Abstract
Objective:
Fibroblast growth factor (FGF)-21 is highly expressed in the liver and regulates glucose and lipid metabolism in rodents. The effects of obesity and fatty liver on circulating FGF-21 levels have been described mainly in adults. Herein, we measured plasma FGF-21 levels in lean and obese adolescents with low and high hepatic fat content (HFF% <5.5% and HFF% ≥5.5%, respectively) and explored their relationship with hepatic fat content, measures of hepatic apoptosis, and insulin sensitivity.
Methods:
A total of 217 lean and obese adolescents with both low and high HFF% (lean = 31; obese low HFF% = 107; and obese high HFF% = 79) underwent an oral glucose tolerance test, a fast gradient magnetic resonance imaging to measure the %HFF and abdominal fat distribution. Cytokeratin 18 levels were measured as a biomarker of liver apoptosis. A subset of adolescents underwent a 2-step hyperinsulinemic-euglycemic clamp, and a liver biopsy (N = 14), to assess insulin sensitivity and steatohepatitis, respectively.
Results:
Compared to controls, FGF-21 levels were higher in obese youth, especially in those with high HFF (P < .001). FGF-21 significantly correlated with adiposity indexes (P < .001), visceral fat (r2 = 0.240, P < .001), hepatic fat content (r2 = 0.278, P < .001), cytokeratin 18 (r2 = 0.217, P < .001), and alanine aminotransferase (r2 = .164, P < .001). In subjects with steatoheaptitis, FGF-21 levels significantly correlated with the nonalcoholic fatty liver disease activity score (r2 = 0.27, P = .04). Stepwise regression analysis indicated that these relationships are independent of body mass index, visceral fat, and insulin sensitivity. An inverse correlation was documented with insulin, hepatic resistance indexes, and adipose resistance indexes, which disappeared after adjusting for hepatic fat content.
Conclusions:
Plasma FGF-21 levels are increased in obese adolescents, particularly in those with fatty liver. FGF-21 concentrations significantly and independently correlate with hepatic fat content and markers of hepatic apoptosis in obese youths.
Fibroblast growth factors (FGFs) play key roles in the regulation of many cellular processes ranging from development to survival in mammals (1, 2). To date, 22 FGF members have been discovered. Among them, FGF-19 (FGF-15 in mice), FGF-21, and FGF-23 were found to similarly lack a conventional heparin binding domain, which allows them to reach the circulation and to act as hormones (1, 2). Instead of heparin, FGF-19, FGF-21, and FGF-23 utilize Klotho cofactor proteins for binding to and activation of fibroblast growth factor receptors. FGF “endocrine” subgroup, and especially FGF-21, have emerged as important endocrine factors involved in glucose and lipid metabolism and energy regulation (3, 4). FGF-21 is primarily expressed by liver, adipose tissue, and pancreas, whereas the enterocytes in the distal part of the small intestine are the main cells producing FGF-19, following the consumption of a meal (5). Both FGFs mediate their effects on tissues through different classic FGF cell surface receptor isomers (1–4, 6, 7). FGF-21 stimulates glucose uptake in adipocytes (7) and regulates energy metabolism and enhanced mitochondrial oxidative function through the activation of AMP-activated protein kinase and sirtuin 1 (8). Transgenic mice that overexpress FGF-21 had higher glucose clearance and were resistant to diet-induced obesity (7). Administration of recombinant FGF-21 to diabetic fatty rats (7) and diabetic rhesus monkeys (9) reduced plasma glucose and triglycerides, lowered low-density lipoprotein cholesterol, increased high-density lipoprotein cholesterol, and caused a modest weight loss. More importantly, FGF-21 mRNA expression in the human liver increases with steatosis grade, and its serum level is significantly increased in adult nonalcoholic fatty liver disease (NAFLD) patients (10).
Associations of this circulating hormone with glucose, insulin, and lipids metabolism as well as hepatic fat content have been described to a large extent in animals, whereas the physiological roles of FGF-21 in humans remain insufficiently understood, particularly in childhood. Although FGF-21 levels have been reported to be paradoxically elevated in adult subjects with fatty liver, one study in obese children showed no difference with young subjects with or without ultrasound-defined NAFLD (11). Therefore, to gain insights in the putative emerging role of FGF-21 in the pathogenesis of NAFLD, we measured circulating plasma FGF-21 levels in a relatively large group of obese youth with low and high hepatic fat content (HFF% <5.5% and HFF% ≥5.5%, respectively) compared to healthy nonobese peers. Macrovesicular liver fat content was measured noninvasively by the fast-magnetic resonance imaging (MRI) in the entire cohort and in a subset of obese youth by a liver biopsy. We further examined the relationships between FGF-21 with both direct and indirect measures of insulin sensitivity, hepatic insulin sensitivity, and suppression of lipolysis in adolescents with varying degrees of obesity and liver fat content.
