Abstract
Context:
Individual variation in the ability to convert excess calories to heat and the effects of dietary macronutrient composition are unclear.
Objective:
Stability and determinants of the energy expenditure (EE) response to overconsumption were assessed.
Design, Setting, and Participants:
Twenty subjects (75% male) with normal glucose regulation were evaluated during 24 hours each of energy balance, fasting, and 5 different diets with 200% energy requirements in a clinical research unit.
Interventions:
Five 1-day overfeeding diets were given in random order: high carbohydrate (75%) and low protein (3%); high carbohydrate and normal protein (20%); high fat (46%) and low protein; high fat (60%) and normal protein; and balanced (50% carbohydrates, 20% protein).
Main Outcome Measures:
The 24-hour EE, sleeping EE, and thermic effect of food (TEF) during each diet were measured with a metabolic chamber. Appetitive hormones were measured before and after the diets.
Results:
The EE response to overfeeding exhibited good intraindividual reproducibility. Similar increases above eucaloric feeding in 24-hour EE (mean 10.7 ± 5.7%, P < .001; range 2.9–18.8%) and sleeping EE (14.4 ± 11.3%, P < .001; range 1.0–45.1%) occurred when overfeeding diets containing 20% protein, despite differences in fat and carbohydrate content, but the EE response during overfeeding diets containing 3% protein was attenuated. The percent body fat negatively correlated with TEF during normal protein overfeeding (r = −0.53, P < .01). Fasting peptide YY negatively correlated with TEF (r = −0.56, P < .01) and the increase in sleeping EE (r = −0.54, P < .01) during overfeeding.
Conclusions:
There is an intrinsic EE response to overfeeding that negatively associates with adiposity, although it represents a small percentage of consumed calories.
In humans, the homeostatic regulation of energy balance is easily overwhelmed by external stimuli such that acute episodes of overeating often occur. For example, in a study in which people were given free access to food, the average daily intake was more than 150% of energy requirements (1). Some individuals seem better able to resist weight change with overfeeding (2–4), possibly due to interindividual variation in the energy costs of weight gain (5, 6). Although humans use only an average of 10% of calories consumed for metabolizing and storing food (7, 8), even small differences between individuals' energy expenditure (EE) response to acute episodes of excess intake may contribute to weight differences.
Food ingestion leads to increases in EE from the digestion, absorption, metabolism, and storage of nutrients as well as a theoretical facultative response that may serve as an energy dissipative mechanism. It is not clear whether the EE response to overconsumption is due primarily to intrinsic factors or the macronutrient composition of the food. Although protein has a higher requisite cost for metabolism than carbohydrates or fat (9, 10), older studies have reported that very low (3%) protein diets may magnify the metabolic response to overfeeding (5, 11). A more recent study of long-term overfeeding, however, has shown that fat gain is similar with low and normal protein diets (6). Although the impact of varying carbohydrate and fat content in calorie restricted diets has been investigated (12), differing consequences of low-carbohydrate or low-fat diets when overeating is not as well studied, and most studies addressing these questions in humans have looked at single meals or alterations of only 1 macronutrient (2, 5, 13–17). The goal of this study was to determine the extent and individual reproducibility of the EE response during 24 hours of overfeeding. We hypothesized that at least some individuals would demonstrate evidence of a facultative 24-hour EE response to overfeeding, that variations in macronutrients might magnify this effect, and that the 24-hour EE response would be associated with appetitive hormones and body composition measures.
Subjects and Methods
Study population
Twenty healthy individuals between the ages of 18 and 51 years were recruited from the Phoenix (Arizona) area between 2008 and 2010, admitted to the clinical research unit, and asked to avoid rigorous activity for the duration of the study. None of the subjects were taking medication or had evidence for comorbidities, including hypertension or metabolic syndrome, on history, physical examination, or laboratory analysis. All women were premenopausal. After 3 days of a weight-maintaining diet, normal glucose tolerance was confirmed by an oral glucose tolerance test (OGTT) (18). Plasma glucose concentrations during the OGTT were determined using an enzymatic oxygen-rate method (Beckman Glucose Analyzer 2; Beckman Instruments, Brea, CA), and concurrent plasma insulin concentrations were measured using an automated immunoenzymometric assay (Tosoh Bioscience Inc, Tessenderlo, Belgium). The weight-maintaining diet consisted of 50% carbohydrates, 30% fat, and 20% protein. This diet was also given on the days between overfeeding diets. Body composition was determined using dual-energy x-ray absorptiometry (DPX-L; Lunar Radiation, Madison, Wisconsin).
All subjects provided written informed consent prior to beginning the study. The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases.
EE measurements
Twenty-four-hour energy expenditure (24h-EE) was measured using whole-room indirect calorimetry. Subjects were asked to remain sedentary while in the respiratory chamber. Chamber temperature averaged 21.9 ± 0.6°C. Air samples from the chamber were analyzed during the last 8 seconds of every minute and compared with reference air that was simultaneously flowing into the chamber. To minimize analyzer noise, each calculated concentration of CO2 and O2 was based on a curve fitted to the preceding 30 minutes of measurements (19). The average CO2 production and O2 consumption per minute during the 23.25 hours in the respiratory chamber were extrapolated to 24 hours. These values were used to calculate the respiratory quotient and the 24h-EE as previously published (20). Quality control assessments done monthly demonstrated an average recovery of predicted O2 consumption and CO2 production of 100.7% [coefficient of variation (CV) 3.9%] and 99.4% (CV 3.6%), respectively. Radar sensors were used to continuously detect subject movement, expressed as a percentage of time in motion. Sleeping EE was calculated as the mean EE between 11:30 and 5:00 am when subject movement was less than 1.5% (<0.9 sec/min). Sleeping EE was extrapolated to a 24-hour period as previously described (20).
