Abstract
Context:
Recent evidence implicates the orphan nuclear receptor, nuclear receptor subfamily 2, group F, member 2 (NR2F2; chicken ovalbumin upstream promoter-transcription factor II) as both a master regulator of angiogenesis and an oncogene in prostate and other human cancers.
Objective:
The objective of the study was to determine whether NR2F2 plays a role in ovarian cancer and dissect its potential mechanisms of action.
Design, Setting, and Patients:
We examined NR2F2 expression in healthy ovary and ovarian cancers using quantitative PCR and immunohistochemistry. NR2F2 expression was targeted in established ovarian cancer cell lines to assess the impact of dysregulated NR2F2 expression in the epithelial compartment of ovarian cancers.
Results:
Our results indicate that NR2F2 is robustly expressed in the stroma of healthy ovary with little or no expression in epithelia lining the ovarian surface, clefts, or crypts. This pattern of NR2F2 expression was markedly disrupted in ovarian cancers, in which decreased levels of stromal expression and ectopic epithelial expression were frequently observed. Ovarian cancers with the most disrupted patterns of NR2F2 were associated with significantly shorter disease-free interval by Kaplan-Meier analysis. Targeting NR2F2 expression in established ovarian cancer cell lines enhanced apoptosis and increased proliferation. In addition, we found that NR2F2 regulates the expression of NEK2, RAI14, and multiple other genes involved in the cell cycle, suggesting potential pathways by which dysregulated expression of NR2F2 impacts ovarian cancer.
Conclusions:
These results uncover novel roles for NR2F2 in ovarian cancer and point to a unique scenario in which a single nuclear receptor plays potentially distinct roles in the stromal and epithelial compartments of the same tissue.
Ovarian cancer is the fifth leading cause of cancer death for women in the United States (1). Most women are not diagnosed with ovarian cancer until it has spread widely throughout the abdomen and pelvis. Recent evidence has demonstrated that a member of the nuclear receptor superfamily, nuclear receptor subfamily 2, group F, member 2 (NR2F2 or chicken ovalbumin upstream promoter-transcription factor II), regulates early events in angiogenesis and plays a critical role in cancer metastasis (2–4). Altered NR2F2 expression has been implicated in multiple human cancers, including carcinomas of the breast (5–8), prostate (2), pancreas (3, 4), colon (9), and lung (10). In the female productive tract, NR2F2 plays a key role in regulating vasculogenesis as the endometrium undergoes repetitive cycles of regrowth (11). In addition, altered levels of NR2F2 expression have also been implicated in infertility (12–15) and endometriosis (16–18). We hypothesized that altered expression of NR2F2 plays a role in ovarian cancer. Using a series of human tissue specimens, we have found that the expression of NR2F2 is disrupted in both the epithelial and stromal components of ovarian cancer and that dysregulated NR2F2 expression regulates apoptosis in epithelial compartment of ovarian cancer via pathways not previously known to be impacted by this receptor.
Materials and Methods
RNA isolation, quantitative real-time PCR, and gene expression profiling
With permission from our institutional review board, specimens of ovarian cancer, distal fallopian tube, healthy ovary, and benign cystadenoma were obtained from the Gynecologic Tissue Biorepository at Baylor College of Medicine. Written informed consent was obtained for all specimens. Total RNA was extracted using the mirVana kit (Life Technologies, Carlsbad, California). RNA quality was assessed using the Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, California).
Quantitative real-time PCR (qPCR) was performed as described (19). Primer sequences are documented in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. Expression of ribosomal gene L19 (RPL19) or 18S was used as an endogenous control. Relative transcript quantity was calculated using the ΔΔCT method (20) and plotted as mean ± SEM. Student's t test was used to assess statistical significance. A value of P < .05 was considered statistically significant.
Global patterns of gene expression were interrogated using Human WG-6 gene chip (version 3.0; Illumina, San Diego, California). The Texas Children's Cancer Genomics and Proteomics Core Laboratory performed all hybridizations. Results were analyzed as previously described (19, 21). Array data have been deposited into the Gene Expression Omnibus (accession number pending).
Cell culture, transfection, and in vitro assays
Ovarian cancer cell lines were obtained from either the American Type Culture Collection (Manassas, Virginia) (TOV-112D, IGROV1, MDAH-2774, TOV-21G, ES-2) or the University of Texas M. D. Anderson Cancer Center (Houston, Texas) (SKOV3ip1, HeyA8) and cultured in American Type Culture Collection-recommended growth media supplemented with 1% penicillin-streptomycin (Life Technologies). The identity of these lines was confirmed by short tandem repeat sequencing. Normal ovarian surface epithelial cells were obtained and cultured as previously described using 1:1 MCDB-105:Media-99 (Sigma, Inc, St Louis, Missouri) supplemented with 15% fetal bovine serum (Life Technologies), 1% penicillin-streptomycin (Life Technologies), and 2 ng/mL epidermal growth factor (Sigma) (21). All cells were cultured in a humidified incubator at 37°C with 5% carbon dioxide.
For small interfering RNA (siRNA) knockdown, ES-2 and TOV-112D cells were seeded at 100 000 cells/mL in a 6-well plate. After 24 hours, cells were transfected using 5 μL of RNAiMax (Life Technologies) and 5 nM (for TOV-112D) or 25 nM (for ES-2) of ON-TARGETplus SMARTpool-human NR2F2 (NM_021005) or ON-TARGETplus Nontargeting number 1 (Dharmacon, Inc, Lafayette, Colorado). NEK2 was similarly targeted using 100 nM (for TOV-112D) or 25 nM (for ES-2) of ON-TARGETplus SMARTpool-human NEK2 (NM_002497). After 48 hours of transfection, cells were lysed for RNA or protein unless otherwise indicated. Proliferation was measured using CellTiter 96 Aqueous One solution cell proliferation assay (Promega, Inc, Madison, Wisconsin) after an initial plating density of 500 cells/well. Measurements were taken in 24-hour increments. After subtracting background absorbance, data are presented as percentage of baseline absorbance. Cells transfected with nontargeting siRNA were used as a control. Apoptosis was measured using Caspase-Glo 3/7 assay (Promega) after plating 500 cells/well.