Materials and Methods
A group of 217 obese and lean adolescents (N = 186 and N = 31, respectively) was recruited from the Yale Pediatric Obesity Clinic and are part of a longitudinal study on the pathophysiology of type 2 diabetes. Obesity and lean subjects were defined by body mass index (BMI) >95th and by BMI <87th percentile for age and gender, respectively (http://www.cdc.gov/GrowthCharts). Subjects with medical conditions or using medications at the time of recruitment that may affect lipid or glucose metabolism were excluded. All subjects were nonsmokers. Information defining the absence of alcohol consumption was obtained using a questionnaire. Autoimmune hepatitis, Wilson disease, α-1-antitrypsin deficiency, hepatitis B and C, and iron overload were excluded in subjects with persistent elevation in alanine aminotransferase.
All subjects underwent an oral glucose tolerance test as previously described (12). Indexes of insulin sensitivity (whole body insulin sensitivity index, WBISI) (12) and glucose tolerance (normal glucose tolerance; impaired glucose tolerance; type 2 diabetes) according to American Diabetes Association criteria were defined (13). Fasting samples were also collected for measuring FGF-21 and cytokeratin 18 (CK18) levels as a marker of liver apoptosis (14). In all subjects total body composition was measured by dual-energy x-ray absorptiometry and the fast gradient magnetic resonance imaging was used to measure the HFF% and abdominal fat mass distribution. Based on the analysis of hepatic fat content (Table 1), all subjects were divided into the following 3 groups: 1) lean controls (N = 31) with low hepatic fat content (HFF < 5.5%); 2) obese adolescents (N = 107) with low hepatic fat content (HFF < 5.5%); 3) obese adolescents (N = 79) with high hepatic content (HFF ≥ 5.5%).
Table 1.
Main Clinical Characteristic and Body Fat Distribution in Lean and Obese Youth with Low and High Hepatic Fat Content
| Lean | Obese with Low Hepatic Fat Content | Obese with High Hepatic Fat Content | P for Trenda | |
|---|---|---|---|---|
| Numerosity | 31 | 107 | 79 | |
| GT (NGT/IGT/T2D) | 31/0/0 | 83/22/2 | 41/33/5 | .001 |
| Ethnicity (C/AA/H) | 17/7/7 | 47/40/20 | 33/11/35 | .001 |
| Gender, M/F | 14/17 | 38/69 | 39/40 | .154 |
| Anthropometric | ||||
| Age, y | 15.7 ± 0.5 | 14.7 ± 0.3 | 14.5 ± 0.4 | .192 |
| BMI, kg/m2 | 21.1 ± 0.3 | 34.04 ± 0.6 | 35.7 ± 0.6 | <.001b,c,d |
| BMI-Z | 0.32 ± 0.11 | 2.12 ± 0.05 | 2.3 ± 0.03 | <.001b,c,d |
| FM, % | 21 ± 1.0 | 41.8 ± 0.8 | 45.5 ± 0.8 | <.001b,c,d |
| LBM, kg | 45.0 ± 1.8 | 52.3 ± 1.1 | 52.7 ± 1.7 | .016b,c |
| FM, kg | 11.8 ± 0.7 | 39.1 ± 1.5 | 44.6 ± 1.6 | <.001b,c,d |
| Body fat distribution | ||||
| Abdominal | ||||
| Visceral fat, cm2 | 20 ± 2 | 52 ± 2 | 74 ± 3 | <.001b,c,d |
| Subcutaneous fat, cm2 | 136 ± 10 | 489 ± 21 | 560 ± 21 | <.001b,c,d |
| Visc/sub ratio | 0.129 ± 0.008 | 0.105 ± 0.005 | 0.129 ± 0.006 | <.001b,d |
| Deep subQ, cm2 | 43.7 ± 4.5 | 163 ± 8 | 195 ± 8 | <.001b,c,d |
| Superficial SubQ, cm2 | 49 ± 3 | 140 ± 5 | 137 ± 7 | <.001b,c |
| Liver fat content, % | 0.48 ± 0.19 | 1.31 ± 0.16 | 18.90 ± 1.37 | <.001c,d |
| Glucose metabolism | ||||
| Fasting insulin, mcU/mL | 14.90 ± 1.19 | 28.56 ± 1.98 | 43.63 ± 2.94 | <.001b,c,d |
| Fasting C-peptide, pmol/L | 660.69 ± 33.86 | 1030.26 ± 50.33 | 1360.42 ± 65.81 | <.001b,c,d |
| Liver enzymes | ||||
| ALT, UI/L | 14 ± 1 | 28 ± 5 | 48 ± 4 | <.001c,d |
| AST, UI/L | 20 ± 1 | 26 ± 2 | 34 ± 2 | .006c,d |
| Marker of apoptosis | ||||
| CK18, UI/L | 115.9 ± 4.4 | 133.5 ± 5.6 | 200.9 ± 18.1 | .004c,d |
Abbreviations: AA, African American; AST, aspartate aminotransferase; BF, body fat; C, Caucasian; F, female; FM, fat mass; GT, glucose tolerance; H, Hispanic; IGT, impaired glucose tolerance; LBM, lean body mass; M, male; NGT, normal glucose tolerance; T2D, type 2 diabetes; Values are expressed as mean ± SEM.