Meals were provided at 7:00 am, 11:00 am, 4:00 pm, and 7:00 pm. Subjects entered the chamber (time 0) approximately 1 hour after eating breakfast and exited 23.25 hours later. The diet-induced thermogenesis (DIT) was calculated by subtracting the 24h-EE during fasting from the 24h-EE during the relevant dietary intervention. The thermic effect of food (TEF%), defined as the percentage of caloric intake used to metabolize the consumed nutrients, was calculated by dividing DIT by the corresponding caloric intake.
The 24h-EE of subjects during eucaloric conditions was measured twice. Energy intake during the first eucaloric measurement was based on unit specific calculations as previously described (20). Intake during the second eucaloric measurement was equal to the 24h-EE calculated from the first assessment. The 24h-EE results from the second eucaloric assessment were considered the baseline measurements. These baseline 24h-EE results were doubled to determine the kilocalories for the overfeeding diets. Assessment of 24h-EE during 24 hours of fasting and during 5 overfeeding diets (each given for 1 day) were done in random order with a 3-day washout period between dietary interventions. The average CV of the subjects' body weight prior to the 24h-EE assessments was 1.2%. The 5 overfeeding diets varied in macronutrient composition were as follows: 1) balanced overfeeding with 50% carbohydrate, 30% fat, and 20% protein; 2) low-protein overfeeding with 51% carbohydrate, 46% fat, and 3% protein; 3) high-fat, normal-protein overfeeding with 20% carbohydrate, 60% fat, and 20% protein; 4) high-carbohydrate, normal-protein overfeeding with 75% carbohydrate, 5% fat, and 20% protein; and 5) high-carbohydrate, low-protein overfeeding with 75% carbohydrate, 22% fat, and 3% protein. The macronutrient composition was determined using The Food Processor software (ESHA Research, Salem, Oregon). Further details of the diets can be found in Table 1. Volunteers were asked to consume all food provided, but any uneaten food was returned to the metabolic kitchen for weighing such that actual intake by macronutrient could be calculated. One measurement associated with a high-fat, normal-protein diet was excluded as less than 95% of food was consumed. Energy balance was calculated by subtracting 24h-EE from caloric intake.
Table 1.
Details of the Dietary Interventions Based on Representative Diets for an Individual Requiring 2000 kcal for Energy Balance and 4000 kcal for Overfeeding
| Diet | Total Weight, g | Energy Density, kcal/g | Total Food Served Warm, % | Protein Provided as Animal Protein, % | Breakfast, kcal | Lunch, kcal | Dinner, kcal | Snack, kcal |
|---|---|---|---|---|---|---|---|---|
| EU | 1330 | 1.51 | 51.5 | 70.7 | 390 | 604 | 604 | 402 |
| BOF | 2811 | 1.42 | 49.6 | 73.5 | 990 | 1098 | 1090 | 822 |
| CNP | 4658 | 0.86 | 31.2 | 70.5 | 1093 | 882 | 1205 | 820 |
| FNP | 2174 | 1.84 | 21.9 | 90.9 | 826 | 870 | 1394 | 910 |
| CLP | 3515 | 1.14 | 20.0 | 25.0 | 992 | 1204 | 1081 | 723 |
| LPF | 2485 | 1.61 | 13.5 | 33.4 | 661 | 813 | 1454 | 1072 |
Abbreviations: BOF, balanced overfeeding with 50% carbohydrates, 30% fat, and 20% protein; CLP, high-carbohydrate, low-protein overfeeding with 75% carbohydrates, 22% fat, and 3% protein; CNP, high-carbohydrate, normal-protein overfeeding with 75% carbohydrates, 5% fat, and 20% protein; EU, eucaloric feeding; FNP, high-fat, normal-protein overfeeding with 20% carbohydrates, 60% fat, and 20% protein; LPF, low-protein overfeeding with 51% carbohydrates, 46% fat, and 3% protein.
Reproducibility substudy
Fourteen subjects had repeat assessment of 24h-EE during both a second overfeeding session with the balanced overfeeding diet and during a second session of fasting for assessment of the consistency of the EE measures. This sample size was chosen to detect a minimum intraclass correlation coefficient (ICC) of 0.6, the lower limit considered to reflect reliability (21). These repeat measurements were also done in random order and with a 3-day period in between assessments. The consistency of 24h-EE, TEF%, and other EE variables with overfeeding and fasting were assessed by computing the coefficients of variation and ICC.
Hormonal measurements
Both before and after each dietary intervention, fasting blood was collected for measurement of total T4 and T3, catecholamines, leptin, active glucagon-like peptide 1, total peptide YY (PYY), and pancreatic peptide.
Thyroid function tests were performed by the Clinical Core Laboratory of the National Institute of Diabetes and Digestive and Kidney Diseases by an ELISA (Calbiotech, Spring Valley, California). Leptin, glucagon-like peptide 1, PYY, and pancreatic peptide were measured by the Clinical Core Laboratory using luminex technology (Millipore, Billerica, Massachusetts). The serum catecholamines were measured by HPLC with electrochemical detection. The concentrations of catecholamines were determined after extraction from plasma using alumina adsorption as described previously (22).
Statistics
Further details of the statistical methods are provided in the Supplemental Material, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. In general, differences between EE measures were evaluated with mixed models to account for repeated measures and included the variables age, race, sex, percentage body fat, and diet. Differences were compared with the baseline eucaloric measures except for percentage changes from baseline values in which all possible comparisons were done. Confirmatory analyses were done after subtracting EE related to spontaneous physical activity from 24h-EE (23), and results were unchanged so only findings using unaltered 24h-EE are reported. To understand the contribution of the dietary interventions vs the individual to TEF%, a linear regression model including all feeding assessments was created with TEF% as the dependent variable and individual and diet as the 2 explanatory factors. Analyses to determine contributors to TEF%, and the percent increase in 24h-EE during overfeeding with the 3 20% protein diets were also done with mixed models. To better understand the association of adiposity with TEF%, in some analyses a categorical value of obesity based on the World Health Organization definition of obesity was substituted for percentage body fat (24). For all hormonal measures, both the baseline fasting concentrations drawn prior to each dietary intervention and the change in concentrations from baseline to the morning after the diet were evaluated as possible contributors to the TEF%, the percentage change in 24h-EE and sleeping EE from baseline using mixed models. All statistical analyses were done using SAS 9.2 and SAS Enterprise Guide 4.2 (SAS Institute, Cary, North Carolina). A P < .01 was considered statistically significant.
