Abstract
Context:
Abnormal cortisol levels are a key pathophysiological indicator of post-traumatic stress disorder (PTSD). Endogenous normalization of cortisol concentration through exercise may be associated with PTSD symptom reduction.
Objective:
The aim of the study was to determine whether mindfulness-based stretching and deep breathing exercise (MBX) normalizes cortisol levels and reduces PTSD symptom severity among individuals with subclinical features of PTSD.
Design and Setting:
A randomized controlled trial was conducted at the University of New Mexico Health Sciences Center.
Participants:
Twenty-nine nurses (28 female) aged 45–66 years participated in the study.
Intervention:
Sixty-minute MBX sessions were conducted semiweekly for 8 weeks.
Main Outcome Measures:
Serum cortisol was measured, and the PTSD Checklist–Civilian version (PCL-C) was performed at baseline and weeks 4, 8, and 16.
Results:
Twenty-nine participants completed the study procedures, 22 (79%) with PTSD symptoms (MBX, n = 11; control, n = 11), and 7 (21%) without PTSD (BASE group). Eight-week outcomes for the MBX group were superior to those for the control group (mean difference for PCL-C scores, −13.6; 95% confidence interval [CI], −25.6, −1.6; P = .01; mean difference for serum cortisol, 5.8; 95% CI, 0.83, 10.8; P = .01). No significant differences were identified between groups in any other items. The changes in the MBX group were maintained at the 16-week follow-up (P = .85 for PCL-C; P = .21 for cortisol). Our data show that improved PTSD scores were associated with normalization of cortisol levels (P < .05).
Conclusions:
The results suggest that MBX appears to reduce the prevalence of PTSD-like symptoms in individuals exhibiting subclinical features of PTSD.
Post-traumatic stress disorder (PTSD) is an anxiety disorder that may develop in some people after exposure to traumatic events (1). In any given year, 7.7 million Americans over the age of 18 are diagnosed with PTSD (2). A recent study of intensive care unit nurses found them to be at high risk for PTSD resulting from repetitive exposure to extreme stressors, including high patient mortality and morbidity, daily exposure to traumatic events, and the inability to adjust to extreme environments, with 24% of participating nurses testing positive for PTSD symptoms (3). In the workplace, persistent PTSD symptoms lead to reduced job satisfaction and productivity, aversion to work (4), increased absenteeism (5), compassion fatigue (6), and burnout (7). Considering the 17% vacancy rate for critical care nurses and a predicted 114 000 vacant critical care nursing positions in the United States by 2015, PTSD may be a significant contributor to the increasing shortage of critical care nurses (3).
Although pharmacological and cognitive therapy interventions have proven efficacy in treating PTSD (8), residual symptoms remain problematic. In 2010, 39% of individuals with PTSD used complementary and alternative medicine interventions, including mind-body practices that incorporate stretching movements and postures with breathing (ie, yoga, tai chi, and qigong) (9). Substantial evidence has shown that mind-body interventions have a positive impact on quality of life, reduce stress, and improve health outcomes among individuals with PTSD (8, 10, 11)
In healthy individuals, the hypothalamic-pituitary-adrenal (HPA) axis functions to adapt the body to acute stressors through a cascade of hormonal responses, including the secretion of CRH in the hypothalamus, release of ACTH in the anterior pituitary gland, and secretion of glucocorticoids in the adrenal cortex (12). Chronic elevation of glucocorticoids, coupled with genetic factors, personal traumatic history, and unhealthy lifestyle, however, has deleterious effects on the body (13), leading to dysregulation of the HPA axis. Dysfunction of the HPA axis (14), characterized by abnormally high levels of CRH and low levels of cortisol, is one of the distinct neuroendocrine profiles that differentiates PTSD from other mental illnesses (15).