P adjusted: age, gender, ethnicity.
P < .05 lean vs obese with low hepatic fat content.
P < .05 lean vs obese with high hepatic fat content.
P < .05 obese with low hepatic fat content vs obese with high hepatic fat content.
A subset of subjects (118 adolescents: 7 lean, 65 obese HFF <5.5%, and 46 obese HFF ≥5.5%) underwent a 2-step hyperinsulinemic-euglycemic clamp with coinfusion of 6,2d-glucose a 5d-gycerol to further assess muscle, hepatic, and adipose insulin sensitivity, respectively, as previously reported (15). In 14 subjects, due to persistent elevations in alanine aminotransferase (ALT) levels, a liver biopsy was obtained that revealed steatohepatitis (NASH). The study was approved by the Yale University Human Investigation Committee. Written informed consent was obtained from the parents and assent from the children and adolescents.
Biochemical analysis
Samples were stored at −80°C until the time of the analysis of serum FGF-21, which was measured using ELISA (quantitative human FGF-21 ELISA; R&D Systems, Minneapolis, Minnesota). The intra-assay and interassay coefficients of variability were 2.8 and 7.0%, respectively. The assay has a minimum limit of detection of 4.6 pg/mL. No samples were below the lower limit of detection.
Plasma glucose was measured using the YSI 2700 STAT Analyzer (Yellow Springs Instruments, Yellow Springs, Ohio) and lipids were measured using an Autoanalyzer (model 747-200; Roche-Hitachi). Plasma insulin and C-peptide levels were measured using double antibody RIA from Millipore (Billerica, Massachusetts) and from Diagnostic Products Corp. (Los Angeles, California), respectively. The intra-assay variation was 4.5% for insulin and 5.9% for C-peptide, and the interassay variation was 10% for insulin and 11% for C-peptide. Liver enzymes were measured using standard automated kinetic enzymatic assays. CK18 was measured using immune-based (ELISA) assay as previously reported by Feldstein et al (14, 16, 17). Plasma-free fatty acids were assayed by a colorimetric method.
Imaging studies
Abdominal MRI and total body composition (dual-energy x-ray absorptiometry)
Multislice abdominal MRI studies were performed on a Siemens Sonata 1.5 T system (Siemens Medical Solutions, Malvern, Pennsylvania) (18). Total body composition was measured by dual-energy x-ray absorptiometry with a Hologic scanner (Boston, Massachusetts).
Fast-MRI: Liver fat content
Measurement of liver fat content was performed by MRI using the 2-point Dixon method as modified by Fishbein et al (19) and reported by Cali et al (20). The fast-MRI was validated against 1H-nuclear magnetic resonance as previously reported (21).
Liver histology
The histological diagnosis of NAFLD was established by study pathologists according to their expertise. The NAFLD activity score was determined for each patient as previously described (22). In detail, steatosis was assessed as the percentage of hepatocytes involved within a lobule (0%–100%, steatosis score) and by using a 4-grade classification modified from Kleiner et al (22): 0, absent; 1, <5%; 2, 5%–33%; 3, 33%–66%; 4, >66%. Staging and grading were performed according to Brunt et al (23). The NAFLD activity score (NAS) was calculated according to Kleiner et al (22). The score is defined as the sum of the scores for steatosis (0, <5%; 1, 5%–33%; 2, 33%–66%; 3, >66%), lobular inflammation (0, none; 1, <2 foci/×200 magnification field; 2, 2–4 foci/×200 magnification field; 3, >4 foci/×200 magnification field), and ballooning (0, none; 1, few; 2, many). Thus, this score ranges from 0 to 8. According to this classification, NASH is defined by a NAS of 5 or more.