Creation of tissue microarray and immunohistochemistry
Formalin-fixed specimens were used to create a paraffin-embedded tissue microarray. This array contained 210 cores from 50 ovarian cancers (37 serous carcinomas, 8 endometrioid carcinomas, 2 mucinous carcinomas, and 3 clear cell carcinomas) along with representative cores from 10 normal ovaries and 10 distinct positive controls. Controls included appendix, lung, breast, prostate, endocervix, liver, nerve, brainstem, and lymph node. Antigen mobilization was performed using 10 mM sodium citrate (pH 6). Nonspecific binding was blocked using PBS supplemented with 5% normal goat serum. Primary antibody specific for NR2F2/chicken ovalbumin upstream promoter-transcription factor II (kind gift of Dr Ming-Jer Tsai, Baylor College of Medicine) was used at a dilution of 1:100. Antigen was visualized using a 3,3-diaminobenzidine-labeled goat antimouse secondary antibody (Sigma-Aldrich). Sections were counterstained with Meyer's hematoxylin (Sigma-Aldrich). Secondary antibody alone was used as a negative control. NR2F2 staining was scored for both intensity and frequency of staining by 2 independent investigators (M.L.A. and S.M.H.) who were blinded to tissue type and pathological diagnosis. Relative staining intensity (0, 1+, 2+, 3+) as well as the proportion of cells expressing antigen (0: 0%–5%; 1: 6%–25%; 2: 26%–50%; 3: 51%–75%; 4: 76%–100%) was recorded. A minimum of 500 cells in each of 3 high-power (×40) fields was examined. Mean expression scores were compared using 2-tailed Student's t tests. Average scoring ± SEM is shown.
Western blotting
Total protein was prepared using radioimmunoprecipitation assay buffer [50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Triton X-100; 1% sodium dodecyl sulfate; and 1 mM EDTA] supplemented with 1× cOmplete mini EDTA-free protease inhibitor cocktail (Roche, Indianapolis, Indiana). Cell pellets were sonicated using a Missonix XL-2000 (Qsonica, Newtown, Connecticut) 3 times for 20 seconds, with a minimum of 1 minute on ice between pulses. Protein concentration was quantified using a BCA protein assay kit (Pierce, Rockford, Illinois) per the manufacturer's instructions. Fifty micrograms of protein were separated using 10% Bis-Tris gels (Life Technologies) and transferred to a polyvinyl difluoride membrane using an iBLOT (Life Technologies) at 20 V for 5 minutes. Blots were blocked using PBS, supplemented with 1% Tween-20 and 5% nonfat dry milk (MP Biomedical, LLC, Solon, Ohio) for 1 hour at room temperature. Blots were incubated with primary antibody to NR2F2 (Perseus Proteomics, Tokyo, Japan) at 1:250 or NEK2 (BD Biosciences, San Jose, California) at 1:100, washed, and incubated with secondary antibody, antimouse (Cell Signal Technology, Danvers, Massachusetts) at 1:2000 or antirabbit (Sigma-Aldrich) at 1:10 000, washed, and detected using SuperSignal West Pico chemiluminescent substrate (Pierce). Blots were stripped with Restore Western blot stripping buffer (Thermo Fisher Scientific, Waltham, Massachusetts) and reprobed using antibody to glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich) at 1:2000 dilution with antirabbit (Sigma-Aldrich) at 1:10 000 dilution.
Statistical analyses
All statistical analyses were performed using SAS software (SAS Institute, Cary, North Carolina). Survival analysis of the abstracted patient data was performed with a log-rank (Mantel-Cox) test and presented as a Kaplan-Meier survival curve using GraphPad Prism (version 5.04; GraphPad Software, Inc, La Jolla, California). Level 3 mRNA expression data were accessed from The Cancer Genome Atlas (TCGA) ovarian cancer data set. Two-sample t test and ANOVA implemented in R (http://www.R-project.org/) were used to evaluate the significance of differentially expressed genes and outcome demographics.
