 95% CI 0.7–9.1) (Figure 2).
Figure 2.
Serum biochemical markers of bone turnover: formation (A) and resorption (B). Boxes indicate the 25th and 75th centiles, central lines indicate the median, whiskers indicate the minimum and maximum values that are not outliers, and circles represent outliers. *, Paired-samples t test (different from controls, P < .05).
In patients with carcinoid syndrome, plasma 5HIAA was positively correlated with OC and CTX. Whole-blood serotonin and urine 5HIAA did not correlate with any of the bone turnover markers. In controls, whole-blood serotonin was positively correlated with OC, PINP, and CTX. Plasma and urine 5HIAA did not correlate with any of the bone turnover markers (Table 3).
Table 3.
Correlation of Serotonin and 5HIAA With Bone Turnover Markers in Patients With Carcinoid Syndrome
| Blood Serotonin, nmol/109 Platelets | Plasma 5HIAA, nmol/L | 24-Hour Urine 5HIAA, mmol per 24 h | |
|---|---|---|---|
| Controls | |||
| OC, ng/mL | 0.42a | 0.13 | 0.15 |
| PINP, ng/mL | 0.45a | 0.14 | 0.27 |
| CTX, ng/mL | 0.47a | 0.26 | 0.08 |
| Carcinoid patients | |||
| OC, ng/mL | 0.36 | 0.43a | 0.31 |
| PINP, ng/mL | 0.24 | 0.23 | 0.14 |
| CTX, ng/mL | 0.32 | 0.40a | 0.29 |
Results given as Spearman's rho R value.
P < .05.
Discussion
We hypothesized that patients with carcinoid syndrome and high circulating serotonin might be expected to have reduced osteoblast activity, with low bone formation, reduced bone density, and poor microarchitecture. However, despite prolonged exposure to very high circulating serotonin, we did not find any clinically significant differences in bone density or microarchitecture between patients with carcinoid syndrome and controls. PINP did not differ between cases and controls, and OC was higher in patients with carcinoid syndrome than controls, suggesting that bone formation is not inhibited in carcinoid syndrome.
Our bone turnover results are similar to those of van Dijk et al (11) who found no difference in PICP, OC, or N-terminal cross-linking telopeptide of type I collagen between patients with carcinoid disease who had high or normal serotonin secretion. They found no correlation between urinary 5HIAA and bone turnover markers, which is consistent with our results.
However, we did find some correlations of bone turnover markers with plasma and blood serotonin measurements; plasma 5HIAA correlated with the bone formation marker OC in patients with carcinoid syndrome, and blood serotonin correlated with the formation markers OC and PINP and the resorption marker CTX in controls. The direction of these relationships is unexpected if serotonin is an inhibitor of bone formation. In carcinoid patients it is possible that patients with more severe disease, and hence higher serotonin, could have higher bone turnover than patients with milder disease due to proinflammatory effects of tumor load or poor nutrition. However, the positive relationship between bone turnover and serotonin was also seen in controls and was seen in healthy controls from the previous study of Lrp5 mutations (8). We do not yet have an explanation for this association.
Other human studies do support an association between circulating serotonin and bone mass. Circulating serotonin is negatively correlated with femoral neck BMD and radius trabecular thickness in healthy women (14). Patients with high bone mass Lrp5 mutations have lower circulating serotonin than controls (3, 8, 9), but their serotonin is not correlated with any bone imaging or biochemical measurements. In healthy controls from the Lrp5 high bone mass study, serotonin was negatively correlated with tibial cortical density (but no other bone measures at the spine, hip, tibia, or radius) (8).
However, treatment of human preosteoblasts with serotonin does not alter differentiation or mineralization (11), suggesting that circulating serotonin may not be a direct regulator of osteoblast function in humans.
Some preclinical models support a role for circulating serotonin in inhibition of bone formation and bone mass, but not all animal studies have produced consistent results. In vitro studies confirm an inhibitory function of serotonin in rodent osteoblasts through the Htr1b receptor (3, 15). Gut-specific Lrp5 knockout mice demonstrate loss of inhibition of Tph-1 serotonin production and high circulating serotonin and have low bone mass due to reduced osteoblast proliferation (3, 15). Reduction of circulating serotonin by a low-tryptophan diet restores their bone mass (3). Conversely, gut-specific Tph-1 knockouts have low circulating serotonin and high bone mass due to increased osteoblast proliferation and increased bone formation (3), consistent with a role for serotonin in inhibition of bone formation.
However, in other models, global Tph-1 knockout mice demonstrate increased bone mass during growth but have a similar bone phenotype to wild type at maturity (6, 16), and activation or inactivation of Lrp5 in mouse intestinal cells does not affect bone mass, Tph-1 expression, or circulating serotonin levels (6).
Inhibition of gut serotonin synthesis through Tph-1 inhibition prevents bone loss and restores bone mass in ovariectomized rodents in a similar manner to parathyroid hormone (4, 5), although this bone anabolic effect was not reproduced by a different Tph-1 inhibitor, despite decreased intestinal serotonin content (6).
In summary, the existing evidence supports an association between Lrp5 mutations and circulating serotonin in humans, but it is not yet clear whether the alterations in serotonin levels are causative of the bone phenotype. It is possible that gut serotonin is an important regulator of bone turnover in rodents, but there are some inconsistencies between animal models.