Statistical analysis
The distribution of continuous variables was examined for skewness and kurtosis and parameters were logarithmically transformed, when appropriate. Data are expressed as means ± SD or SE. Differences in gender were analyzed by Fisher exact test, whereas dichotomous variables were analyzed by χ2 test.
Differences across the 3 groups (lean, obese with low hepatic fat at baseline, and obese with high hepatic fat at baseline) were analyzed by one-way ANOVA test and post-hoc pairwise comparisons were made, adjusting the level of significance for multiple comparisons (age, gender, ethnicity, and body mass index Z score [BMI-Z]) with the Bonferroni correction. To evaluate the relationship between FGF-21 concentrations and liver steatosis as well the main metabolic and indexes of hepatic apoptosis, a Pearson correlation was performed, adjusting for age, gender, ethnicity, and BMI-Z. In the subgroup of subjects who underwent a liver biopsy, a Pearson correlation was also performed to explore the relationship between FGF-21 levels and NAS score. To further characterize the relationship between FGF-21 concentrations and obesity, a linear regression was performed using FGF-21 as dependent variables, whereas BMI, visceral fat content, age, gender, and ethnicity were used as independent variables. Finally, to characterize the relationship between FGF-21 concentrations and both liver steatosis and apoptosis further, a linear regression was also performed using 2 models using HFF and CK18 values, respectively, as the dependent variables. In the models, FGF-21, BMI-Z, WBISI, visceral fat content, age, gender, and ethnicity were used as independent variables. All analyses were performed using SPSS 19.0 for Windows (SPSS, Inc, Chicago, Illinois) and statistical significance was assessed at the 2-tailed 0.05 threshold.
Results
Anthropometric and circulating FGF-21 levels: Effects of obesity and hepatic fat content (Table 1 and Figure 1)
Figure 1.
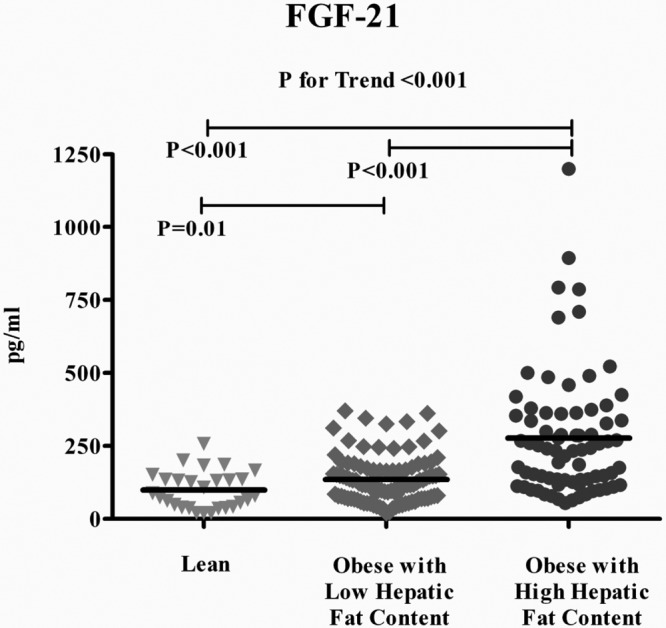
FGF-21 concentrations in lean and obese youth with low and high hepatic fat content.
As shown in Table 1, based on BMI, the cohort was stratified into a lean and obese group; the latter was than further divided into 2 groups of obese with normal and high hepatic content. Age and gender distributions across the 3 groups were similar. As expected, a significant and progressive increase in BMI, BMI-Z, body fat, lean body mass, and fat mass was found across lean, obese with normal, and obese with high hepatic fat content. The visceral, subcutaneous, deep, and superficial subcutaneous significantly and progressively increased across the 3 groups. By design, hepatic fat content was significantly increased across the 3 groups (Table 1). These differences in the hepatic fat content were paralleled by higher levels of liver enzymes and CK18 levels across the 3 groups (Table 1). Fasting and 2-hour glucose levels increased significantly across the 3 groups of adolescents, as did fasting insulin, and C-peptide (data not shown). However, no difference was found in terms of fasting glucose between lean and obese adolescent with low hepatic fat content. Insulin stimulated glucose disposal (“M” = mg/lean body mass/min) was significantly lower across the 3 groups (see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Fasting hepatic glucose production was significantly greater in those adolescents with fatty liver as well as percentage of FFA suppression and the hepatic and adipocyte resistance index (see Supplemental Table 1).