Results
Subject characteristics
Thirty-three subjects were evaluated for eligibility for the study; 20 completed the study (see Supplemental Figure 1). Characteristics of these 20 subjects (15 males, 5 females), all with normal glucose regulation, are shown in Table 2. Three self-identified themselves as African American, 2 as Hispanic, 7 as Caucasian, and 8 as Native American. Twelve subjects (60%) were classified as obese. Average caloric content of the overfeeding diets was 4488 ± 110 kcal/d. All diets were well tolerated.
Table 2.
Characteristics of the 20 Subjects Including Eucaloric EE Measurements
| Variable | All (n = 20) | Males (n = 15) | Females (n = 5) |
|---|---|---|---|
| Race | 3 AA, 7C, 2 H, 8 NA | 2 AA, 5 C, 2 H, 6 NA | 1 AA, 2 C, 2 NA |
| Age, y | 36.7 ± 8.9 (18.3, 50.7) | 38.6 ± 7.8 (25.5, 50.7) | 30.7 ± 10.3 (18.3, 44.8) |
| Height, cm | 173.7 ± 9.8 (154.5, 196.4) | 177.8 ± 7.3 (169.4, 196.4) | 161.5 ± 4.9 (154.5, 166)a |
| Weight, kg | 83.8 ± 11.7 (56.4, 107.8) | 84.1 ± 8.8 (73.9, 104.8) | 82.9 ± 19.6 (56.4, 107.8) |
| BMI, kg/m2 | 27.9 ± 4.4 (20.7, 39.1) | 26.6 ± 1.9 (23.7, 30.3) | 31.8 ± 7.4 (20.7, 39.1) |
| Percent Body Fat, % | 30.5 ± 11.2 (6.9, 52.8) | 26.3 ± 7.3 (6.9, 36.4) | 43.2 ± 12.1 (24.2, 52.8)a |
| Fasting glucose, mg/dL | 95 ± 6 (80, 99) | 95 ± 6 (80, 99) | 97 ± 5 (90, 99) |
| 2 h glucose, mg/dL | 101 ± 21 (65, 138) | 100 ± 25 (65, 138) | 112 ± 21 (80, 133) |
| Fasting insulin, μIU/mL | 7.3 ± 6.0 (2.0, 29.7) | 5.6 ± 2.2 (2.0, 10.0) | 13.8 ± 11.0 (4.5, 29.7)a |
| 2-Hour insulin, μIU/mL | 49.4 ± 64.7 (4.0, 292.8) | 32.7 ± 27.8 (4.0, 88.0) | 111.7 ± 122.2 (25.0, 292.8)a |
| TSH, mIU/L | 0.42 ± 0.22 (0.30, 1.15) | 0.42 ± 0.23 (0.30, 1.15) | 0.54 ± 0.17 (0.43, 0.79) |
| Total T4, μg/dL | 7.5 ± 1.2 (6.3, 10.7) | 7.5 ± 1.2 (6.3, 10.7) | 7.7 ± 1.6 (6.5, 10.0) |
| Energy balance, kcal/d | 7 ± 95 (-180, 169) | 12 ± 76 (-117, 159) | −6 ± 149 (-180, 169) |
| 24-Hour EE, kcal/d | 2266 ± 239 (1848, 2810) | 2342 ± 201 (2094, 2810) | 2038 ± 212 (1848, 2316)a |
| TEF% | 9.7 ± 6.0 (1.3, 20.9) | 9.7 ± 4.8 (2.5, 20.6) | 11.1 ± 7.2 (1.3, 20.9) |
Abbreviations: AA, African American, C, Caucasian, H, Hispanic, NA, Native American. Data are presented as mean ± SD, with minimum and maximum values in parentheses.
P < .01 for the difference between males and females as assessed by Student's t test (insulin levels were logged prior to statistical analyses).
Reproducibility of the energy response to fasting and overfeeding
Fourteen subjects had repeat assessments during fasting and balanced overfeeding to assess reproducibility of the EE measurements. The ICC for 24h-EE during overfeeding was 0.88 with a CV of 3.2%, and during fasting was 0.82 with a CV of 3.9%. The ICCs and CVs for all variables during the repeat assessments are presented in Supplemental Table 1. The ICC for the TEF% during the 2 identical overfeeding diets was 0.65 (n = 14). The ICC for the TEF% during all 3 overfeeding diets with 20% protein, including the balanced, high-carbohydrate, and high-fat overfeeding diets, for all 20 individuals was similar at 0.65.