Half of our cases were receiving treatment with somatostatin analogs but still had high plasma serotonin. There are no published data on the effects of somatostatin analogues on bone turnover in carcinoid syndrome, but when used for treatment of acromegaly, they reduce osteocalcin through reduction in IGF-I (17). They might also affect bone metabolism through other mechanisms. In our study those patients on somatostatin analogues (n = 12) had numerically lower BMD by DXA and HR-pQCT compared with those not on treatment, but this was not statistically significant. Nevertheless, this suggests that inclusion of treated patients would be more likely to exaggerate than diminish BMD differences between patients and controls.
We recognize that this is a small sample, but we have maximized the power of the sample to detect differences by using individually matched controls and paired-samples t tests. The power calculation was based on a clinically significant BMD difference of 0.5 SD. It is possible that there are smaller BMD differences than this between cases and controls, which we would not have detected.
Carcinoid syndrome is an extreme model of serotonin excess in humans, and carcinoid syndrome is a complex disorder. Tumors secrete factors other than serotonin, and it is possible that other tumor factors protect the skeleton from deleterious effects of high serotonin, but the commonly cosecreted factor from enterochromaffin cells is histamine, which is proresorptive so would tend to reduce BMD.
However, the combined DXA, HR-pQCT, and bone turnover results suggest that patients with carcinoid syndrome do not have significantly reduced bone formation despite prolonged exposure to high circulating serotonin. This adds important information to the ongoing investigation of the effects of serotonin on bone metabolism.
In conclusion, prolonged exposure to serotonin excess in carcinoid syndrome is not associated with clinically significant reductions in bone density, poor bone microarchitecture, or reduced biochemical markers of bone formation.
The regulation of bone by the gut, and particularly by gut-derived serotonin, is a rapidly developing area of knowledge and may have therapeutic potential. However, the inconsistencies between models show that it is a complex system that may behave differently under different conditions. A clearer understanding may be needed before its therapeutic potential can be evaluated.
Acknowledgments
We thank Mike Bradburn for statistical support and Fatma Gossiel for the bone turnover marker measurements. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research.
This work was supported by a National Institute for Health Research Biomedical Research Unit grant.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CTX
- C-terminal telopeptide of type I collagen
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- 5HIAA
- 5-hydroxyindoleacetic acid
- HR-pQCT
- high-resolution peripheral quantitative computed tomography
- Lrp5
- lipoprotein receptor-related protein 5
- OC
- osteocalcin
- PINP
- amino-terminal propeptide of type I procollagen
- Tph-1
- tryptophan hydroxylase 1.
References
- 1. Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521 [DOI] [PubMed] [Google Scholar]
- 2. Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development Cell. 2001;107:513–523 [DOI] [PubMed] [Google Scholar]
- 3. Yadav VK, Ryu JH, Suda N, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum Cell. 2008;135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Inose H, Zhou B, Yadav VK, Guo XE, Karsenty G, Ducy P. Efficacy of serotonin inhibition in mouse models of bone loss. J Bone Miner Res. 2011;26:2002–2011 [DOI] [PubMed] [Google Scholar]
- 5. Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29:477–486 [DOI] [PubMed] [Google Scholar]
- 8. Frost M, Andersen T, Gossiel F, et al. Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high-bone-mass phenotype due to a mutation in Lrp5 J. Bone Miner Res. 2011;26:1721–1728 [DOI] [PubMed] [Google Scholar]
- 9. Frost M, Andersen TE, Yadav V, Brixen K, Karsenty G, Kassem M. Patients with high-bone-mass phenotype owing to Lrp5–T253I mutation have low plasma levels of serotonin. J Bone Miner Res. 2010;25:673–675 [DOI] [PubMed] [Google Scholar]
- 10. Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9:61–72 [DOI] [PubMed] [Google Scholar]
- 11. van Dijk SC, de Herder WW, Kwekkeboom DJ, et al. 5-HIAA excretion is not associated with bone metabolism in carcinoid syndrome patients. Bone. 2012;50:1260–1265 [DOI] [PubMed] [Google Scholar]
- 12. Miller AG, Brown H, Degg T, Allen K, Keevil BG. Measurement of plasma 5-hydroxyindole acetic acid by liquid chromatography tandem mass spectrometry—comparison with HPLC methodology. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:695–699 [DOI] [PubMed] [Google Scholar]
- 13. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Modder UI, Achenbach SJ, Amin S, Riggs BL, Melton LJ, III, Khosla S. Relation of serum serotonin levels to bone density and structural parameters in women. J Bone Miner Res. 2010;25:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kode A, Mosialou I, Silva BC, et al. FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin. J Clin Invest. 2012;122:3490–3503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chabbi-Achengli Y, Coudert AE, Callebert J, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci USA. 2012;109:2567–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terzolo M, Piovesan A, Osella G, et al. Serum levels of bone Gla protein (osteocalcin, BGP) and carboxyterminal propeptide of type I procollagen (PICP) in acromegaly: effects of long-term octreotide treatment. Calcif Tissue Int. 1993;52:188–191 [DOI] [PubMed] [Google Scholar]