Cortisol plays a key role in the pathophysiology of PTSD. In healthy individuals, an increase in basal cortisol levels is associated with improvement in declarative memory and performance accompanied by normalized glucose metabolism in the limbic system (16). Individuals with PTSD symptoms have decreased basal cortisol levels (17), although not all studies agree with this finding (18–20), and a greater sensitivity of cortisol negative feedback inhibition in the HPA axis compared to those without PTSD (15). In persons with chronic PTSD, basal cortisol level increases are associated with symptom improvement (21).
Exercise is associated with transient increases in plasma cortisol (22) and improved cognitive function (16) among healthy individuals. Low- to moderate-intensity mind-body interventions have shown positive effects on PTSD symptoms (11). The question is whether there is an accompanying change in basal cortisol levels associated with the improvement in PTSD symptoms.
Individuals with PTSD have abnormally high secretion of CRH (23) but a low level of ACTH, the hormone responsible for inducing secretion of glucocorticoids in the adrenal cortex (24). Blunted ACTH response to CRH may lead to hyposecretion of ACTH in the pituitary gland, resulting in a decrease of cortisol production in the adrenal cortex (24). Studies indicate that dehydroepiandrosterone sulfate (DHEAS), an anabolic steroid molecule, may enhance resilience to stress via neuroprotection against cell death and behavioral deficits (25), and that exercise significantly elevates DHEAS levels (26). We therefore chose to measure plasma ACTH and serum DHEAS to examine whether a relationship between an exercise-induced decrease in PTSD symptom severity and an increase in the levels of serum DHEAS and plasma ACTH exists as a result of exercise.
The primary purpose of this study was to explore the underlying neuroendocrinological mechanism(s) of the improvement in symptom severity associated with mindfulness-based stretching and deep breathing exercise (MBX) by examining the changes in cortisol levels. We investigated whether low- to moderate-intensity exercise can induce endogenous normalization of basal cortisol. The intervention included a mindfulness component to enhance emotional regulation and cognitive function. We hypothesized that resting morning serum cortisol levels would normalize and PTSD symptom severity would decrease after an 8-week course of low- to moderate-intensity exercise.
Subjects and Methods
Study design and subjects
We conducted a 3-arm randomized controlled study. Nurse volunteers were recruited from the University of New Mexico (UNM) Hospital through advertisement, and they gave their informed written consent before participation. Inclusion criteria were age greater than 18 years and employment as a nurse at the UNM Hospital. Exclusion criteria included an inability to participate in the exercise program, a positive answer to any of the 7 screening questions on the Physical Activity Readiness Questionnaire, or current use of systemic glucocorticoid.
Subjects were screened for PTSD symptoms; those with PTSD Checklist–Civilian version (PCL-C) scores of at least 28 and a score of 3 or higher on 1 or more individual items were randomized, by a coin flip, into the exercise (MBX) group or the control (CON) group. Two researchers alternated flipping a coin, allowing the tossed coin to clatter to the floor. We chose to use this method for simplicity and convenience, although recent studies show that it may compromise the validity of the randomization (27). Participants who were not positive for PTSD were assigned to a healthy group (BASE) to collect normative data for cortisol in this population.
PTSD symptoms and fasting serum cortisol were assessed at an individually standardized time (around 8 am) at baseline and at weeks 4, 8, and 16. Participants were instructed to refrain from drinking alcohol, taking nonprescription drugs, and engaging in vigorous exercise for 72 hours before blood sampling. Compliance was verified by self-report. Study protocol and procedures were approved by the Human Research Protections Office at the UNM Health Sciences Center.
Mind-body intervention
A series of 16 standardized, semiweekly 60-minute MBX sessions were led by a trained instructor and conducted in the UNM Health Sciences Center Clinical and Translational Science Center. The intervention, created by the first author, consisted of stretching and balancing movements combined with breathing and a focus on mindfulness. Mindfulness is a quality of consciousness that is associated with control of attention and awareness (28), promoting a direct awareness of bodily movement, sensations, and surroundings, thus often inducing positive psychological and behavioral responses. Although mindfulness-based stress reduction has been cited as a “gold standard” (29), it was impractical for our target population due to its intensive time and resource requirements. Based on over 20 years of experience in teaching martial arts for persons with high stress levels, the first author developed the intervention. During the sessions, the participants were instructed to attend to the flow of each movement at the present moment, focusing on conscious regulation of inhalation, retention, and exhalation of the breath. Over the course of 8 weeks, the intensity of the exercise increased, but the sequence of the movements was the same.