EE response to fasting or overfeeding with various macronutrients
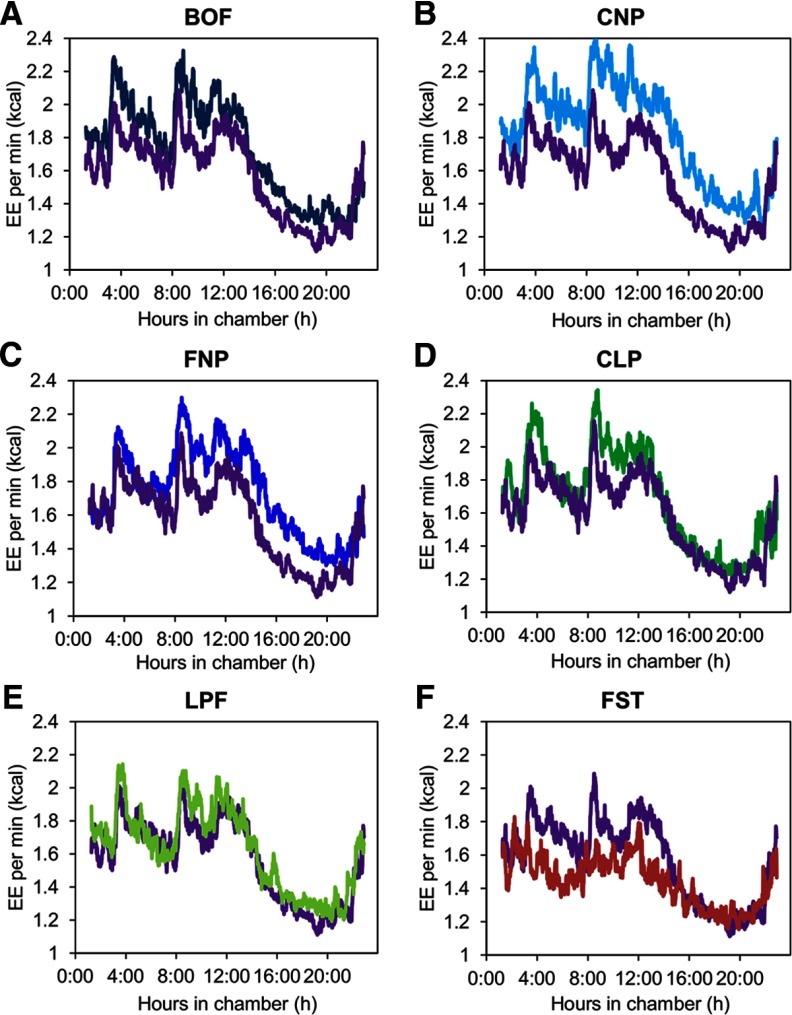
Compared with EE during energy balance, the 24h-EE decreased with fasting and increased with overfeeding of diets containing 20% protein (Figure 1 and Table 3). During the balanced overfeeding diet, which contained double the calories given for the eucaloric measurements but equivalent macronutrient proportions, 24h-EE increased by a percentage similar to the decrease observed during fasting (Table 3). Overfeeding with diets containing 3% protein did not lead to a significant increase in 24h-EE above baseline (Figure 2, C and E, and Table 3). The spike in EE after high-carbohydrate meals rapidly returned to baseline when combined with low protein, in contrast with normal protein in which the EE never completely returned to baseline (Figure 1, B and D). The percentage increases in 24h-EE and sleeping EE above baseline observed with all 3 diets containing 20% protein were not different from one another, regardless of differences in fat and carbohydrate intake. Comparisons of macronutrient oxidation are shown in Supplemental Table 2.
Figure 1.
EE per minute over 23.25 hours during all dietary interventions and compared with eucaloric feeding (shown in purple on every graph). The EE per minute during overfeeding is shown with the balanced overfeeding diet (BOF) (n = 20) with 50% carbohydrates (C), 30% fat (F), and 20% protein (P) in dark blue in (panel A); with the high carbohydrate, normal-protein diet (CNP) (n = 20) with 75% C, 5% F, 20% P in light blue (panel B); with the high-fat, normal-protein diet (FNP) (n = 19) with 20% C, 60% F, 20% P in blue (panel C); with the high-carbohydrate, low-protein diet (CLP) (n = 20) with 75% C, 22% F, 3% P in green (panel D); and with the low-protein diet (LPF) (n = 20) with 51% C, 46% F, 3% P in light green (panel E). The EE per minute during fasting (FST) (n = 20) is shown in red (panel F). The 0:00 time period indicates entry into the respiratory chamber (1 hour after consuming breakfast); lunch was given at the 3-hour mark, dinner at the 8-hour mark, and snack at the 11-hour mark. Participants were asked to be in bed from the 15-hour mark to at least the 21-hour mark in the chamber and to limit unnecessary activity throughout the 24-hour period. The trajectories were significantly different from eucaloric feeding (P < .001) during fasting and overfeeding with the BOF, CNP, and FNP diets.
Table 3.
Extent of EE Responses During Eucaloric Feeding, 200% Overfeeding With Diets Varying in Macronutrient Content, and Fasting
| 24h-EE, kcal/d | Change in 24h-EE, %a | Sleeping EE, kcal/db | Change in Sleeping EE, % | DIT, kcal/d | TEF%, % | 24-Hour SPA, % | |
|---|---|---|---|---|---|---|---|
| EU (n = 20) | 2200 ± 234 (2151, 2250) | N/A | 1757 ± 222 (1694, 1821) | N/A | 223 ± 141 (171, 274) | 9.7 ± 6.0 (8.4, 11.1) | 4.3 ± 1.1 (3.9, 4.7) |
| BOF (n = 20) | 2399 ± 276 (2349, 2448)c | 9.8 ± 7.0 (7.5, 12.1)c | 1989 ± 278 (1926, 2053)c | 13.8 ± 14.7 (9.9, 17.7)c | 441 ± 185 (389, 493)c | 9.8 ± 4.2 (8.4, 11.2) | 4.8 ± 1.8 (4.4, 5.2) |
| CNP (n = 20) | 2506 ± 284 (2456, 2556)c | 14.4 ± 5.8 (12.1, 16.7)c | 1988 ± 293 (1925, 2052)c | 13.4 ± 11.7 (9.5, 17.2)c | 548 ± 187 (497, 600)c | 12.3 ± 4.1 (10.9, 13.6) | 4.8 ± 1.2 (4.4, 5.2) |
| FNP (n = 19) | 2361 ± 337 (2310, 2412)c | 7.6 ± 7.9 (5.2, 10.0)c | 2034 ± 318 (1968, 2099)c | 16.0 ± 13.8 (12.0, 20.0)c | 398 ± 230 (344, 451)c | 8.7 ± 4.8 (7.3, 10.1) | 4.2 ± 1.1 (3.8, 4.7) |
| CLP (n = 20) | 2314 ± 199 (2256, 2373) | 5.6 ± 5.0 (2.9, 8.3) | 1809 ± 171 (1734, 1884) | 4.0 ± 10.4 (−0.6, 8.6) | 340 ± 143 (279, 401) | 7.7 ± 3.1 (6.0, 9.3) | 4.1 ± 0.8 (3.7, 4.6) |
| LPF (n = 20) | 2262 ± 234 (2212, 2311) | 3.3 ± 5.4 (1.0, 5.6) | 1872 ± 198 (1808, 1935) | 7.2 ± 7.7 (3.3, 11.1) | 293 ± 115 (241, 345) | 6.6 ± 2.5 (5.2, 7.9)c | 4.2 ± 1.2 (3.8, 4.6) |
| FST (n = 20) | 1981 ± 220 (1932, 2031)c | −9.7 ± 6.0 (−7.4, −12.0)c,d | 1726 ± 202 (1662, 1789) | −1.4 ± 7.9 (−5.3, 2.5)e | N/A | N/A | 3.8 ± 1.4 (3.4, 4.2) |
Abbreviations: BOF, balanced overfeeding with 50% carbohydrates, 30% fat, and 20% protein; CLP, high-carbohydrate, low-protein overfeeding with 75% carbohydrates, 22% fat, and 3% protein; CNP, high-carbohydrate, normal-protein overfeeding with 75% carbohydrates, 5% fat, and 20% protein; EU, eucaloric feeding; FNP, high-fat, normal-protein overfeeding with 20% carbohydrates, 60% fat, and 20% protein; LPF, low-protein overfeeding with 51% carbohydrates, 46% fat, and 3% protein; N/A, not available. Data are shown as mean ± SD, with 95% CI in parentheses.