Results
Expression of NR2F2 in epithelial ovarian cancers
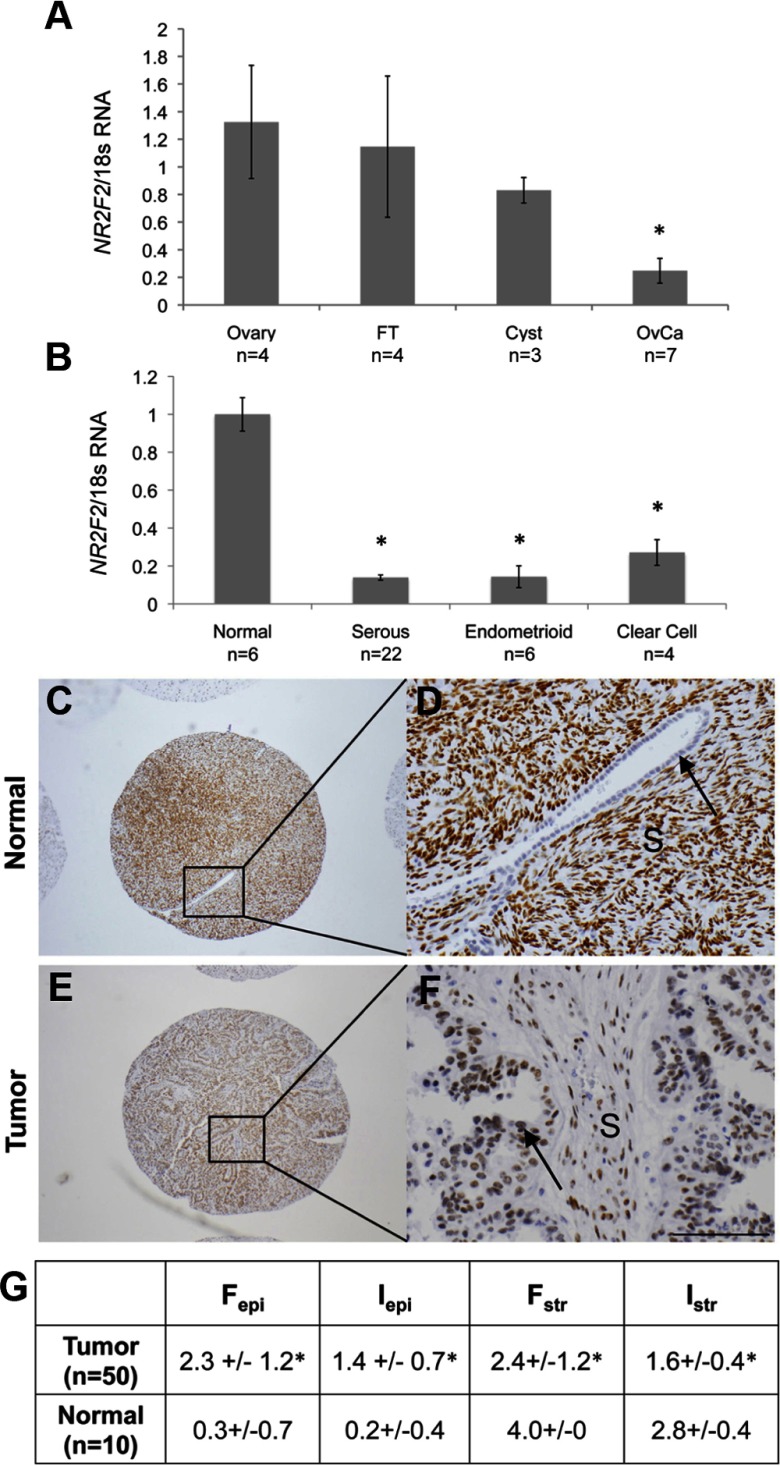
Previous unvalidated microarray studies have suggested that NR2F2 is down-regulated in ovarian cancers (22). To confirm this observation, we compared NR2F2 expression in specimens of high-grade serous cancers to normal ovary, fallopian tube, and serous cystadenomas. We found that levels of both NR2F2 transcript and protein were significantly lower in serous cancers than ovary or fallopian tube (Figure 1A and Supplemental Figure 1). Low levels of NR2F2 expression were also observed in the homogenized specimens of other ovarian cancer histotypes, including endometrioid and clear cell cancers (Figure 1B). No difference in NR2F2 expression was observed between different ovarian cancer histotypes. NR2F2 expression was similar in specimens of pre- and postmenopausal ovary (data not shown).
Figure 1.
Expression and localization of NR2F2 in ovarian cancer. A, NR2F2 expression is decreased in serous ovarian cancers compared with normal and benign tissues. B, NR2F2 is decreased across all histotypes of epithelial ovarian cancers. In both A and B, the relative expression of NR2F2 transcript is normalized to levels of 18s in the normal ovary. C–F, Immunohistochemical staining for NR2F2. Magnification of ×4 cores (C and E) and ×20X cores (D and F) of normal ovary with epithelial cleft (C-D) or serous ovarian cancer (E and F). G, Composite results from tissue microarray scoring. *, P < .05. Cyst, serous cystadenoma; Fepi, frequency of staining in epithelium; Fstr, frequency of staining in stroma; FT, fallopian tube; Iepi, intensity of staining in epithelium; Istr, intensity of staining in stroma; OvCa, serous ovarian cancer; S, stroma. Arrows, Epithelium.
Localization of NR2F2 in ovarian cancers and normal ovary
Previous studies in human ovary have shown that NR2F2 is not typically expressed in the ovarian epithelia (23, 24). However, NR2F2 localization within the ovarian cancer complex has not been examined. We used a tissue microarray containing triplicate cores from 50 epithelial ovarian cancers and 10 normal ovaries to further localize and quantify NR2F2 expression in ovarian cancer. Robust NR2F2 staining was routinely observed in the stroma of normal ovary with only minimal NR2F2 staining in epithelia lining the ovarian surface, clefts, or crypts (Figure 1, C and D). In contrast to normal ovary, NR2F2 staining was frequently found in epithelia of ovarian cancers. Staining for NR2F2 in ovarian cancer stroma was much less intense than the stroma of normal ovary (Figure 1, E–G).
NR2F2 and ovarian cancer outcomes
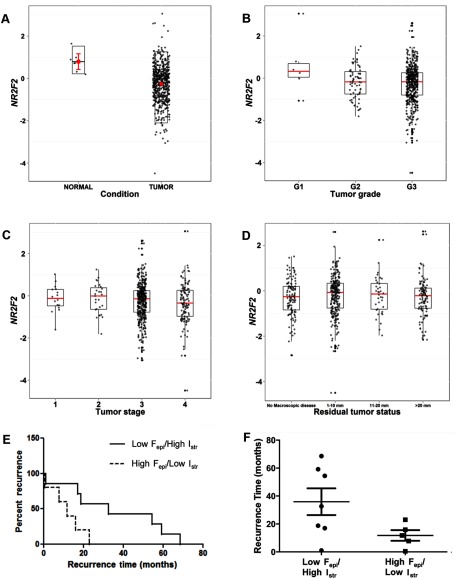
Next, we examined NR2F2 expression in ovarian cancers using data from the TCGA (25). Examination of level 3 TCGA data confirmed that NR2F2 expression is significantly lower in ovarian cancers than normal fallopian tube (P < .001, Figure 2A). Although a trend toward lower levels of NR2F2 was observed with higher tumor grade (Figure 2B, P < .054, ANOVA), overall levels of NR2F2 transcript assessed in ovarian cancers by the TCGA did not correlate with other clinically recognized prognostic features of this disease such as tumor stage (Figure 2C), volume of residual tumor present after debulking surgery (Figure 2D), or age at diagnosis (not shown).
