Abstract
Context:
It is unknown whether intensive lifestyle modification (ILS) or metformin changes sex steroids among premenopausal women without a history of polycystic ovarian syndrome (PCOS).
Objectives:
We examined 1-year intervention impact on sex steroids (estradiol, testosterone, dehydroepiandrosterone, and androstenedione [A4]) and SHBG and differences by race/ethnicity.
Participants:
A subgroup of Diabetes Prevention Program participants who were premenopausal, not using estrogen, without a history of PCOS or irregular menses, and who reported non-Hispanic white (NHW), Hispanic, or African-American race/ethnicity (n = 301).
Interventions:
Randomization arms were 1) ILS with the goals of weight reduction of 7% of initial weight and 150 minutes per week of moderate intensity exercise, 2) metformin 850 mg twice a day, or 3) placebo.
Results:
Neither intervention changed sex steroids compared to placebo. ILS, but not metformin, increased median SHBG by 3.1 nmol/L (∼11%) compared to decreases of 1.1 nmol/L in the placebo arm (P < .05). This comparison remained significant after adjustment for changes in covariates including waist circumference. However, associations with glucose were not significant. Median baseline A4 was lower in Hispanics compared to NHWs (5.7 nmol/L vs 6.5 nmol/L, P < .05) and increases in A4 were greater in Hispanics compared to NHWs (3.0 nmol/ vs 1.2 nmol/L, P < .05), and these differences did not differ significantly by intervention arm. No other racial/ethnic differences were significant.
Conclusions:
Among premenopausal glucose-intolerant women, no intervention changed sex steroids. ILS increased SHBG, although associations with glucose were not significant. SHBG and sex steroids were similar by race/ethnicity, with the possible exception of lower baseline A4 levels in Hispanics compared to NHWs.
Among premenopausal women, endogenous sex steroid levels are associated with increased risk of common comorbidities, including breast cancer (1) and type 2 diabetes (2). Women who are overweight and insulin-resistant are at particularly high risk for these conditions (3, 4). Thus, it is important to examine whether endogenous steroid profiles are altered with lifestyle interventions and other treatments such as metformin that target insulin resistance and/or weight. To date, most randomized studies in premenopausal women focus on those with a diagnosis of polycystic ovarian syndrome (PCOS), and there are few studies among premenopausal women without this diagnosis. A recent meta-analysis of 6 randomized controlled trials in women with PCOS reported that lifestyle change reduced weight, waist circumference, and total testosterone (T), with only minimal effects on SHBG (5). In contrast, in one trial that enrolled eumenorrheic premenopausal women, lifestyle change led to increases in SHBG and decreases in estradiol (E2), and T was not reported (6). Women with PCOS tend to have elevated androgen levels and decreased SHBG levels compared to women without PCOS (7) and thus lifestyle modification may affect sex steroids and SHBG depending on PCOS diagnosis. Similarly, metformin reduces androgen levels among premenopausal women with PCOS (8–10) but metformin effects in premenopausal women without PCOS have not been reported.
Due to the associated increased risks in morbidity, it is also important to determine whether endogenous sex steroid profiles vary by race/ethnicity. However, only a few studies have reported racial/ethnic hormone variations in premenopausal women (11–13). In one study of 54 premenopausal women, non-Hispanic whites (NHW) had lower E2 than African-Americans (AA) across the menstrual cycle (11). In another cohort, (12) NHWs and AAs had similar E2 levels, and NHWs had higher T than Hispanics, but sex steroid levels varied most by body mass index (BMI). No studies have examined whether sex steroid and SHBG levels vary by race/ethnicity among women who are overweight and glucose-intolerant. Because adiposity decreases SHBG as well as affecting sex steroids (14), racial/ethnic differences may be minimal among overweight women. No studies have reported racial/ethnic variation in SHBG and sex steroid levels in response to lifestyle interventions.
The Diabetes Prevention Program (DPP) randomized racially and ethnically diverse overweight, nondiabetic glucose-intolerant participants to a program of intensive lifestyle modification (ILS), metformin, or placebo (4). Participants randomized to ILS and metformin had maximal weight loss and reductions in glucose at 1 year after randomization (4). We conducted a secondary analysis of premenopausal women participating in the DPP who were not using estrogen therapy and who did not have a history of PCOS or irregular menses. Specifically, we examined the association between randomization assignment and changes in SHBG and sex steroids including E2 and androgens (dehydroepiandrosterone [DHEA], androstenedione [A4], and T) between baseline and 1 year. We hypothesized that ILS or metformin would reduce sex steroid levels and increase SHBG, and further, that associations between interventions with sex steroids and SHBG would be attenuated after adjustment for other covariates, particularly changes in waist circumference. Finally, we hypothesized that racial/ethnic variations in SHBG and sex steroid levels would be minimal in our overweight, glucose-intolerant participants.