With the increase in both obesity and hepatic fat content, FGF-21 levels increased progressively in lean (99 ± 12 pg/mL), obese with low (135 ± 8 pg/mL), and obese with high hepatic fat content (277 ± 21 pg/mL, P < .001) (Figure 1).
Relationship between FGF-21 and obesity (Table 2)
Table 2.
Stepwise Linear Regression Analysis for Predicting FGF-21 Levels in the Entire Cohort
| Independent Variables | FGF-21 |
|||
|---|---|---|---|---|
| β | P | β | P | |
| Age, y | −.012 | .866 | −.067 | .290 |
| Gender | −.004 | .950 | −.031 | .630 |
| Ethnicity | .005 | .946 | .014 | .831 |
| BMI-Z | .351 | <.001 | ||
| Visceral fat content, cm2 | .497 | <.001 | ||
| R2 | .123 | .247 | ||
To characterize the relationship between FGF-21 concentrations and obesity further, a linear regression was performed using FGF-21 as dependent variables, whereas BMI, visceral fat content, age, gender, and ethnicity were used as independent variables. As shown in Table 2, BMI degree and visceral obesity are strongly related to FGF-21 levels.
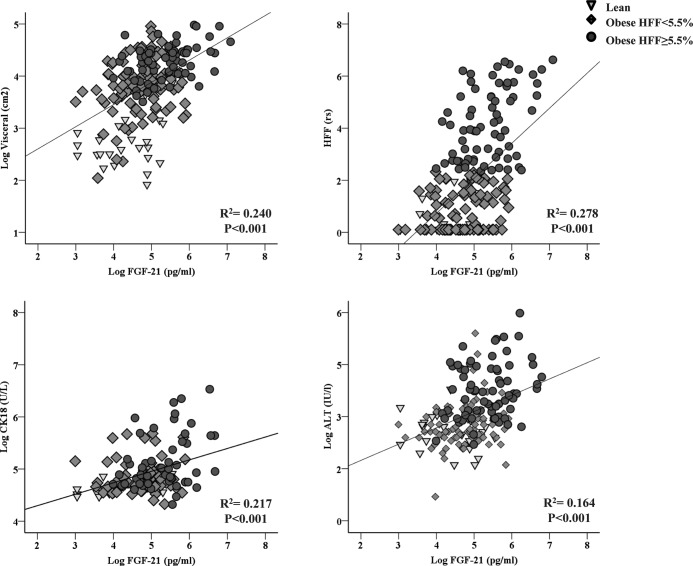
Relationships between FGF-21, hepatic fat content, and biomarkers of liver apoptosis and injury (Figure 2 and Table 3)
Figure 2.
Correlation between FGF-21 and visceral fat, HFF%, CK18, and ALT in the study cohort.
Table 3.
Stepwise Linear Regression Analysis for Predicting Hepatic Fat Content in the Entire Cohort
| Independent Variables | β | P | β | P | β | P | β | P | β | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Hepatic fat content | ||||||||||
| Age, y | −.016 | .819 | .034 | .606 | −.131 | .056 | −.091 | .150 | −.105 | .092 |
| Gender | −.027 | .681 | −.091 | .156 | −.034 | .567 | −.052 | .392 | −.085 | .143 |
| Ethnicity | .067 | .317 | .028 | .659 | .079 | .184 | .096 | .119 | .076 | .197 |
| BMI-Z | .409 | <.001 | .176 | .028 | −.070 | .450 | .236 | <.001 | −.100 | .254 |
| WBISI | −.381 | <.001 | −.200 | .004 | ||||||
| Visceral fat content, cm2 | .562 | <.001 | .294 | <.001 | ||||||
| FGF-21, pg/mL | .455 | <.001 | .312 | <.001 | ||||||
| R2 | .167 | .259 | .289 | .340 | .431 | |||||
| CK18 | ||||||||||
| Age, y | .004 | .965 | .058 | .522 | −.018 | .841 | −.052 | .533 | .005 | .959 |
| Gender | −.095 | .270 | −.098 | .296 | −.088 | .310 | −.077 | .362 | −.108 | .204 |
| Ethnicity | .161 | .065 | .126 | .152 | .149 | .085 | .177 | .034 | .079 | .374 |
| BMI-Z | .270 | .002 | .119 | .262 | .064 | .593 | .110 | .208 | .001 | .998 |
| WBISI | −.305 | <.001 | −.201 | .023 | ||||||
| Visceral fat content, cm2 | .303 | .001 | .010 | .928 | ||||||
| FGF-21, pg/mL | .411 | <.001 | .373 | <.001 | ||||||
| R2 | .073 | .093 | .092 | .210 | .215 | |||||
FGF-21 levels were directly correlated with hepatic fat content (r2 = 0.278, P < .001), marker of hepatic apoptosis CK18 (r2 = 0.217, P < .001), and ALT (r2 = 0.164, P < .001).