Figure 2.
Clinical significance of NR2F2 expression. A–D, Box plot of TCGA ovarian cancer and normal data sets with NR2F2 expression. A, NR2F2 transcript level in serous ovarian cancer was 0.48-fold less than levels reported for normal fallopian tube (P = .0002). B, NR2F2 transcript level according to tumor grade. C, NR2F2 transcript levels based on FIGO tumor stage. D, NR2F2 transcript level based on residual tumor status. Analysis of disease-free interval as a function of NR2F2 localization. E, Kaplan-Meier analysis, comparing disease-free intervals of patients with low staining frequency in the epithelia and high staining intensity in the stroma (n = 7, solid line) with patients with high staining frequency in the epithelia and low staining intensity in the stroma (n = 5, dashed line) (P = .03, HR 0.17, 95% CI 0.04–0.83). F, Scatter plot of patient disease-free intervals. Patients with low staining frequency in the epithelia and high staining intensity in the stroma (left panel) had a median disease-free interval of 32.66 months (range 1.02–68.53 months). Patients with high staining frequency in the epithelia and low staining intensity in the stroma (right panel) had a median disease-free interval of 11.76 months (range 0.46–23.06 months). Fepi, frequency of staining in epithelium; Fstr, frequency of staining in stroma; G1, grade 1; G2, grade 2; G3, grade 3; Iepi, intensity of staining in epithelium; Istr, intensity of staining in stroma.
Using our original immunohistochemical data, we asked whether NR2F2 localization within a cancer rather than its overall levels in homogenized tissue specimens correlate with outcome. Linear regression was used to test relationships between NR2F2 localization in the epithelial and stromal components of ovarian cancer and key outcome demographics. The clinical characteristics of patients from whom specimens were collected are summarized in Supplemental Table 2. For the purposes of this analysis, we considered staining frequency and intensity as separate variables. No correlation was observed between NR2F2 staining in ovarian cancer epithelia and stroma in the same specimens (r = 0.2). However, the frequency of NR2F2 staining in the epithelium of metastatic ovarian cancers [≥ Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) stage II] was significantly greater than the levels observed in disease confined only to the ovaries (FIGO stage I) (Wilcox rank sum test, P = .03). We also found that frequency of NR2F2 staining in ovarian cancer epithelia was associated with a trend toward greater likelihood of disease recurrence [hazard risk (HR) 1.51; 95% confidence interval (CI) 0.99–2.30, P = .055)]. When stromal NR2F2 expression was examined, we observed a trend toward higher stromal staining intensity in optimally debulked cancers (Wilcox rank sum test, P = .09).
To explore these data further, we examined outcomes while simultaneously considering NR2F2 expression in epithelia and stroma. Patients whose tumoral NR2F2 expression qualified them within the lowest quintile of epithelial staining (low Fepi; score < 1.67) and highest quintile of stromal staining intensity (high Istr; score > 3.4) were compared with the subset of patients with the highest quintile of epithelial staining (high Fepi; score > 3.67) and the lowest quintile of stromal staining intensity (low Istr; score < 1.67). This comparison defined 2 groups most (low Fepi/high Istr) and least (high Fepi/low Istr) similar to normal ovary. We found that the median disease-free interval for the low Fepi/high Istr subset of patients was 32.7 months, significantly longer than the 11.8 months observed in high Fepi/low Istr subset of patients (log rank, P = .03) (Figure 2, E and F). This difference translated into a significantly decreased hazard of disease recurrence (HR 0.17, 95% CI 0.04–0.83) for low Fepi/high Istr patients. No significant associations between localization of NR2F2 expression, serum CA-125 levels, overall survival, tumor grade, presence of malignant ascites, presence or absence of nodal metastases, or menopausal status at the time of initial laparotomy were noted.
Biological impact of targeting NR2F2 expression in vitro
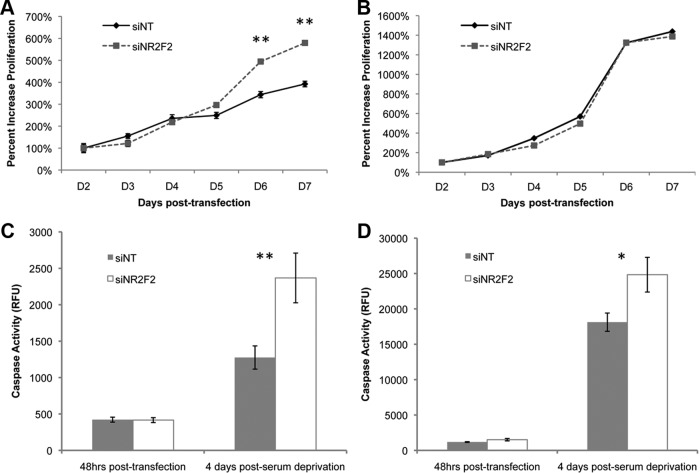
To assess potential biological roles for NR2F2 in ovarian cancer, we examined the effect of NR2F2 depletion on cellular function. We targeted NR2F2 in ES-2 and TOV-112D cells, 2 cell lines with significant NR2F2 expression (Supplemental Figure 2). Western blot confirmed an efficient knockdown of NR2F2 expression, which could be detected at 48 h (Supplemental Figure 3) and persisted for at least 6 days (data not shown). Knockdown of NR2F2 resulted in increased rates of apoptosis in serum starved cultures of both ES-2 and TOV-112D cells (Figure 3, C and D). Loss of NR2F2 was accompanied by increased rates of proliferation in ES-2 but not TOV-112D cells (Figure 3, A and B).