Materials and Methods
Characteristics of DPP participants have been reported (4). Briefly, the DPP inclusion criteria included age ≥25 years, fasting plasma glucose of 95–125 mg/dL, 2-hour plasma glucose of 140–200 mg/dL after a 75-glucose load, and BMI ≥24 kg/m2 (≥22 kg/m2 for Asian Americans). Written informed consent was obtained from all participants before screening, consistent with the guidelines of each participating center's institutional review board. Eligible participants were randomly assigned to 1 of 3 interventions: 850 mg metformin twice daily, placebo twice daily, or ILS. The goals of ILS were to achieve and maintain a weight reduction of at least 7% through consumption of a low-calorie, low-fat diet, plus moderate physical activity for at least 150 minutes per week (4). Weight was measured semiannually and waist circumference was measured annually. All participants had an annual oral glucose tolerance test and semiannual fasting plasma glucose test. At the time of randomization, all women completed a questionnaire about their menses, gynecological history including surgeries, and estrogen use (contraceptive and postmenopausal therapy). Medication use was reassessed every 6 months. For this ancillary study, women were eligible to participate if they had consented to participate in ancillary studies, were aged 25–47 years, they were premenopausal (no history of bilateral oophorectomy or hysterectomy and ongoing menses), they had no history of irregular menses, they had no use of exogenous estrogen at randomization, and they were located at a center that agreed to participate; 2 centers opted not to participate. Of the 1981 women in the DPP, 575 women met eligibility criteria, and 54% of eligible women (n = 311) chose to participate in this opportunistic sample. For this report, 10 additional women were excluded due to use of exogenous estrogen at the first follow-up year, for a total of 301 participants for this report.
Ancillary study participants underwent identical randomization and measurements as other DPP participants. Glucose and insulin were obtained and measured as previously reported (15). Briefly, women were instructed to consume their usual diet. An oral glucose tolerance test was performed between 7 am and 10:30 am after an overnight fast. Venous blood was sampled before and 2 hours after a 75 g oral glucose load (Trutol 75; Custom Laboratories, Baltimore, Maryland). Plasma glucose was measured fasting and at 2 hours; plasma insulin was measured fasting. Insulin sensitivity was assessed using inverse fasting insulin levels (1/fasting insulin) (15). Measurements were performed at a central Biochemistry Laboratory (University of Washington, Seattle, Washington). Plasma glucose was measured by the glucokinase method. Insulin measurements were performed by a RIA method using an anti–guinea pig antibody that measures total immunoreactive insulin. The assay is a 48-hour polyethylene glycol–accelerated method with coefficients of variation (CVs) of 4.5% for high-concentration quality control samples and 6.9% for low-concentration quality control samples. The CV for masked split duplicates in this assay was <8.5%. SHBG, FSH, LH, T, E2, and A4 were measured by the Endocrine Laboratories of the University of Tennessee Health Science Center in Memphis and DHEA was measured at St. Luke's Roosevelt Hospital Obesity/Nutrition Center of Columbia University. A4 was determined by RIA using reagents from Diagnostic Systems Laboratories (Webster, Texas). A solid-phase, 2-site sequential chemiluminescent immunometric assay on the DPC Immulite analyzer (Diagnostics Products Corporation, Los Angeles, California) was used for other hormones (16). The CVs of the assays were all less than 5%.
Statistical Analysis
Baseline characteristics were described using percentages for categorical variables and means (SD) for quantitative variables. For variables where the distribution was skewed, log-transformed values and median values were also examined. For unadjusted comparisons of continuous variables, we used t tests and Wilcoxon rank-sum tests for skewed distributions. For each sex steroid measure and for SHBG, we excluded outliers consistent with hyperandrogenism (for T, n = 33; for DHEA, n = 12; for A4, n = 2) or exogenous estrogen use (for SHBG, n = 5; for E2, n = 18) as well as women with FSH values ≥40 nmol/L at baseline or 1-year follow-up (n = 17). For each sex steroid measure and for SHBG, we used t tests or Wilcoxon rank-sum tests to compare levels of change by randomization arm. Change was calculated as year 1 hormone level minus baseline hormone level. To determine if effects were confounded by age, race/ethnicity, waist circumference, or insulin sensitivity (represented by 1/fasting insulin), we also constructed linear regression models that adjusted for each of these factors. To determine if the observed changes in sex steroids and SHBG between baseline and year 1 were associated with changes in either fasting glucose or postchallenge glucose between baseline and year 1, we created a series of linear regression models stratified by randomization arm in which change in glucose levels was the dependent variable. Models examined associations with changes in SHBG and additional models adjusted for age, race/ethnicity, waist circumference, and 1/fasting insulin. Similar models were constructed for individual sex steroids. We compared baseline SHBG and sex steroids and changes in these measures by race/ethnicity, before and after adjustment for age, waist circumference, and 1/fasting insulin. Models examining changes between baseline and year 1 also adjusted for randomization arm. Finally, a single model pooled all women and included randomization arm as a covariate. In a post-hoc power calculation, the analysis had 87% power at α = .005 to detect a difference between ILS vs placebo with respect to sex hormone changes. The SAS analysis system was used for all analyses (SAS Institute, Cary, North Carolina).