To investigate the association of FGF-21 further with more advanced stages of fatty liver disease such as steato-hepatitis, we analyzed the data obtained in a subgroup of subjects who underwent a liver biopsy. Circulating FGF-21 levels were significantly and positively associated with NAS score (r2 = 0.27, P = .04).
To investigate the relationship between FGF-21 and hepatic fat content and markers of hepatic apoptosis further, a multiple stepwise analysis was performed. As shown in Table 3, a significant association between the FGF-21 and both hepatic fat content, defined by HFF values, and hepatic cells apoptosis, as defined by CK18 concentrations, was found in the entire cohort, even after adjusting for relevant confounding factors, confirming a relevant contribution of the FGF-21 to hepatic alteration in obese youth.
Relationship between FGF-21 and whole-body, hepatic, and adipocyte insulin resistance (Figure 3)
Figure 3.
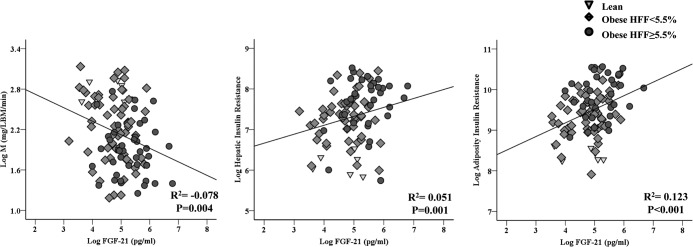
Correlation between FGF-21 and insulin sensitivity (glucose disposal, M), hepatic insulin resistance, and adipose insulin resistance. LBM, lean body mass.
The insulin-stimulated rate of glucose disposal (Rd) correlated inversely with plasma FGF-21 concentration (r2 = −0.078, P = .004). A positive correlation also was observed between FGF-21 level and hepatic insulin resistance index (r2 = 0.123, P < .001) and adipocyte insulin resistance index (r2 = 0.051, P = .001). These correlations however were no longer significant when adjusting for hepatic fat content in a multiple stepwise analysis, suggesting that there is no independent effect of FGF-21 on peripheral and hepatic insulin sensitivity but rather the weak relationships maybe totally mediated by the coexistence of fatty liver disease.
Discussion
In this study we showed that, compared to healthy nonobese peers, obese youth have significantly increased circulating plasma FGF-21 levels. In addition to obesity, however, those with increased liver fat content displayed a further elevation in FGF-21 levels. The novel finding is, however, the independent relationships between FGF-21 not only with liver fat content but also with a marker of hepatic apoptosis, CK18 levels. In contrast, weak relationships between FGF-21, peripheral, hepatic, and adipocyte insulin sensitivity were found across the groups, which disappeared after adjusting for relevant confounders such as hepatic fat content.
Previous studies in adults reported an association between FGF-21 levels and indirect measure of adiposity, such as BMI values (24–28). Such a similar association has been also recently reported in youth by Reinehr et al (11). In our study we confirm an association between both BMI and BMI-Z with circulating FGF-21. In addition, we also described a significant association between FGF-21 concentrations and direct measures adiposity and adipose tissue distribution, evaluated by using the total body composition (dual-energy x-ray absorptiometry) and abdominal MRI.
The FGF “endocrine” subgroup, and especially FGF-21, has also been shown to be key factors in glucose and lipid metabolism and energy regulation (3, 4). In a group of adult patients with newly diagnosed type 2 diabetes, Chen et al reported significantly higher plasma FGF-21 concentrations compared to nondiabetic control subjects, and that FGF-21 negatively correlated with fasting plasma glucose (29). In a later study, Zhang et al (16) documented that circulating levels of FGF-21 correlated positively with fasting insulin and negatively with high-density lipoprotein cholesterol (26). Conversely, in patients with anorexia nervosa, plasma FGF-21 concentrations are decreased, and increased following weight gain (30). In contrast with these results, a recent study by Reinehr et al failed to show a similar association between FGF-21 and homeostasis model of assessment-insulin resistance in children and adolescents. However, in that study only fasting indexes of insulin sensitivity were used (11). In the current study we used not only indirect but also more direct and robust measures of insulin sensitivity to assess any potential relationship with FGF-21 in lean and obese adolescents. Indirect relationships were found with peripheral insulin-stimulated glucose disposal and direct relationships with both hepatic and adipocyte insulin sensitivity. These relationships were weak, however, and not independent of hepatic fat content.