Figure 3.
Cellular impact of NR2F2 knockdown. Cells were transfected with siRNA to NR2F2 (siNR2F2) or nontargeting siRNA (siNT) as indicated. After knockdown was confirmed (Supplemental Figure 3) (A and B), proliferation was measured in either ES-2 (A) or TOV-112D (B) cells transfected with either siNR2F2 or siNT (n = 3). Results are standardized to day 2. C and D, Apoptosis was measured in either serum-starved cultures of either ES-2 (C) or TOV-112D (D) cells transfected with either siNR2F2 or siNT (n = 3). Caspase-3/7 is reported in relative fluorescence units. *, P < .05; **, P < .01.
Impact of targeting NR2F2 on gene expression
Multiple prior studies have suggested that NR2F2 plays a significant role in the developmental steps of vasculogenesis (3, 4, 7, 8, 14, 26–29) and that its expression supports cancer angiogenesis (2–4, 7, 10). Therefore, we hypothesized that expression of NR2F2 contributes to ovarian cancer by promoting the expression of gene products mediating tumor angiogenesis. To our surprise, knockdown of NR2F2 did not impact expression of any angiogenic gene products we examined in vitro. These included angiopoietin-1 (ANG1) and -2 (ANG2), plasminogen activator urokinase (PLAU), vascular endothelial growth factor (VEGF) D (VEGFD), tyrosine kinase with immunoglobulin-like and EGF-like domains (TIE1), tyrosine kinase endothelial (TEK, also known as TIE2), and enhancer of zeste homolog 2 (EZH2) in either ES-2 or TOV-112D cells (Supplemental Figure 3). In mouse uterus, loss of Nr2f2 leads to a significant decrease in the expression of bone morphogenetic protein 2 (Bmp2) (13). However, loss of NR2F2 in ovarian cancer cell lines did not significantly impact BMP2 expression (Supplemental Figure 3).
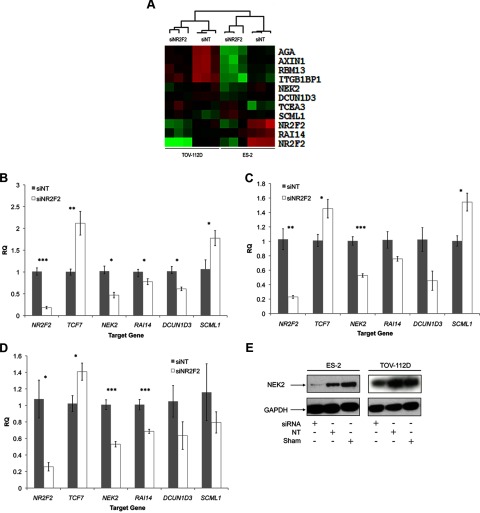
To better understand NR2F2-regulated pathways in ovarian cancer, we used whole-genome expression microarrays to examine established ovarian cancer cell lines in which NR2F2 was targeted by siRNA. Three biological replicates in 2 different cell lines (ES-2 and TOV-112D) were studied. We discovered 659 distinct gene products differentially expressed (>1.5-fold, P < .05) in either cell line when NR2F2 expression was knocked down (Supplemental Table 3). Expression of 19 gene products was altered when NR2F2 expression was targeted in either ES-2 or TOV-112D cells. A heat map of the 10 genes whose transcripts were differentially expressed in common between the 2 lines is found in Figure 4A. Using independently transfected cultures, we confirmed that the expression of never in mitosis gene a-related kinase 2 (NEK2) was altered in response to NR2F2 knockdown when assessed both by qPCR (Figure 4, B–D) and Western blot (Figure 4E). Altered expression of retinoic acid induced 14 (RAI14), defective in cullin neddylation 1, domain containing 3 (Saccharomyces cerevisiae) (DCUN1D3), and sex comb on midleg-like 1 (Drosophila) (SCML1) could be similarly validated (Figure 4, B and C). To determine whether these genes are regulated by NR2F2 in other ovarian cancer cell lines, we transfected TOV-21G cells with siRNA targeting either NR2F2 or a nontargeting control. We found that the expression of RAI14 and NEK2 were reduced by NR2F2-specific siRNA (Figure 4D), with DCUN1D3 and SCML1 following a similar trend. Thus, the subset of genes we identified appears to be regulated by NR2F2 in common across multiple ovarian cancer cell lines.
Figure 4.
Whole-genome profiling of NR2F2-regulated gene expression in ovarian cancer cell lines. A, Heat map representing differentially expressed genes in both ES-2 and TOV-112D (P < .05, fold change > 1.5) with Euclidian hierarchical clustering. Rows, Gene; columns, profiled samples. As a quality control (QC), results for 2 different probes specific for NR2F2 are included. B, qPCR validation of genes differentially expressed in ES-2 (n = 4). C, qPCR validation of genes differentially expressed in TOV-112D (n = 4). D, qPCR validation of genes differentially expression in TOV-21G (n = 4). E, Western blot for NEK2. *, P < .05; **, P < .01; ***, P < .001. Green, up-regulated; red, down-regulated.