Results
Participant characteristics by randomization arm are shown in Table 1. At baseline, there were no significant differences by study arm except that women randomized to metformin had higher fasting insulin levels than women randomized to placebo. The mean age of the cohort was 41 years, and approximately one-half were NHW, a quarter were African-American, and a quarter were Hispanic. BMI, waist circumference, levels of fasting and postchallenge glucose, FSH, LH, SHBG, and sex steroid levels were similar across randomization arms.
Table 1.
Baseline Characteristics of Participants
| Overall | Lifestyle | Metformin | Placebo | |
|---|---|---|---|---|
| (n = 301) | (n = 105) | (n = 97) | (n = 99) | |
| Age, y | 40.6 (4.5) | 40.4 (4.8) | 40.8 (4.4) | 40.8 (4.3) |
| Race/ethnicity, % | ||||
| Non-Hispanic white | 48 | 50 | 44 | 48 |
| African-American | 26 | 27 | 28 | 22 |
| Hispanic | 27 | 24 | 28 | 29 |
| Weight, kg | 97.4 (21.1) | 97.0 (20.3) | 97.7 (21.0) | 97.5 (22.5) |
| Waist circumference, cm | 105.7 (14.6) | 106.0 (14.9) | 104.4 (14.1) | 106.8 (15.0) |
| BMI, kg/m2 | 36.5 (7.4) | 35.9 (6.8) | 36.3 (7.3) | 37.2 (8.0) |
| Fasting insulin, IU/L, median (IQR) | 29.0 (19.0) | 29.0 (19.0) | 30.0 (19.0) | 25.0 (18.0) |
| Fasting plasma glucose, mg/dL | 106.0 (8.3) | 106.9 (7.7) | 107.5 (8.0) | 107.4 (9.1) |
| 2-h glucose, mg/dL | 164.9 (16.9) | 163.6 (16.8) | 166.1 (16.9) | 164.9 (17.1) |
| FSH, IU/L, median (IQR) | 3.9 (3.2) | 3.7 (2.9) | 3.9 (3.4) | 4.1 (3.4) |
| LH, IU/L, median (IQR) | 2.9 (3.3) | 2.7 (3.4) | 3.1 (3.3) | 3.0 (3.5) |
| SHBG, nmol/L, median (IQR) | 29.1 (19.5) | 29.0 (18.7) | 28.3 (16.3) | 32.8 (21.4) |
| DHEA, nmol/L, median (IQR) | 21.5 (12.9) | 22.4 (13.9) | 21.2 (13.6) | 20.9 (11.8) |
| E2, pg/mL, median (IQR) | 39.2 (35.4) | 41.2 (36.1) | 35.4 (32.0) | 40.4 (39.3) |
| T, nmol/L, median (IQR) | 2.0 (1.5) | 2.0 (1.4) | 1.9 (1.4) | 2.1 (1.5) |
| A4, nmol/L, median (IQR) | 6.2 (3.7) | 6.4 (4.1) | 6.1 (3.7) | 6.1 (3.9) |
Means (SD) or percentages or medians (interquartile ranges or IQR) are shown. Bold type indicates significant difference (P < .05) between intervention and placebo.
Between baseline and year 1, women randomized to ILS, metformin, and placebo lost an average of 6.0 kg, 2.8 kg, and 1.2 kg, respectively (P < .01 for differences between interventions and placebo, P < .01 for lifestyle vs metformin) and had waist circumference reductions of 5.3 cm, 2.2 cm, and 1.7 cm, respectively (P < .01 for ILS vs placebo and P = .61 for metformin vs placebo, P < .01 for lifestyle vs metformin). Women randomized to ILS and metformin had reductions in FPG of 4.4 mg/dL and 6.9 mg/dL, respectively, while women randomized to placebo had increases in FPG of 3.3 mg/dL (P < .01 for differences between interventions and placebo, P = .13 for lifestyle vs metformin). Women randomized to ILS, metformin, and placebo had reductions in 2-hour glucose of 17.1 mg/dL, 10.8 mg/dL, and 3.9 mg/dL, respectively (P = .04 for ILS vs placebo and P = .25 for metformin vs placebo, P = .21 for lifestyle vs metformin).