Because the liver is the major site for FGF-21 expression and hepatic steatosis is highly correlated with impairment of glucose and lipid metabolism in humans, the relationship between hepatic steatosis and FGF-21 has been investigated in several recent studies. Li et al (10) reported that serum FGF-21 levels were significantly higher in the NAFLD group compared with the controls and had a high positive correlation with intrahepatic triglyceride content (r = 0.662, P < .001). This study, along with recent reports by Dushay et al (31) and Yilmaz et al (32), contributed greatly to expand our knowledge on plasma FGF-21 levels in patients with NAFLD and indicates the role of FGF-21 in regulating hepatic lipid metabolism. Only one recent study by Reinehr et al also reported such an association between ultrasound-detected hepatic fat content and FGF-21 levels (11). Although the aforementioned studies suggest that FGF-21 could be a potential biomarker to screen or monitor NAFLD patients (33), the methods used to assess the severity of hepatic steatosis, such as B-mode ultrasound or pathological score system, were qualitative or semi-quantitative and did not reflect the quantitative association between serum FGF-21 and hepatic fat content accurately. Moreover, in the study by Li et al, liver biopsies were obtained from patients undergoing resection for benign liver disease and the number of patients with precise information of hepatic fat content was rather small, which might preclude a reliable conclusion (10). In a recent study in adults using 1H magnetic resonance spectroscopy to quantify hepatic fat content, Yan et al (34) showed that FGF-21 was strongly correlated with the hepatic fat content in people with mild or moderate hepatic steatosis and could better reflect hepatic fat content than any known serum parameters. In the present study, by using MRI and biopsy-derived measures of hepatic fat content and apoptosis, we documented a relevant correlation between FGF-21 and obesity-related liver alteration. In addition, by using multiple stepwise linear regression analysis and testing the variables of interest singularly in the model, we documented that FGF-21, BMI-Z, WBISI, and visceral fat were significantly associated with both hepatic fat content and CK18 levels, a index of hepatic apoptosis. More importantly, when all the variables were included in the model, FGF-21 levels were shown to be the best predictor. Finally, FGF-21 was directly and significantly correlated with the NAS score in the subgroup of youth who performed a liver biopsy.
The strength of the current study is a result of a relatively large sample size and the availability of both indirect and direct measures of adiposity, insulin sensitivity, and especially of liver hepatic fat content. Limitations are mainly related to the fact that we used a clinic-based sample of obese youths, which may not be a true representative of the general population. In addition, although our data suggest that FGFs could represent a possible marker of NAFLD in obese youth, due to the study design, the cause and effect relationships cannot be evaluated by the present cross-sectional clinical study. In addition, although we documented a correlation between FGF-21 values and NAS score, it should be acknowledged that in our study a persistent elevation in ALT level of the population was documented in 14 subjects and therefore a relatively small sample size of young subjects underwent liver biopsy. We acknowledge also that CK18 might not be specific for NASH because it can be elevated in a number of other conditions and thus may sometimes give spurious results (35). In fact, it has been shown that one possible limitation of the use of CK18 for the prediction of NASH is its intrinsic inability to discriminate between NASH and other chronic diseases that involve apoptosis, such as cholangitis and cholestasis, chronic hepatitis, cancer, and trauma (35).
Conclusions
Compared to healthy nonobese peers, circulating FGF-21 levels are increased in obese youth and are associated with fatty liver and markers of hepatic apoptosis. These associations are independent of overall obesity, visceral fat, and insulin sensitivity.
Acknowledgments
C.G. analyzed the data and wrote the manuscript. A.F., N.S., G.K., R.K., and B.P. researched data. S.C. wrote the manuscript and reviewed and edited the manuscript. S.C. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by the National Institutes of Health (NIH) Grants (R01-HD-40787, R01-HD-28016, and K24-HD-01464 to S.C.) and by the National Center for Research Resources, NIH (CTSA Grant UL1-RR-0249139), Yale Diabetes Endocrinology Research Center P30 DK045735, European Society of Pediatric Endocrinology (ESPE) Full Research Fellowship (to C.G.), NIH Grants DK076852 and DK082451 (to A.F.). N.S. is supported by the American Heart Association (13SDG14640038).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- ALT
- alanine aminotransferase
- BMI
- body mass index
- BMI-Z
- body mass index Z score
- CK18
- cytokeratin 18
- FGF
- fibroblast growth factor
- HFF%
- high hepatic fat content
- MRI
- magnetic resonance imaging
- NAFLD
- nonalcoholic fatty liver disease
- NAS
- nonalcoholic fatty liver disease activity
- NASH
- non-alcoholic steatohepatitis
- WBISI
- whole body insulin sensitivity index.