The percent change in EE during the diets containing 3% protein (LPF, CLP) were significantly different from the overfeeding diets with 20% protein (FNP, CNP, BOF) but not from each other (P < .01).
Sleeping EE was extrapolated to 24 hours for comparison.
P < .01 compared with energy balance (EU). Comparisons were done using mixed models to account for repeated measures. The P values were corrected for multiple comparisons with Dunnett's test for all models except the percent changes from baseline. In the models for the percent changes in 24h-EE and sleeping EE from baseline, all dietary interventions were compared and correction for multiple comparisons was done with the Tukey-Kramer method.
The percentage change in EE during the fasting (FST) intervention was significantly different from all dietary interventions (P < .01).
The percent change in sleeping EE during the fasting (FST) intervention was significantly different from that observed when overfeeding the diets containing 20% protein (FNP, CNP, BOF) but not the diets containing 3% protein (CLP, LPF) (P < .01).
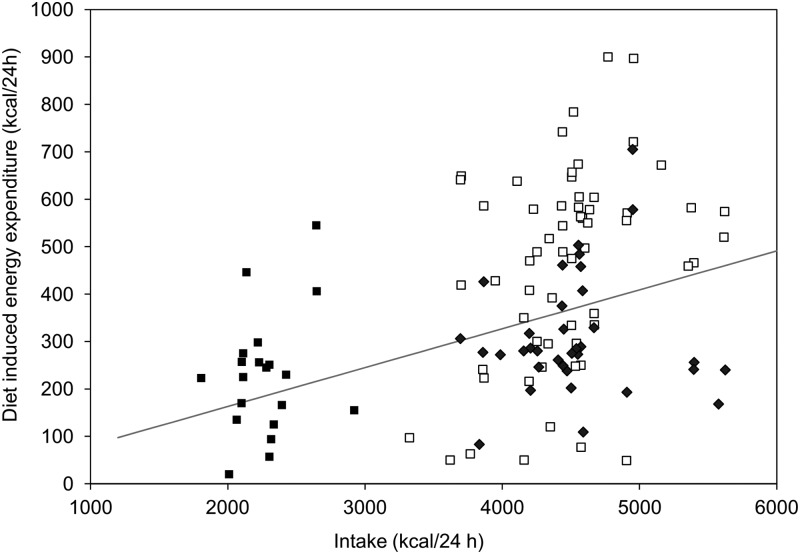
Figure 2.
Relationship between DIT and dietary intake. DIT was calculated as the difference between the 24h-EE during each feeding intervention and the fasting assessment [n = 20 individuals represented 6 times each (1 individual is represented only 5 times)]. Solid squares indicate feeding during energy balance and open squares indicate normal protein overfeeding. Solid diamonds indicate low-protein overfeeding. The gray line indicates the expected dose-response curve, assuming a strictly linear relationship, between DIT and intake based on feeding studies from D'Alessio et al. (7).
DIT and dietary intake were correlated (Figure 2) with an intercept close to 0 [DIT (kilocalories per day) = −0.7+0.1 (intake)], although DIT generated during low-protein overfeeding was consistently lower than predicted from prior literature (7) (Figure 2). Conversely, some individuals' DIT was higher than predicted with consumption of the 20% protein diets (Figure 2). However, on average, the TEF% for the overfeeding diets was similar to the TEF% during energy balance (Table 3). The exception was low protein, high-fat overfeeding.
The thermic effect of food during overfeeding is related to percentage body fat
Although, on average, TEF% was not different during diets containing 20% protein, TEF% was highly variable with a range of 2%–21% (during overfeeding) between subjects. Individual TEF% in energy balance correlated with both TEF% during normal protein overfeeding (r = 0.7, P = .001) and during low-protein overfeeding (r = 0.5, P = .01). In a model of TEF% from all dietary interventions, the contribution of the individual response explained 49% of the variance in TEF% (partial r2 = 0.49; P < .001), whereas the diet given explained an additional 16% of the variance (partial r2 = 0.16; P < .001). In a mixed model, the TEF% during overfeeding with diets containing 20% protein was negatively associated with percentage body fat, even after adjustment for age, sex, race, and diet [β = −.23; 95% confidence interval (CI) −0.06, −0.39%; P = .009]. The low-protein diets were not included in this analysis because these diets are not representative of usual free-living protein consumption. When volunteers were categorized as either obese or lean, lean subjects had an overfeeding TEF% that was 4.3% higher (95% CI for the difference 0.9, 7.7%; P = .004). Similarly, lean subjects were more likely to have a positive residual variance from the regression line between DIT and caloric intake (84 ± 46 vs −55 ± 37 kcal/d; P = .02; 95% CI for the difference: 21, 258 kcal/d). The TEF% during energy balance did not correlate with percent body fat (r = −0.01; P = .9) or vary between lean and obese subjects (11.4 ± 5.2 vs 8.6 ± 6.5%; P = .3; 95% CI for the difference: −3.0, 8.6%).