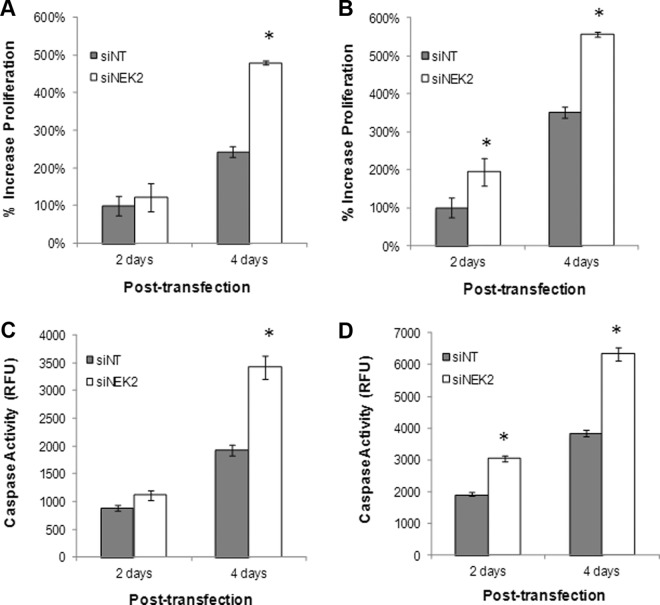
To determine whether the function of the genes we identified is consistent with the biological impact of NR2F2 in ovarian cancer cells, we targeted NEK2 in cultures of ES-2 and TOV-112D cells (Supplemental Figure 4). Depletion of NEK2 in both cell lines resulted in increased proliferation as predicted by our earlier experiments targeting NR2F2 (Figure 5, A and B). Similarly, targeting NEK2 resulted in increased rates of apoptosis (Figure 5, C and D). Both observations are consistent with our hypothesis that the impact of NR2F2 in the epithelial compartment of ovarian cancer is in part mediated through NEK2, although direct interaction has not been tested.
Figure 5.
Cellular effect of NEK2 knockdown. Cells were transfected with siRNA to NEK2 (siNEK2) or nontargeting siRNA (siNT) as indicated. After knockdown was confirmed (Supplemental Figure 4), proliferation was measured in either ES-2 (A) or TOV-112D (A and B), and cells were transfected with either siNEK2 or siNT (B) (n = 3). Results are standardized to day 2. C and D, Apoptosis was measured in cultures of ES-2 (C) or TOV-112D (D) cells transfected with either siNEK2 or siNT (n = 3). Caspase-3/7 is reported in relative fluorescence units. *, P < .05.
Pathway analysis of NR2F2-regulated gene expression
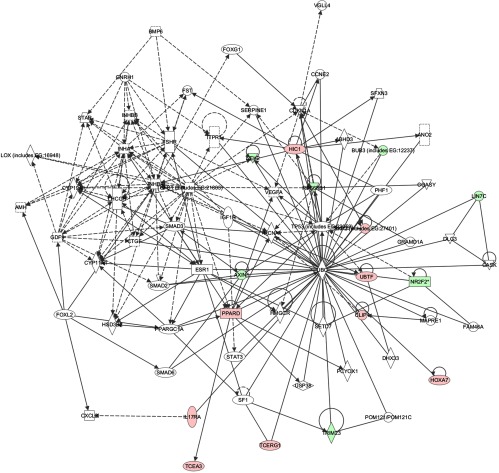
Because ES-2 cells are derived from a clear cell tumor (30) and TOV-112D cells are derived from an endometrioid ovarian cancer (31), it is possible that NR2F2 has distinct functions within the unique genetic contexts of these 2 cell lines. Euclidian hierarchical clustering revealed that patterns of gene expression with NR2F2 knockdown are unique to each cell line studied (Figure 4A). Database for Annotation, Visualization, and Integrated Discovery (DAVID) functional annotation clustering (32, 33) and ingenuity pathway analysis (IPA) (Ingenuity Systems, Redwood, California) were used to characterize gene networks affected by NR2F2 knockdown (19, 34). Importantly, DAVID analysis of gene functions potentially regulated by NR2F2 in ES-2 cells revealed several cell structure annotation clusters as well as regulation of cell cycle and WNT/β-catenin signaling pathways. The annotation clusters identified by DAVID in TOV-112D cells were related chiefly to cell migration and germ cell development. Overlapping the top 2 networks identified by IPA and DAVID analysis in each cell line revealed a unique network centered on the tumor suppressor TP53 (tumor protein 53) and ubiquitin C (Figure 6). A role for NR2F2 in regulating ubiquitin C has not been previously described. Neither DAVID nor IPA revealed any functional annotation involved in angiogenesis.
Figure 6.
Pathway analysis. Merged IPA network analysis of ES-2 and TOV-112D profiled microarray samples.
Discussion
NR2F2 plays a role in vasculogenesis during normal human development. A number of recent studies have also suggested that this orphan nuclear receptor plays a key role in promoting cancer metastasis (2–4, 7, 8, 14, 26–29). Based on this, we hypothesized that NR2F2 plays an important role in ovarian cancer. The findings demonstrated here indicate that dysregulated NR2F2 expression is a robust feature of ovarian cancer and plays an important role in determining its clinical outcome.
A unique aspect of these observations is that the association between NR2F2 and time to disease recurrence was revealed only when both the epithelial and stromal compartments of ovarian cancer are individually considered. This suggests that dysregulated NR2F2 expression in each ovarian cancer compartment is important. Our inability to confirm these observations using recently released TCGA data does not detract from their potential significance because levels of NR2F2 and other genes reported by TCGA investigators were generated using homogenized specimens. TCGA specimens included varying amounts of epithelial and stromal tissue, which likely confounds their use to examine relationships between NR2F2 levels and outcomes. These relationships become further confounded when NR2F2 expression changes in opposite directions in the epithelial and stromal compartments. An important limitation to our current work is that we have been able to examine only a relatively limited number of ovarian cancer specimens by immunohistochemistry. Post hoc power calculations indicate that our pilot study was significantly underpowered (0.42) to achieve statistical significance (P < .05) when examining changes in NR2F2 expression in both the stromal and epithelial compartments. It is possible that more subtle associations between NR2F2 localization and ovarian cancer outcomes have been missed by our analyses. Thus, it will be important to confirm our observations in the future using a larger pool of ovarian cancer specimens.