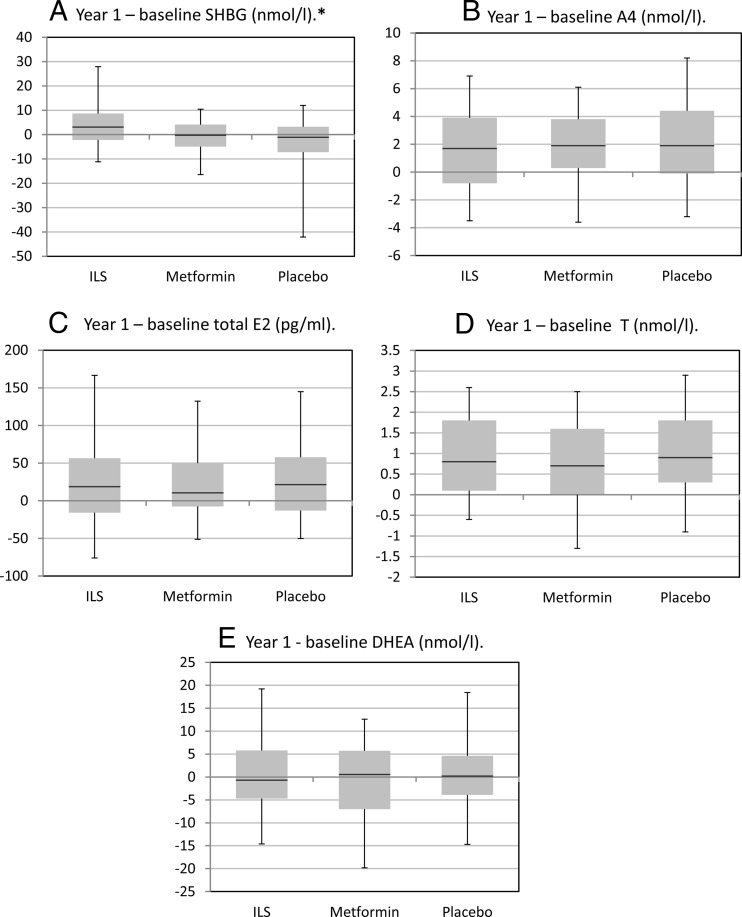
Changes in SHBG and sex steroid levels by randomization arm are shown in Figure 1. SHBG levels increased significantly among women randomized to ILS vs placebo, but not among women randomized to metformin vs placebo. Changes in sex steroid levels between baseline and year 1 did not differ by randomization arm for other sex hormones (Figure 1). Results from adjusted analyses were similar to those from unadjusted analyses. Specifically, the association between randomization to ILS vs placebo for increases in the change in log SHBG between baseline and year 1 persisted after adjustment for covariates including waist circumference and 1/fasting insulin as well as age and race/ethnicity (P < .01 in fully adjusted models), as did the lack of change in sex steroid levels by randomization arm (P > .05 in all unadjusted and fully adjusted models).
Figure 1.
Unadjusted changes in SHBG and sex steroid levels by randomization assignment. Box plots illustrate medians and interquartile ranges (25th percentile and 75th percentile) and whiskers represent 5th and 95th percentiles. *, Significant differences between ILS and placebo arms for SHBG. There were no significant differences between metformin and placebo arms regarding other sex hormones.
We examined associations between changes in SHBG and changes in FPG by randomization arm, and changes in SHBG and changes in 2-hour glucose by randomization arm. Before and after adjustment for covariates including waist circumference changes, changes in SHBG were not associated with changes in FPG. In unadjusted models, among women randomized to ILS, increases in SHBG were associated with declines in 2-hour glucose, but this association did not persist after inclusion of change in waist circumference (β-coefficient −0.31, P = .35). Results were similar in models that pooled all women and adjusted for randomization.
Participant characteristics are shown by race/ethnicity in Table 2. About a third of each racial/ethnic group was randomized to ILS, metformin, and placebo. Hispanics were younger and had lower baseline weights compared to NHWs. AAs had higher fasting insulin and glucose levels than NHWs. Both AAs and Hispanics had lower baseline FSH values than NHWs, although maximum FSH values were less than 13 nmol/L in all racial/ethnic groups. Hispanics had lower A4 levels than NHWs. Other baseline sex steroid levels and SHBG were similar across racial/ethnic groups. When we examined whether these racial/ethnic differences in baseline levels changed after adjustment for covariates, including age, waist circumference, and 1/fasting insulin, we observed no change in associations. Specifically, Hispanics still had lower baseline log A4 levels before and after adjustment for these covariates compared to NHWs (P < .01 for all comparisons).
Table 2.
Baseline Participant Characteristics by Race/Ethnicity
| Non-Hispanic White (n = 143) | African-American (n = 77) | Hispanic (n = 81) | |
|---|---|---|---|
| Randomization arm, % | |||
| ILS | 36 | 36 | 31 |
| Metformin | 30 | 35 | 33 |
| Placebo | 34 | 29 | 36 |
| Age, y | 41.4 (4.0) | 41.2 (4.5) | 38.8 (4.9) |
| Weight, kg | 94.0 (26.5) | 104.3 (22.6) | 88.6 (17.5) |
| Waist circumference, cm | 105.9 (14.7) | 109.3 (15.6) | 102.1 (12.8) |
| BMI, kg/m2 | 36.3 (7.4) | 37.9 (8.1) | 35.4 (6.6) |
| Fasting insulin levels, IU/L, median (IQR) | 31.0 (20.0) | 34.0 (23.0) | 25.0 (17.0) |
| Fasting plasma glucose, mg/dL | 106.5 (7.8) | 109.6 (9.4) | 106.2 (7.6) |
| 2-h glucose, mg/dL | 164.2 (16.0) | 167.1 (18.4) | 163.9 (17.1) |
| FSH, IU/L, median (IQR) | 4.4 (3.8) | 3.5 (3.3) | 3.5 (2.6) |
| LH, IU/L, median (IQR) | 2.8 (3.0) | 2.9 (3.4) | 3.0 (4.1) |
| SHBG, nmol/L, median (IQR) | 27.9 (20.0) | 30.0 (18.4) | 31.5 (20.4) |
| DHEA, nmol/L, median (IQR) | 22.3 (12.0) | 21.8 (11.7) | 20.6 (15.4) |
| E2, pg/mLmedian (IQR) | 40.5 (35.4) | 36.4 (34.7) | 39.4 (36.1) |
| T, nmol/L, median (IQR) | 1.9 (1.4) | 2.1 (1.2) | 1.9 (1.5) |
| A4, nmol/L, median (IQR) | 6.5 (4.2) | 6.1 (3.0) | 5.7 (4.5) |
Means (SD) or percentages shown unless otherwise indicated. Bold type indicates significant difference at P < .05 from non-Hispanic whites.