Hormonal measures
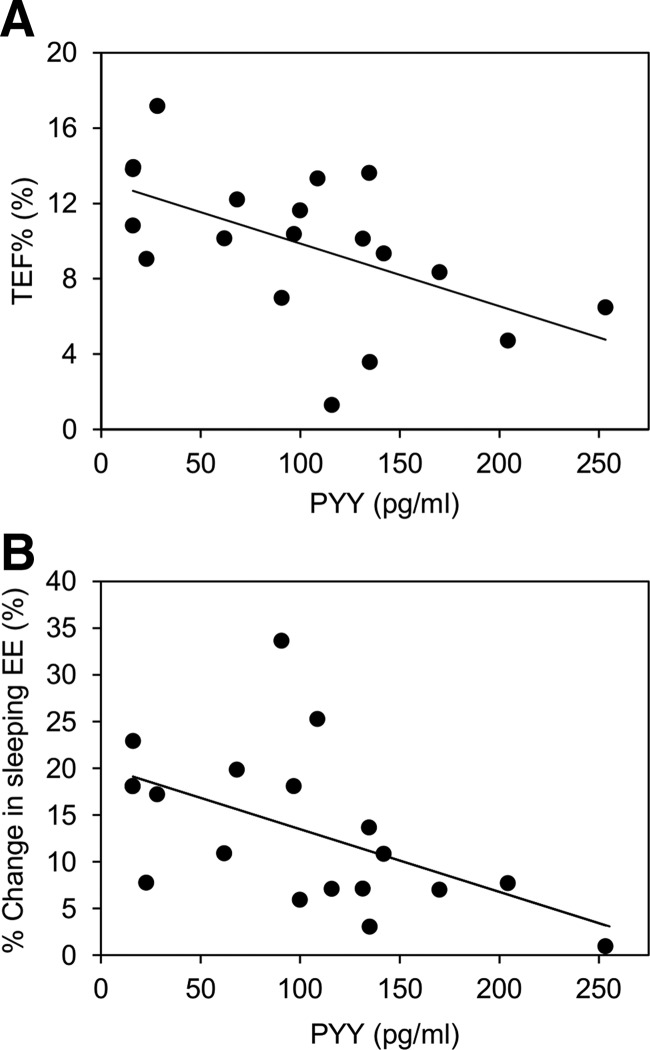
See the Supplemental Material and Supplemental Table 3 for more results of hormonal measures. Baseline fasting PYY concentrations were negatively correlated with TEF%, DIT, and the proportional changes in EE and sleeping EE that occurred with 20% protein overfeeding (Figure 3). Even after adjusting for covariates, fasting PYY was associated with TEF% (β = −.03 ± .01% per picograms per milliliter; 95% CI −0.01, −0.05; P = .008), DIT (β = −1.2 ± 0.4 kcal/d·pg per milliliter; 95% CI −0.4, −2.1; P = .006) and percentage increase in sleeping EE (β = −.06 ± 0.02% per picograms per milliliter; 95% CI −0.01, −0.1; P = .009) but not with percentage increase in total 24h-EE (β = −.02 ± .01% per picograms per milliliter; 95% CI 0.01, −0.04; P = .09). Two-hour insulin concentrations during the OGTT were negatively correlated with the TEF% during overfeeding (ρ = −0.55; P = .002), but this relationship was not independent of percentage body fat.
Figure 3.
Correlation between mean thermic effect of food (TEF%) (Pearson r = −0.56, P < .01) (A) and percent change in sleeping EE (r = −0.54, P < .01) (B) during normal protein overfeeding (mean from 3 normal protein overfeeding diets) and mean fasting PYY in the 20 participants. PYY was drawn prior to beginning the overfeeding diets.
Of the catecholamines measured, 3, 4-dihydroxyphenylglycol, norepinephrine, and 3,4-dihydroxyphenylalanine were increased on the mornings after the energy balanced diet, fasting, and low-protein overfeeding diets (see Supplemental Figure 2) but not after the overfeeding diets with 20% protein. Epinephrine, dopamine, and 3,4,-dihydroxyphenyl acetic acid did not change with the diets. Neither the baseline concentrations nor the changes in catecholamines were related to any changes in EE.
Discussion
We found that 24h-EE decreases by an average of 10% during fasting and increases by a similar amount with 100% caloric excess. The response to overfeeding was reproducible within an individual, even with alterations in macronutrient content of the diets, with larger interindividual than intraindividual variation, in part due to differences in adiposity and serum PYY concentrations. The dietary contribution to the EE response to 24 hours of overfeeding was dominated by the protein content of the diet, with no measurable difference in response to differences in fat or carbohydrate content, and an attenuated DIT with low-protein diets.
Prior studies have assessed the ability of humans to increase EE after food intake (2, 5, 13, 25–28) as well as the link between TEF% and adiposity (8, 9, 17, 27, 29, 30), but few studies have determined the reproducibility of these measurements with overfeeding and fasting or the effects of variations in protein, fat, and carbohydrates during overfeeding. Although our study has limitations including a relatively small, predominantly male sample and a lack of specific data about physical fitness and socioeconomic status, we used the gold standard of 24h-EE measurement and overfed participants with 5 different diets. The increase in food intake to twice energy requirements was chosen to maximize potential thermogenic responses. We were primarily interested in the thermogenic response to short-term episodes of overeating as might occur on a holiday or other celebratory event.