Serum cortisol
Serum cortisol was measured after morning phlebotomy at baseline and weeks 4 and 8 for all groups. For the MBX group, serum cortisol was also measured at week 16. Serum cortisol was analyzed using the ADVIA Centaur XP Immunoassay System (Bayer HealthCare, Tarrytown, New York) at TriCore Reference Laboratories (Albuquerque, New Mexico).
ACTH and DHEAS
Plasma ACTH and serum DHEAS were measured after morning phlebotomy at baseline and postintervention and were analyzed at TriCore Reference Laboratories using the Immulite 2000 Immunoassay System (Siemens, Erlangen, Germany).
PCL-C scores
The PCL-C is a 17-item self-report instrument that measures the 17 Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) symptoms of PTSD, and it is commonly used to screen for PTSD (7). The instrument has been previously validated in individuals with PTSD, and it showed good test–retest reliability and internal consistency (30, 31). The criteria for inclusion in the PTSD symptomatic group were a PCL-C total score of at least 28 with a score of 3 or higher on 1 or more items. Participants did not undergo further clinical diagnostic testing for PTSD.
Sample size
To detect a reduction in PTSD symptom severity with a 2-sided 5% significance level and a power of 80%, the mean difference of PCL-C scores of 5.16 or greater requires a sample size of 20 participants for MBX and CON groups, given an anticipated dropout rate of 10%.
Statistical analysis
Data analyses were conducted using an a priori intention-to-treat approach including all participants who were randomly assigned. We conducted the Shapiro-Wilk W-tests for the assumption of normality and found no departure from normality in our data. To find the between-group differences of the intervention, we conducted t-tests comparing MBX and CON groups at postintervention. The analysis for the within-group difference was conducted using repeated measures ANOVA for both groups at baseline and week 8. We analyzed the potential effects of confounders using multivariate regression, employing the covariates of age, gender, ethnicity, education, marital status, smoking status, body mass index, and nursing experience (Table 1). For a dropout in the CON group, we used the conservative, last-observation-carried-forward method, using baseline values to replace missing postintervention outcome values.
Table 1.
Baseline Demographic and Clinical Characteristics
| MBX Group | CON Group | BASE Group | |
|---|---|---|---|
| n | 11 | 11 | 7 |
| Age, y | 47.6 (7.7) | 45.0 (10.0) | 44.6 (16.2) |
| BMI, kg/m2 | 26.9 (5.1) | 27.4 (7.3) | 25.5 (3.2) |
| Sex (female) | 10 (91%) | 11 (100%) | 7 (100%) |
| Smoking status | |||
| Ever smoked | 5 (45%) | 4 (36%) | 1 (14%) |
| Never smoked | 6 (55%) | 7 (64%) | 6 (86%) |
| Ethnic origin | |||
| White (not Hispanic or Latino) | 6 (55%) | 7 (64%) | 2 (29%) |
| Hispanic or Latino | 4 (36%) | 3 (27%) | 4 (57%) |
| African American | 1 (9%) | 1 (14%) | |
| American Indian | 1 (9%) | ||
| Education | |||
| College degree | 9 (82%) | 9 (82%) | 4 (57%) |
| Graduate degree | 2 (18%) | 2 (18%) | 3 (43%) |
| Marital status | |||
| Married/partnered | 6 (55%) | 6 (55%) | 3 (43%) |
| Unmarried/single | 5 (45%) | 5 (45%) | 4 (57%) |
| Nursing experience | |||
| Less than 5 y | 3 (27%) | 2 (18%) | 2 (29%) |
| 6–10 y | 2 (18%) | 1 (14%) | |
| 11–15 y | 1 (14%) | ||
| More than 15 y | 8 (73%) | 7 (64%) | 3 (43%) |
| PCL-C score | 43.1 (11.2) | 42.6 (12.7) | 21.8 (3.4) |
| Cortisol (μg/dL) | 9.6 (4.1) | 12.9 (6.9) | 13.9 (5.8) |
| ACTH (pg/mL) | 19.8 (10.8) | 11.8 (4.6) | 14.8 (5.4) |
| DHEAS (μg/dL) | 85.6 (33.7) | 100.7 (62.6) | 90.6 (66.0) |
Data are expressed as means (standard deviation) or number (percentage). P > .05 for all comparisons. BMI, body mass index.