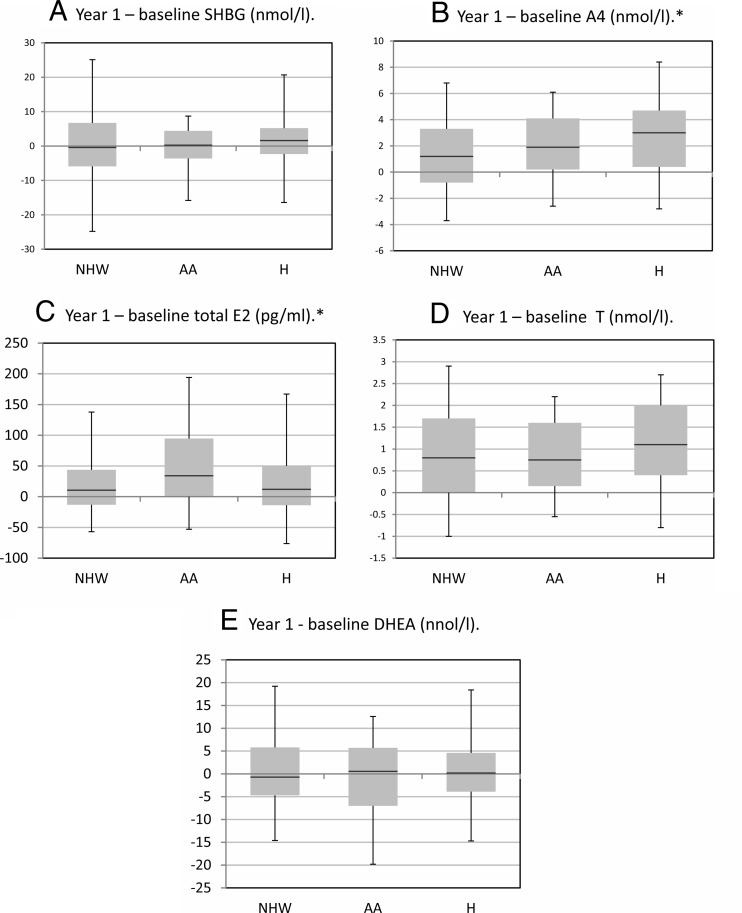
Changes in SHBG and sex steroids by race/ethnicity are shown in Figure 2. In unadjusted comparisons, Hispanics had larger increases in A4 levels than NHWs (P < .01). When we examined whether these racial/ethnic differences in baseline sex steroid levels changed after adjustment for covariates, including randomization assignment, age, waist circumference, and 1/fasting insulin, we observed that Hispanics still had larger increases in log A4 levels than NHWs (P < .01). The greater increases in A4 in Hispanics vs NHWs did not differ significantly by intervention arm, suggesting that interventions had minimal effect on A4 levels and intervention effects did not differ by race/ethnicity.
Figure 2.
Unadjusted changes in sex hormone binding globulin (SHBG) and sex steroid levels by race/ethnicity. AA, African-American; H, Hispanic; NHW, non-Hispanic white. Box plots illustrate medians and interquartile ranges (25th percentile and 75th percentile) and whiskers represent 5th and 95th percentiles. *, Significant differences for AA vs NHW regarding E2 changes (P < .05) and between Hispanics vs NHW vs androstenedione (A4) changes (P < .05).
Discussion
Among premenopausal glucose-intolerant overweight women, we found that randomization to lifestyle change increased SHBG compared to placebo. These changes were independent of changes in waist circumference as well as other variables, including age, race/ethnicity, and baseline insulin sensitivity. Lifestyle change did not alter sex steroid levels compared to placebo, and metformin did not alter SHBG and sex steroids compared to placebo. The increases in SHBG were not associated with improvements in glucose after adjustment for waist circumference, suggesting that in our overweight, dysglycemic sample, SHBG was not an independent mediator of lifestyle effects on glucose. At baseline, Hispanics had lower A4 levels than NHWs, even after consideration of the younger age and lower body mass of Hispanics compared to NHWs. Hispanics had greater increases in A4 than NHWs that did not differ significantly by randomization arm, and the magnitude of these differences was small.