In most endotherms, TEF% averages about 10% of caloric intake (31). However, our data demonstrate that TEF% varies widely between individuals and is well correlated within an individual during energy balance and overfeeding. We found a similar degree of reproducibility in TEF% during overfeeding as other studies found with energy balance (23). Our study also demonstrates that TEF% is consistent both with the same overfeeding diet and diets with large differences in carbohydrate or fat content. It is possible, however, that with more power, the larger point estimate of the TEF% with high-carbohydrate, normal-protein overfeeding may have reached significance. The relative stability of an individual's TEF% over wide variations in caloric intake and diet composition support the concept that much of the EE response to feeding is intrinsic, likely from individual differences in factors such as gut motility, efficiency of absorption and cellular processes, and body composition. This variation may potentially contribute to the differences in excess energy stored when people overeat.
There is no need to activate thermogenic mechanisms to dissipate excess calories when a person is in energy balance. However, it may be that each person has both a certain efficiency of metabolizing food and an additional interrelated capacity for facultative thermogenesis when energy needs are exceeded. The observed DIT with overconsumption of 20% protein diets was greater in some individuals than might be expected from a strictly linear relationship with food intake. Although most studies report a linear association between DIT and intake (31), most studies do not overfeed to the degree ours did. Others have reported that the EE response to overeating requires multiple days to fully develop (32). We observed a relatively high response in a single day, most likely due to the large degree of overfeeding. The source of deviation from a linear relationship is unclear but could be from an increased cost of protein storage or activation of brown adipose tissue. The increased EE during sleep after overeating a normal protein diet indicates a prolonged thermogenic response. The TEF% during overfeeding, but not energy balance, was negatively related to percentage body fat, which may reflect adaptive responses only observable with excess caloric intake. Other studies have reported that obese individuals have a reduced TEF% (8, 23, 29, 30), but this finding has not been consistent (7, 16, 33). The relationship between adiposity and TEF% has been attributed to increased insulin resistance (8, 34). We tried to minimize this effect by including only individuals with normal glucose tolerance. The lack of a sustained increase in EE during low-protein overfeeding, even with a large increase in carbohydrate consumption, argues against the role of insulin or brown adipose tissue in the EE response to short-term overfeeding. A decrease in TEF% in obese individuals has also been attributed to a reduced ability to dissipate heat because of insulation provided by excess adipose tissue (35). Conversely, other studies found that TEF% was unchanged with weight loss (36). A complex interrelationship may exist in which TEF% is limited by body habitus, increasing the risk of further weight gain. Our cross-sectional data can not differentiate whether obesity is the cause or consequence of the relatively reduced TEF% seen with overeating.
Early studies reported that lean subjects demonstrate the greatest caloric cost of weight gain with long-term overfeeding of a low-protein diet (5, 11). However, a study comparing the effects of 8 weeks of overfeeding with low protein vs normal-protein diets found that although weight gain with the low-protein diet was less, body fat accumulation was similar between diets, and EE did not increase with low-protein overfeeding (6). Our study confirmed that EE during low-protein overfeeding was not different from EE in energy balance, no matter the contribution from other macronutrients.
Baseline total PYY concentrations were negatively correlated with both TEF% and percentage increase in sleeping EE. PYY is implicated as a satiety hormone and postprandial PYY response correlates positively with DIT (37), although we were not able to assess postprandial changes in gut hormones while participants were within the chamber. However, because EE does not increase with infusion of PYY into humans (38), it seems unlikely that PYY has a direct effect on DIT. PYY has been shown to inhibit gastrointestinal motility and secretion (39). It may be that, prior to the postprandial rise in PYY, a lower fasting PYY concentration permits a greater amount of gut motility and activity, leading to more EE during meal consumption. The sympathetic nervous system has been hypothesized to contribute to DIT in humans by activating brown adipose tissue (40). We observed an increase in catecholamines only after fasting, energy balance, and the low-protein diets. Thus, our results do not support this hypothesis and, if anything, indicate a catecholamine response to low protein intake that is separate from any observed EE changes.
In conclusion, our results demonstrate the limited ability for humans to dissipate excess energy intake as heat during near-maximal, short-term overfeeding. Both high-carbohydrate and high-fat diets have been implicated as contributors to obesity; however, our data indicate there is little difference in EE between these diets. The exception is diets with very low protein content, which have a lower EE response. The correlation between TEF% during energy balance and overfeeding, the association with PYY concentrations, and the reproducibility of the measures indicate that much of the efficiency of nutrient metabolism may be intrinsic. The increase in nocturnal EE plus the association of overfeeding TEF% with adiposity may indicate a small, but important, capacity for additional thermogenesis in some people.
Acknowledgments
We thank the clinical research staff of the Obesity and Diabetes Clinical Research Section of the Phoenix Epidemiology and Clinical Research Branch for their excellent care of the participants. The study had a Clinical Trial registration number of NCT00523627 (www.clinicaltrials.gov).
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- CV
- coefficient of variation
- DIT
- diet-induced thermogenesis
- EE
- energy expenditure
- 24h-EE
- 24-hour energy expenditure
- ICC
- intraclass correlation coefficient
- OGTT
- oral glucose tolerance test
- PYY
- peptide YY
- TEF%
- thermic effect of food.