Results
Study participants
Of 63 nurses screened, 29 met the inclusion criteria (Table 1). Of those, 22 were found to be PTSD symptomatic and were randomized into MBX or CON groups; 7 were assigned to the non-PTSD BASE group. Twenty-eight participants completed the study; 1 CON group member withdrew due to family problems (Figure 1). There was no significant difference in age, ethnicity, education, marital status, smoking status, or nursing experience between the MBX and CON groups.
Figure 1.
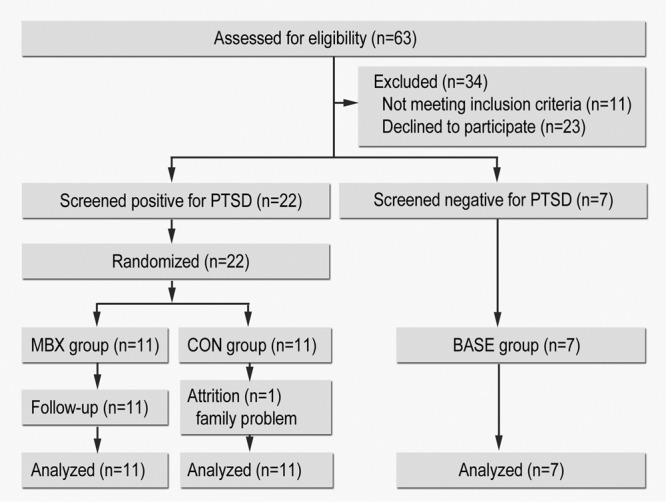
Flow diagram of the study progress through enrollment, intervention, follow-up, and data analysis.
As shown in Table 1, compliance among participants in the intervention was high, with 28 participants attending at least 75% of the 16 classes. More specifically, 1 participant (9%) attended 12 (75%) classes, 6 (55%) attended 13 (81%) classes, 3 (27%) attended 14 (88%) classes, and 1 (9%) attended 16 (100%) classes.
Effect of MBX on PTSD symptom severity
Eight-week outcomes for the MBX group were superior to those for the CON group (mean difference for PCL-C scores, −13.6; 95% confidence interval [CI], −25.6, −1.6; P = .01; mean difference for serum cortisol concentration, 5.8; 95% CI, 0.83, 10.8; P = .01). No significant differences were identified between groups in any other items. The repeated measures ANOVA on PCL-C scores was significant (P < .001), revealing a significant difference over time between groups. In the within-group analysis using t-testing (Table 2 and Figure 2A), we found that participation in the 8-week MBX significantly reduced PTSD symptom severity in the MBX group as measured by PCL-C (43.1 ± 11.2 vs 24.3 ± 3.3; 95% CI, 11.7, 25.9; P < .001). The changes in the MBX group were maintained at the 16-week follow-up (mean difference for PCL-C scores, 0.3; 95% CI, −2.9, 3.4; P = .85). Conversely, PCL-C scores in the CON group showed no significant change at 8 weeks (42.6 ± 12.7 vs 41.0 ± 16.3; 95% CI, −6.3, 9.5; P = .657).