Our report is unique in that we examined premenopausal women without PCOS, in whom trials of lifestyle modification that examine sex steroids are few. Unlike trials in women with PCOS (5), we did not find that lifestyle or metformin affected androgen profiles or other sex steroids such as E2. Because women with PCOS often have greater relative hyperandrogenism than the women enrolled in the DPP, women may experience greater absolute reductions in sex steroids in response to interventions. Moreover, among women with PCOS, interactions between adiposity, insulin sensitivity, and ovarian sex steroid production may differ from women without PCOS (7). It is also possible that the greater body mass in DPP women obscured changes in sex steroids, as observational studies have reported that the associations between sex steroids and adiposity are reduced among obese women (17). Our results are similar to previous trials in postmenopausal women (18–20), including the DPP (21), that reported that a lifestyle intervention increased SHBG levels independent of anthropometric changes. Theoretically, lifestyle change could preferentially decrease hepatic fat deposition to a greater extent than visceral or subcutaneous fat deposition and subsequently increase SHBG production through that mechanism (22). More specifically, reduction in overall caloric intake as well as increased metabolic fitness (both of which were components in the ILS intervention) could potentially lead to increased SHBG production (23). Reduced hepatic lipogenesis increases levels of hepatocyte nuclear factor-4α, a key transcriptional regulator of SHBG expression, and could also alter peroxisome proliferator-activated receptors, another potential transcriptional regulator of SHBG expression (24). In postmenopausal DPP women, we have previously reported that we found no associations between ILS or metformin and E2 and T, although both interventions were associated with significant weight loss (21).
Although SHBG was associated with glucose, this association was not independent of improvements in waist circumference. This is in contrast to what we have previously reported in postmenopausal women in the DPP (21) or in observational studies of postmenopausal women (25–28). The DPP has previously reported that ILS led to greater reductions in glucose among older and NHW participants (29). Premenopausal women in the DPP were younger and more often Hispanic than the overall cohort, and with the reduced effect of lifestyle change, our power to observe associations with SHBG may have been reduced. We may not have observed an effect of lifestyle change on SHBG and sex steroids and subsequently on glucose levels due to the small magnitude of SHBG change; in previous reports in the DPP (21) as well as other cohorts (2), differences in SHBG of approximately 10 nmol/L were associated with reductions in glucose and diabetes incidence. Additionally, waist circumference and SHBG are closely interrelated, and longitudinal analyses in perimenopausal women suggest that changes in waist circumference may precede changes in SHBG (30). Premenopausal women in the DPP had elevated visceral adiposity in proportion to body mass compared to other DPP subpopulations (31), and thus waist circumference may have been a particularly important confounder in our analysis. Finally, it is also possible that the effects of SHBG on glucose are relatively reduced among women with higher endogenous steroid production, as is the case for premenopausal women. Sex steroids inhibit the binding of SHBG to cell membrane receptors in vitro (32), and the higher endogenous sex steroid production in premenopausal women may reduce the availability of active SHBG. In summary, the impact of the small changes observed in SHBG may have been further reduced by the visceral adiposity and high endogenous sex steroid production in this subgroup of women.
We did not observe that interventions changed sex steroids. Our findings suggest that changes in sex steroids did not mediate the observed changes in glucose, in contrast to previous reports that have found cross-sectional correlations between androgens and hyperinsulinism (33), as well as hyperglycemia (34), particularly among AA women, suggesting that androgens contributed to racial/ethnic variation in diabetes (34). In the DPP, such associations may have been minimized by the fact that all DPP participants were already dysglycemic, combined with the fact that the previous smaller reports examined racial/ethnic differences in a single center.
In our overweight and glucose-intolerant cohort, we did not find that baseline sex steroid levels varied by race/ethnicity, with the exception of the lower A4 levels in Hispanics. We also did not find that 1-year changes in sex steroids varied by race/ethnicity, with the exception of the slightly greater A4 increases in Hispanics than NHWs. Greater adiposity and insulin resistance may have obscured racial/ethnic differences in sex steroids aside from A4, which have a probable bidirectional relationship with adiposity (17). In contrast, racial/ethnic variation in A4 remained after adjustment for age, race/ethnicity, waist circumference, and 1/fasting insulin. A4 production occurs in the adrenal glands as well as the gonads in premenopausal women and is a precursor of both T and E2, with elevations beyond those observed in this report consistent with congenital adrenal hyperplasia, adrenal tumors, or PCOS. To our knowledge, no studies have previously reported comparisons in NHWs and Hispanics in A4 except in pregnancy, where the mean levels of A4 (as well as T) were lower in NHWs compared to Hispanics (35), and among women with PCOS, where NHWs and Hispanics had similar levels (36). As A4 differences between race/ethnicities were small and values within normal range, the clinical implications of this finding are not known.