References
- 1. Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr. 2007;86:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diaz EO, Prentice AM, Goldberg GR, Murgatroyd PR, Coward WA. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am J Clin Nutr. 1992;56:641–655 [DOI] [PubMed] [Google Scholar]
- 3. Lammert O, Grunnet N, Faber P, et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84:233–245 [PubMed] [Google Scholar]
- 4. Bouchard C, Tremblay A, Després JP, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482 [DOI] [PubMed] [Google Scholar]
- 5. Dulloo AG, Jacquet J. Low-protein overfeeding: a tool to unmask susceptibility to obesity in humans. Int J Obes Relat Metab Disord. 1999;23:1118–1121 [DOI] [PubMed] [Google Scholar]
- 6. Bray GA, Smith SR, de Jonge L, et al. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating. JAMA. 2012;307:47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D'Alessio DA, Kavle EC, Mozzoli MA, et al. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res. 1997;5:622–631 [DOI] [PubMed] [Google Scholar]
- 9. Jequier E. Thermogenic responses induced by nutrients in man: their importance in energy balance regulation. Experientia Suppl. 1983;44:26–44 [DOI] [PubMed] [Google Scholar]
- 10. Webb P, Annis JF. Adaptation to overeating in lean and overweight men and women. Hum Nutr Clin Nutr. 1983;37:117–131 [PubMed] [Google Scholar]
- 11. Miller DS, Mumford P. Gluttony. 1. An experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967;20:1212–1222 [DOI] [PubMed] [Google Scholar]
- 12. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–241 [DOI] [PubMed] [Google Scholar]
- 13. Forbes GB, Brown MR, Welle SL, Lipinski BA. Deliberate overfeeding in women and men: energy cost and composition of the weight gain. Br J Nutr. 1986;56:1–9 [DOI] [PubMed] [Google Scholar]
- 14. Hamilton JS. Heat increments of diets balanced and unbalanced with respect to protein. J Nutr. 1939;17:583–599 [Google Scholar]
- 15. Schutz Y, Bessard T, Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr. 1984;40:542–552 [DOI] [PubMed] [Google Scholar]
- 16. Schwartz RS, Ravussin E, Massari M, O'Connell M, Robbins DC. The thermic effect of carbohydrate versus fat feeding in man. Metabolism. 1985;34:285–293 [DOI] [PubMed] [Google Scholar]
- 17. Swaminathan R, King RF, Holmfield J, Siwek RA, Baker M, Wales JK. Thermic effect of feeding carbohydrate, fat, protein and mixed meal in lean and obese subjects. Am J Clin Nutr. 1985;42:177–181 [DOI] [PubMed] [Google Scholar]
- 18. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(suppl 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 19. Nguyen T, de Jonge L, Smith SR, Bray GA. Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min. Med Biol Eng Comput. 2003;41:572–578 [DOI] [PubMed] [Google Scholar]
- 20. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174 [PubMed] [Google Scholar]
- 22. Eisenhofer G, Goldstein DS, Stull R, et al. Simultaneous liquid-chromatographic determination of 3,4-dihydroxyphenylglycol, catecholamines, and 3,4-dihydroxyphenylalanine in plasma, and their responses to inhibition of monoamine oxidase. Clin Chem. 1986;32:2030–2033 [PubMed] [Google Scholar]
- 23. Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr. 1995;61:1013–1019 [DOI] [PubMed] [Google Scholar]
- 24. WHO Expert Committee Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452 [PubMed] [Google Scholar]
- 25. Weyer C, Vozarova B, Ravussin E, Tataranni PA. Changes in energy metabolism in response to 48 h of overfeeding and fasting in Caucasians and Pima Indians. Int J Obes Relat Metab Disord. 2001;25:593–600 [DOI] [PubMed] [Google Scholar]
- 26. Deriaz O, Fournier G, Tremblay A, Despres JP, Bouchard C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am J Clin Nutr. 1992;56:840–847 [DOI] [PubMed] [Google Scholar]
- 27. Goldman RF, Haisman MF, Bynum G, Horton ES, Sims EAH. Experimental obesity in man: metabolic rate in relation to dietary intake. In: Bray GA, ed. Obesity in Perspective. DHEW publication number (NIH) 75-708 Washington DC: US Government Printing Office; 1975;165–186 [Google Scholar]
- 28. Joosen AM, Bakker AH, Westerterp KR. Metabolic efficiency and energy expenditure during short-term overfeeding. Physiol Behav. 2005;85:593–597 [DOI] [PubMed] [Google Scholar]
- 29. Bessard T, Schutz Y, Jequier E. Energy expenditure and postprandial thermogenesis in obese women before and after weight loss. Am J Clin Nutr. 1983;38:680–693 [DOI] [PubMed] [Google Scholar]
- 30. Weinsier RL, Nelson KM, Hensrud DD, Darnell BE, Hunter GR, Schutz Y. Metabolic predictors of obesity. Contribution of resting energy expenditure, thermic effect of food, and fuel utilization to four-year weight gain of post-obese and never-obese women. J Clin Invest. 1995;95:980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Secor SM. Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B. 2009;179:1–56 [DOI] [PubMed] [Google Scholar]
- 32. Jebb SA, Prentice AM, Goldberg GR, Murgatroyd PR, Black AE, Coward WA. Changes in macronutrient balance during over- and underfeeding assessed by 12-d continuous whole-body calorimetry. Am J Clin Nutr. 1996;64:259–266 [DOI] [PubMed] [Google Scholar]
- 33. Granata GP, Brandon LJ. The thermic effect of food and obesity: discrepant results and methodological variations. Nutr Rev. 2002;60:223–233 [DOI] [PubMed] [Google Scholar]
- 34. Weststrate JA. Resting metabolic rate and diet-induced thermogenesis: a methodological reappraisal. Am J Clin Nutr. 1993;58:592–601 [DOI] [PubMed] [Google Scholar]
- 35. Brundin T, Thorne A, Wahren J. Heat leakage across the abdominal wall and meal-induced thermogenesis in normal-weight and obese subjects. Metabolism. 1992;41:49–55 [DOI] [PubMed] [Google Scholar]
- 36. Nelson KM, Weinsier RL, James LD, Darnell B, Hunter G, Long CL. Effect of weight reduction on resting energy expenditure, substrate utilization, and the thermic effect of food in moderately obese women. Am J Clin Nutr. 1992;55:924–933 [DOI] [PubMed] [Google Scholar]
- 37. Doucet E, Laviolette M, Imbeault P, Strychar I, Rabasa-Lhoret R, Prud'homme D. Total peptide YY is a correlate of postprandial energy expenditure but not of appetite or energy intake in healthy women. Metabolism. 2008;57:1458–1464 [DOI] [PubMed] [Google Scholar]
- 38. Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–E1068 [DOI] [PubMed] [Google Scholar]
- 39. Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Welle S. Sympathetic nervous system response to intake. Am J Clin Nutr. 1995;62:1118S–1122S [DOI] [PubMed] [Google Scholar]