Table 2.
Summary Results of PCL-C Scores and Cortisol at Pre- and Postintervention
| MBX Group (n = 11) |
Difference (95% CI) at 8 wk | Control Group (n = 11) |
Difference (95% CI) at 8 wk | |||
|---|---|---|---|---|---|---|
| Baseline | 8 wk | Baseline | 8 wk | |||
| Primary endpoint | ||||||
| PCL-C total | 43.1 (11.2) | 24.3 (3.3) | 18.8 (11.7 to 25.9) | 42.6 (12.7) | 41.0 (16.3) | 1.6 (−6.3 to 9.5) |
| PCL-C subtypes | ||||||
| Re-experiencing | 2.5 (0.7) | 1.4 (0.3) | − 1.1 (− 1.5 to − 0.6) | 2.6 (1.1) | 2.4 (1.1) | −0.2 (−0.9 to 0.4) |
| Avoidance | 2.5 (0.8) | 1.4 (0.3) | − 1.1 (− 1.6 to − 0.6) | 2.5 (0.6) | 2.5 (0.8) | −3.6 (−0.4 to 0.4) |
| Hyperarousal | 2.7 (0.9) | 1.5 (0.4) | − 1.2 (− 1.7 to − 0.6) | 2.8 (0.8) | 2.7 (1.1) | −0.1 (−0.6 to 0.4) |
| Secondary endpoint | ||||||
| Cortisol (μg/dL) | 9.6 (4.1) | 14.6 (5.7) | 5.1 (2.0 to 8.1) | 12.9 (6.9) | 13.8 (5.7) | 0.8 (−2.2 to 3.8) |
| ACTH (pg/mL) | 19.8 (10.8) | 22.2 (10.5) | 2.4 (−0.8 to 5.6) | 12.1 (5.1) | 15.5 (8.4) | 3.4 (−1.6 to 8.4) |
| DHEAS (μg/dL) | 85.6 (33.7) | 94.8 (46.4) | 9.2 (−5.4 to 23.7) | 91.9 (43.0) | 89.6 (43.2) | −2.2 (−27.1 to 22.6) |
Data are expressed as means (standard deviation).
Figure 2.
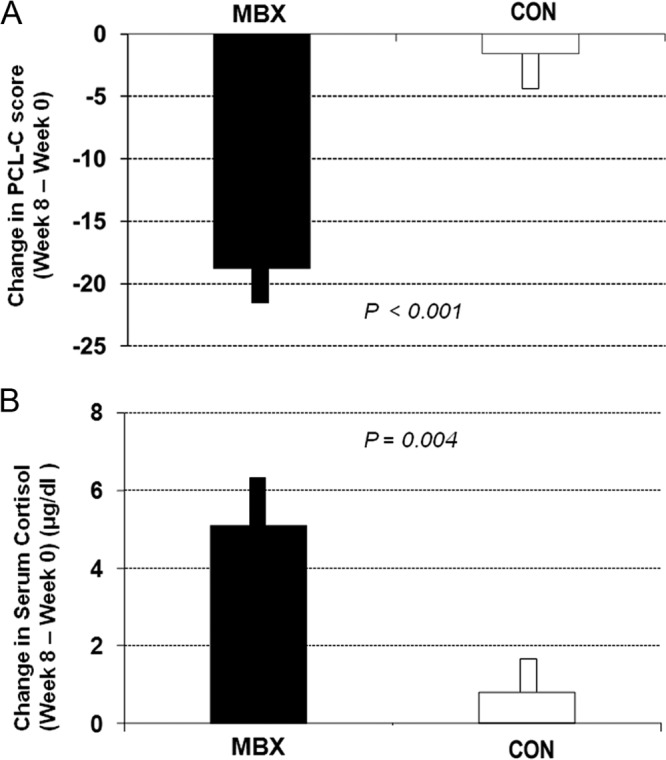
Change in PCL-C score (A) and serum cortisol (B) for the MBX (black bars) and CON (white bars) groups. Data are shown as mean ± SEM.