The strengths of our report include its randomized clinical trial design, with interventions that successfully caused statistically and clinically significant amounts of weight loss, measurement of a wide range of hormones, and a racially and ethnically diverse cohort. It is possible that residual confounding from adiposity or insulin sensitivity occurred, because the proxy measures used were not direct measures. Although we used RIA, which may be less sensitive than mass spectrometry at lower levels of sex steroids, sex steroid levels are higher in premenopausal women than postmenopausal women. The blood draws were not timed to the menstrual cycle. Therefore it is possible that the intracycle variation in sex steroids was greater than the differences between women by randomization arm. T levels are believed to peak around ovulation, with similar levels before and after ovulation; however, day-to-day variation in T is greater and independent of menstrual cycle, such that peaks are usually not discernible within women (37). Therefore, the lack of association between interventions and T likely reflected the lack of impact of interventions on day to day or average T, rather than bias to the null due to menstrual cycle variation. Correlation coefficients between the average value across the menstrual cycle with any individual day have been reported to be ≥0.85 for A4; 0.92 for DHEA; 0.75 for T, and 0.91 for SHBG (38), also suggesting that the lack of association was not due to variations in hormones within the menstrual cycle. As would be expected, variation in E2 was the highest within the menstrual cycle, although correlation coefficients were still ≥0.53 (38). Thus, particularly with E2, our results may have been biased to the null. Finally, this study was a secondary analysis of a randomized trial not designed a priori to assess the impact of lifestyle or metformin on sex steroid and SHBG concentrations nor racial/ethnic variation in sex hormones.
We conclude that lifestyle changes have favorable effects on SHBG in premenopausal overweight and glucose-intolerant women, even as interventions have minimal effects on sex steroids. However, in our premenopausal sample, the resulting SHBG changes had minimal impact on fasting and postchallenge glucose levels, suggesting that this mechanism may be less important for dysglycemia in women with significant endogenous sex steroid production. In addition, racial/ethnic variation in sex steroids and SHBG was minimal in the population studied, with the exception of A4. This suggests that modifiable factors such as body shape and size as well as insulin sensitivity may have greater impact on sex hormone profile than other factors specific to race/ethnicity. Replication of NHW and Hispanic differences in A4 and study of additional mechanisms are needed.
Acknowledgments
The investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The opinions expressed are those of the investigators and do not reflect the views of the Indian Health Service or other funding agencies. A complete list of DPP centers, investigators, and staff can be found in the Appendix. We are grateful to the principal investigators and program coordinators and to the DPP volunteers who agreed to participate in this ancillary study. We are particularly indebted to the program coordinators who assisted in the data collection and coordination of this study. The following 20 DPP centers were involved in this study: Pennington Biomedical Research Center/Louisiana State University, The University of Miami, The University of Texas Health Science Center (San Antonio), The University of Washington and Veteran's Affairs Puget Sound Health Care System, University of Tennessee Health Science Center (Memphis), Northwestern University Medical School, Massachusetts General Hospital, The University of California (Los Angeles), St Luke's-Roosevelt Hospital, Indiana University, Medstar Research Institute, Washington University (St. Louis), The University of New Mexico (Albuquerque), Albert Einstein College of Medicine, The University of Chicago, Jefferson Medical College, The University of California (San Diego), The University of Pittsburgh, Joslin Diabetes Center, and The University of Colorado.
The project described was supported by Award Numbers U01DK048489, R01DK083297, R01DK53061, GCRC RR00211, and K23DK071552 from The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The NIDDK provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; collection, management, analysis, and interpretation of the DPP. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding for data collection and participant support were also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the Office of Research on Women's Health, the Office of Research on Minority Health, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc, Health O Meter, Hoechst Marion Roussel, Inc, Merck-Medco Managed Care, Inc, Merck and Co, Nike Sports Marketing, Slim Fast Foods Co, and Quaker Oats Co donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp, Matthews Media Group, Inc, and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies.
Disclosure Summary: The authors declare no conflict of interest.
Footnotes
- A4
- androstenedione
- AA
- African-American
- BMI
- body mass index
- CVs
- coefficients of variation
- DHEA
- dehydroepiandrosterone
- DPP
- Diabetes Prevention Program
- E2
- estradiol
- ILS
- intensive lifestyle modification
- NHW
- non-Hispanic white
- PCOS
- polycystic ovarian syndrome
- T
- testosterone.