References
- 1. Yeoh JS, de Haan G. Fibroblast growth factors as regulators of stem cell self-renewal and aging. Mech Ageing Dev. 2007;128:17–24 [DOI] [PubMed] [Google Scholar]
- 2. Kharitonenkov A. FGFs and metabolism. Curr Opin Pharmacol. 2009;9:805–810 [DOI] [PubMed] [Google Scholar]
- 3. Kralisch S, Fasshauer M. Fibroblast growth factor 21: effects on carbohydrate and lipid metabolism in health and disease. Curr Opin Clin Nutr Metab Care. 2011;14:354–359 [DOI] [PubMed] [Google Scholar]
- 4. Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S–257S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schaap FG. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care. 2012;15:386–391 [DOI] [PubMed] [Google Scholar]
- 6. Ogawa Y, Kurosu H, Yamamoto M, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA. 2007;104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA. 2010;107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kharitonenkov A, Wroblewski VJ, Koester A, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781 [DOI] [PubMed] [Google Scholar]
- 10. Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940 [DOI] [PubMed] [Google Scholar]
- 11. Reinehr T, Woelfle J, Wunsch R, Roth CL. Fibroblast growth factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: a longitudinal analysis. J Clin Endocrinol Metab. 2012;97:2143–2150 [DOI] [PubMed] [Google Scholar]
- 12. Yeckel CW, Taksali SE, Dziura J, et al. The normal glucose tolerance continuum in obese youth: evidence for impairment in beta-cell function independent of insulin resistance. J Clin Endocrinol Metab. 2005;90:747–754 [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association Executive summary: standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S4–S10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamimi TI, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011;54:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fitzpatrick E, Mitry RR, Quaglia A, Hussain MJ, DeBruyne R, Dhawan A. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr. 2010;51:500–506 [DOI] [PubMed] [Google Scholar]
- 18. Burgert TS, Taksali SE, Dziura J, et al. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287–4294 [DOI] [PubMed] [Google Scholar]
- 19. Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15:287–293 [DOI] [PubMed] [Google Scholar]
- 20. Cali AM, De Oliveira AM, Kim H, et al. Glucose dysregulation and hepatic steatosis in obese adolescents: is there a link? Hepatology. 2009;49:1896–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H, Taksali SE, Dufour S, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008;59:521–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 23. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474 [DOI] [PubMed] [Google Scholar]
- 24. Tyynismaa H, Raivio T, Hakkarainen A, et al. Liver fat but not other adiposity measures influence circulating FGF21 levels in healthy young adult twins. J Clin Endocrinol Metab. 2011;96:E351–E355 [DOI] [PubMed] [Google Scholar]
- 25. Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf). 2009;71:369–375 [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57:1246–1253 [DOI] [PubMed] [Google Scholar]
- 27. Semba RD, Sun K, Egan JM, Crasto C, Carlson OD, Ferrucci L. Relationship of serum fibroblast growth factor 21 with abnormal glucose metabolism and insulin resistance: the Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2012;97:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eren F, Kurt R, Ermis F, Atug O, Imeryuz N, Yilmaz Y. Preliminary evidence of a reduced serum level of fibroblast growth factor 19 in patients with biopsy-proven nonalcoholic fatty liver disease. Clin Biochem. 2012;45:655–658 [DOI] [PubMed] [Google Scholar]
- 29. Chen WW, Li L, Yang GY, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:65–68 [DOI] [PubMed] [Google Scholar]
- 30. Dostálová I, Kaválkova P, Haluzíková D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab. 2008;93:3627–3632 [DOI] [PubMed] [Google Scholar]
- 31. Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;139:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yilmaz Y, Eren F, Yonal O, et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest. 2010;40:887–892 [DOI] [PubMed] [Google Scholar]
- 33. Morris-Stiff G, Feldstein AE. Fibroblast growth factor 21 as a biomarker for NAFLD: integrating pathobiology into clinical practice. J Hepatol. 2010;53:795–796 [DOI] [PubMed] [Google Scholar]
- 34. Yan H, Xia M, Chang X, et al. Circulating fibroblast growth factor 21 levels are closely associated with hepatic fat content: a cross-sectional study. PLoS One. 2011;6:e24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Younossi ZM, Page S, Rafiq N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg. 2011;21:431–439 [DOI] [PubMed] [Google Scholar]