We chose to initially study the function of dysregulated NR2F2 expression in ovarian cancer using cell lines derived from epithelial compartment of ovarian cancers. This decision was made largely because in vitro models suitable for studying the stromal compartment of different cancers are limited, and NR2F2 has a biologically effect in the epithelial compartment of colorectal cancer (9). Our results point toward a novel role for NR2F2 in regulating proliferation and apoptosis in ovarian cancer epithelia via NEK2. We have confirmed that the relationship between NR2F2 and NEK2 we observed on cDNA microarray is biologically relevant. As shown in Figure 5, targeting NEK2 in ovarian cancer cell lines results in increased rates of proliferation and apoptosis as predicted by our initial experiments examining the impact of NR2F2 knockdown. These observations are also consistent with recent reports indicating that the loss of Nr2f2 in the mesonephros of mice leads to increased rates of programmed cell death (35).
Our observations are also supported by a number of recent reports examining the role of NEK2 and RAI14 in other cells. NEK2 has been shown to mediate the ability of Ras to promote centrosome amplification and genomic instability in mammary epithelial cells (36) and breast cancer growth (37) and apoptosis in colon cancers (38). RAI14, also known as NORPEG, has been previously reported in rapidly proliferating cells (39). Given the intimate associations between cell cycle and apoptosis, both genes are reasonable candidates for mediating the impact of NR2F2 on ovarian cancer. The ability of NR2F2 to impact the expression of these 2 gene products may also explain the novel relationship between NR2F2 and TP53 uncovered by our pathway analyses. TP53 is frequently mutated in ovarian cancers, and it is well known that TP53-regulated signaling pathways are intimately linked to apoptosis as well as the cell cycle (25). It is not currently clear how altered expression of NR2F2, NEK2, or RAI14 function within the context of dysregulated TP53 signaling in ovarian cancer. From our in vitro proliferation and apoptosis data, the mechanisms by which NR2F2 contributes to ovarian cancer appear much more complex than we initially hypothesized. The direct effects of NR2F2 to regulate NEK2 and/or RAI14 are still unknown.
It is intriguing that NR2F2 impacted only proliferation in TP53 wild-type ES-2 cells whereas targeting NR2F2 has no impact on the proliferation of TP53 mutant TOV-112D cells. Because TOV-112D cells contain mutant β-catenin (40), the inability of NR2F2 to impact proliferation may stem from defects in the WNT signaling cascade inherent to these cells. NR2F2 has recently been shown to affect phosphatase and tensin homolog deleted from chromosome 10-mutant prostate cancers more aggressively (2). Thus, the specific genetic context in which NR2F2 exists is likely important and may help to explain why targeting NR2F2 failed to impact proliferation in TOV-112D cells despite its ability to impact NEK2 expression. It will be important to further dissect this genetic relationship in the future.
In conclusion, our observations have uncovered distinct patterns of dysregulated NR2F2 expression that appear to be important for promoting ovarian cancer. Future work will help to better understand the unique role of NR2F2 in each ovarian cancer compartment.
Acknowledgments
We thank Dr Ming-Jer Tsai for the NR2F2 antibody, Brooke Middlebrook for copyediting, and Dr Jan Rohozinski for reading the manuscript.
This work was supported by the Arthur Faris Resident Research Fund, an Idea Award, Women's Reproductive Health Research Grant 5K12HD050128, Partnership for Baylor College of Medicine, Young Texans Against Cancer, Cancer Fighters Fund, National Institutes of Health/National Institute of General Medical Sciences Grant T32 GM88129, and the Liz Tilberis Scholarship Ovarian Cancer Research Fund through the Estate of Agatha Fort.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- FIGO
- Fédération Internationale de Gynécologie et d'Obstétrique
- HR
- hazard risk
- IPA
- ingenuity pathway analysis
- NR2F2
- nuclear receptor subfamily 2, group F, member 2
- qPCR
- quantitative real-time PCR
- siRNA
- small interfering RNA
- TCGA
- The Cancer Genome Atlas.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90 [DOI] [PubMed] [Google Scholar]
- 2. Qin J, Wu SP, Creighton CJ, et al. COUP-TFII inhibits TGF-β-induced growth barrier to promote prostate tumorigenesis. Nature. 2013;493(7431):236–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin J, Chen X, Xie X, Tsai MJ, Tsai SY. COUP-TFII regulates tumor growth and metastasis by modulating tumor angiogenesis. Proc Natl Acad Sci USA. 2010;107(8):3687–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin J, Chen X, Yu-Lee LY, Tsai MJ, Tsai SY. Nuclear receptor COUP-TFII controls pancreatic islet tumor angiogenesis by regulating vascular endothelial growth factor/vascular endothelial growth factor receptor-2 signaling. Cancer Res. 2010;70(21):8812–8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le Dily F, Metivier R, Gueguen MM, et al. COUP-TFI modulates estrogen signaling and influences proliferation, survival and migration of breast cancer cells. Breast Cancer Res Treat. 2008;110(1):69–83 [DOI] [PubMed] [Google Scholar]
- 6. Litchfield LM, Riggs KA, Hockenberry AM, et al. Identification and characterization of nucleolin as a COUP-TFII coactivator of retinoic acid receptor β transcription in breast cancer cells. PloS one. 2012;7(5):e38278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schafer G, Wissmann C, Hertel J, Lunyak V, Hocker M. Regulation of vascular endothelial growth factor D by orphan receptors hepatocyte nuclear factor-4 α and chicken ovalbumin upstream promoter transcription factors 1 and 2. Cancer Res. 2008;68(2):457–466 [DOI] [PubMed] [Google Scholar]