References
- 1. Eliassen AH, Missmer SA, Tworoger SS, et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415 [DOI] [PubMed] [Google Scholar]
- 2. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299 [DOI] [PubMed] [Google Scholar]
- 3. Endogenous Hormones and Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, et al. Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. Br J Cancer. 2011;105:709–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knowler WC, Barrett-Connor E, Fowler SE, et al. , and the Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moran L, Hutchinson S, Norman R, Teede H. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;CD007506. [DOI] [PubMed] [Google Scholar]
- 6. Williams NI, Reed JL, Leidy HJ, Legro RS, De Souza MJ. Estrogen and progesterone exposure is reduced in response to energy deficiency in women aged 25–40 years. Hum Reprod. 2010;25:2328–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehrmann D. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 8. Elkind-Hirsch K, Marrioneaux O, Bhushan M, Vernor D, Bhushan R. Comparison of single and combined treatment with exenatide and metformin on menstrual cyclicity in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:2670–2678 [DOI] [PubMed] [Google Scholar]
- 9. Gambineri A, Patton L, Vaccina A, et al. Treatment with flutamide, metformin, and their combination added to a hypocaloric diet in overweight-obese women with polycystic ovary syndrome: a randomized, 12-month, placebo-controlled study. J Clin Endocrinol Metab. 2006;91:3970–3980 [DOI] [PubMed] [Google Scholar]
- 10. Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767–2774 [DOI] [PubMed] [Google Scholar]
- 11. Marsh EE, Shaw ND, Klingman KM, et al. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 2011;96:3199–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522 [DOI] [PubMed] [Google Scholar]
- 13. Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561 [DOI] [PubMed] [Google Scholar]
- 14. Wildman R, Wang D, Fernandez I, et al. Associations of testosterone and sex hormone binding globulin with adipose tissue hormones in midlife women. Obesity (Silver Spring). In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitabchi AE, Temprosa M, Knowler WC, et al. , The Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086 [DOI] [PubMed] [Google Scholar]
- 17. Liedtke S, Schmidt M, Vrieling A, et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity (Silver Spring). 2012;20:1088–1095 [DOI] [PubMed] [Google Scholar]
- 18. Friedenreich CM, Woolcott CG, McTiernan A, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McTiernan A, Tworoger S, Ulrich C, et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Canc Res. 2004;64:2923–2928 [DOI] [PubMed] [Google Scholar]
- 20. Berrino F, Bellati C, Secreto G, et al. Reducing bioavailable sex hormones through a comprehensive change in diet: the diet and androgens (DIANA) randomized trial. Cancer Epidemiol Biomarkers Prev. 2001;10:25–33 [PubMed] [Google Scholar]
- 21. Kim C, Nan B, Laughlin GA, et al. Endogenous sex hormone changes in postmenopausal women in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2012;97:2853–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peter A, Kantartzis K, Machann J, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumagai S, Shono N, Kondo Y, Nishizumi M. The effect of endurance training on the relationships between sex hormone binding globulin, high density lipoprotein cholesterol, apoprotein A1c and physical fitness in pre-menopausal women with mild obesity. Int J Obes Relat Metab Disord. 1994;18:249–254 [PubMed] [Google Scholar]
- 24. Selva DM, Hammond GL. Peroxisome-proliferator receptor γ represses hepatic sex hormone binding globulin expression. Endocrinology. 2009;150:2183–2189 [DOI] [PubMed] [Google Scholar]
- 25. Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25:55–60 [DOI] [PubMed] [Google Scholar]
- 26. Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torrens J, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women's Health Across the Nation. Menopause. 2009;16:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Delahanty L, Peyrot M, Shrader P, Williamson D, Meigs J, Nathan D. Pretreatment, psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP). Diabetes Care. 2013;36:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wildman R, Tepper P, Crawford S, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2012;97:E1695–E704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ratner RE, Christophi CA, Metzger B, et al. , Diabetes Prevention Program Research Group Prevention of diabetes in women with a history of gestational diabetes:; effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hryjb DJ, Khan MS, Romas NA, Rosner W. The control of the interaction of sex hormone-binding globulin with its receptor by steroid hormones. J Biol Chem. 1990;265:6048–6054 [PubMed] [Google Scholar]
- 33. Burghen GA, Givens JR, Kitabchi AE. Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J Clin Endocrinol Metab. 1980;50:113–116 [DOI] [PubMed] [Google Scholar]
- 34. Kitabchi AE, Imseis RE, Bush AJ, Williams-Cleaves B, Pourmotabbed G. Racial differences in the correlation between gonadal androgens and serum insulin levels. Diabetes Care. 1999;22:1524–1529 [DOI] [PubMed] [Google Scholar]
- 35. Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE. Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2005;14:2147–2153 [DOI] [PubMed] [Google Scholar]
- 36. Welt CK, Arason G, Gudmundsson JA, et al. Defining constant versus variable phenotypic features of women with polcystic ovary syndrome using different ethnic groups and populations. J Clin Endocrinol Metab. 2006;91:4361–4368 [DOI] [PubMed] [Google Scholar]
- 37. Bui HN, Sluss PM, Blincko S, Knol DL, Blankenstein MA, Heijboer AC. Dynamics of serum testosterone during the menstrual cycle evaluated by daily measurements with an ID-LC-MS/MS method and a 2nd generation automated immunoassay. Steroids. 2013;78:96–101 [DOI] [PubMed] [Google Scholar]
- 38. Ahmad N, Pollard T, Unwin N. The optimal timing of blood collection during menstrual cycle for the assessment of endogenous sex hormones: can interindividual differences in levels over the whole cycle be assessed on a single day. Cancer Epidemiol Biomarkers Prev. 2002;11:147–151 [PubMed] [Google Scholar]