- 8. Prahalad P, Dakshanamurthy S, Ressom H, Byers SW. Retinoic acid mediates regulation of network formation by COUP-TFII and VE-cadherin expression by TGFβ receptor kinase in breast cancer cells. PloS one. 2010;5(4):e10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin SW, Kwon HC, Rho MS, Choi HJ, Kwak JY, Park JI. Clinical significance of chicken ovalbumin upstream promoter-transcription factor II expression in human colorectal cancer. Oncol Rep. 2009;21(1):101–106 [PubMed] [Google Scholar]
- 10. Navab R, Gonzalez-Santos JM, Johnston MR, et al. Expression of chicken ovalbumin upstream promoter-transcription factor II enhances invasiveness of human lung carcinoma cells. Cancer Res. 2004;64(15):5097–5105 [DOI] [PubMed] [Google Scholar]
- 11. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting element. Mol Endocrinol. 1999;13(2):239–253 [DOI] [PubMed] [Google Scholar]
- 12. Lee DK, Kurihara I, Jeong JW, et al. Suppression of ERα activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24(5):930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kurihara I, Lee DK, Petit FG, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petit FG, Jamin SP, Kurihara I, et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci USA. 2007;104(15):6293–6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19(9):2299–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeitoun K, Takayama K, Michael MD, Bulun SE. Stimulation of aromatase P450 promoter (II) activity in endometriosis and its inhibition in endometrium are regulated by competitive binding of steroidogenic factor-1 and chicken ovalbumin upstream promoter transcription factor to the same cis-acting elements. Mol Endocrinol. 2006;13:239–253 [DOI] [PubMed] [Google Scholar]
- 17. Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J Biol Chem. 1997;272(50):31465–31474 [DOI] [PubMed] [Google Scholar]
- 18. Castro J, Torres M, Sovino H, Fuentes A, Boric MA, Johnson MC. P450Arom induction in isolated control endometrial cells by peritoneal fluid from women with endometriosis. Fertil Steril. 2010;94(7):2521–2527 [DOI] [PubMed] [Google Scholar]
- 19. Hawkins SM, Creighton CJ, Han DY, et al. Functional microRNA involved in endometriosis. Mol Endocrinol. 2011;25(5):821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-δ δ C(T)] method. Methods (San Diego, CA 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 21. Creighton CJ, Fountain MD, Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70(5):1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee BC, Cha K, Avraham S, Avraham HK. Microarray analysis of differentially expressed genes associated with human ovarian cancer. Int J Oncol. 2004;24(4):847–851 [PubMed] [Google Scholar]
- 23. Suzuki T, Moriya T, Darnel AD, Takeyama J, Sasano H. Immunohistochemical distribution of chicken ovalbumin upstream promoter transcription factor II in human tissues. Mol Cell Endocrinol. 2000;164(1–2):69–75 [DOI] [PubMed] [Google Scholar]
- 24. Sato Y, Suzuki T, Hidaka K, et al. Immunolocalization of nuclear transcription factors, DAX-1 and COUP-TF II, in the normal human ovary: correlation with adrenal 4 binding protein/steroidogenic factor-1 immunolocalization during the menstrual cycle. J Clin Endocrinol Metab. 2003;88(7):3415–3420 [DOI] [PubMed] [Google Scholar]
- 25. Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira FA, Tsai MJ, Tsai SY. COUP-TF orphan nuclear receptors in development and differentiation. Cell Mol Life Sci. 2000;57(10):1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mancini ML, Terzic A, Conley BA, Oxburgh LH, Nicola T, Vary CP. Endoglin plays distinct roles in vascular smooth muscle cell recruitment and regulation of arteriovenous identity during angiogenesis. Dev Dyn. 2009;238(10):2479–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diez H, Fischer A, Winkler A, et al. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313(1):1–9 [DOI] [PubMed] [Google Scholar]
- 29. Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104(5):576–588 [DOI] [PubMed] [Google Scholar]
- 30. Lau DH, Lewis AD, Ehsan MN, Sikic BI. Multifactorial mechanisms associated with broad cross-resistance of ovarian carcinoma cells selected by cyanomorpholino doxorubicin. Cancer Res. 1991;51(19):5181–5187 [PubMed] [Google Scholar]
- 31. Provencher DM, Lounis H, Champoux L, et al. Characterization of four novel epithelial ovarian cancer cell lines. In Vitro Cell Dev Biol Anim. 2000;36(6):357–361 [DOI] [PubMed] [Google Scholar]
- 32. Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 33. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57 [DOI] [PubMed] [Google Scholar]
- 34. Hawkins SM, Andreu-Vieyra CV, Jeong J-W, et al. Dysregulation of uterine signaling pathways in progesterone receptor-cre knockout of Dicer. Mol Endocrinol. 2012;26(9):1552–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu CT, Tang K, Suh JM, Jiang R, Tsai SY, Tsai MJ. COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival. Development. 2012;139(13):2330–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zeng X, Shaikh FY, Harrison MK, et al. The Ras oncogene signals centrosome amplification in mammary epithelial cells through cyclin D1/Cdk4 and Nek2. Oncogene. 2010;29(36):5103–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang S, Li W, Liu N, et al. Nek2A contributes to tumorigenic growth and possibly functions as potential therapeutic target for human breast cancer. J Cell Biochem. 2012;113(6):1904–1914 [DOI] [PubMed] [Google Scholar]
- 38. Suzuki K, Kokuryo T, Senga T, Yokoyama Y, Nagino M, Hamaguchi M. Novel combination treatment for colorectal cancer using Nek2 siRNA and cisplatin. Cancer Sci. 2010;101(5):1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kutty RK, Chen S, Samuel W, et al. Cell density-dependent nuclear/cytoplasmic localization of NORPEG (RAI14) protein. Biochem Biophys Res Commun. 2006;345(4):1333–1341 [DOI] [PubMed] [Google Scholar]
- 40. Samouelian V, Maugard CM, Jolicoeur M, et al. Chemosensitivity and radiosensitivity profiles of four new human epithelial ovarian cancer cell lines exhibiting genetic alterations in BRCA2, TGFβ-RII, KRAS2, TP53 and/or CDNK2A. Cancer Chemother Pharmacol. 2004;54(6):497–504 [DOI] [PubMed] [Google Scholar]