The PCL-C scores were regressed on selected variables to examine possible predictors. After conducting a backward selection regression with all potential predictors included, the model was significant (P = .003), with 31% of the variability in postintervention PCL-C scores explained by the model (adjusted R2 = 0.31). Participation in the MBX intervention (B = −15.18; P = .014) was the only significant predictor in the model.
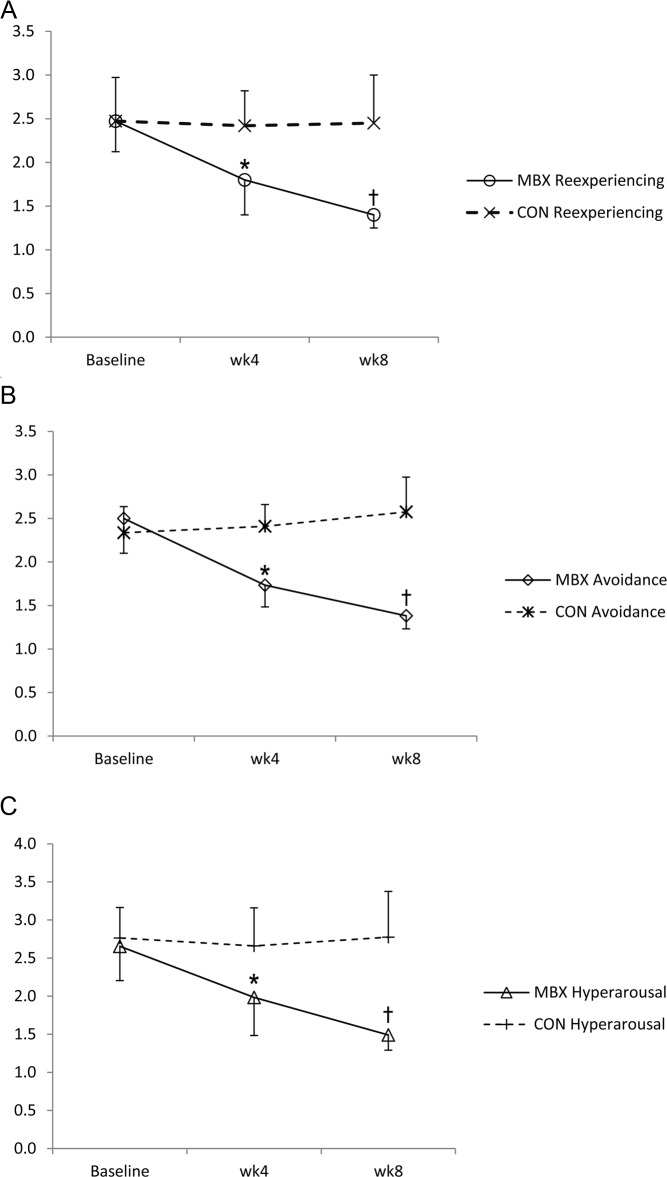
The 3 PTSD symptom subtypes of re-experiencing, avoidance, and hyperarousal were evaluated separately. As shown in Figure 3, the MBX group showed a significant reduction in each of the 3 PCL-C symptom subtypes. Mean re-experiencing scores decreased from 2.5 ± 0.7 to 1.4 ± 0.3 (P < .001), mean avoidance scores fell from 2.5 ± 0.8 to 1.4 ± 0.3 (P < .001), and mean hyperarousal scores decreased from 2.7 ± 0.9 to 1.5 ± 0.4 (P < .001). In the CON group, none of the PCL-C symptom subtypes were significantly changed from baseline (Table 2).
Figure 3.
Changes in PTSD symptom subtypes in the MBX group measured at baseline and weeks 4 and 8. A, Re-experiencing; B, avoidance; and C, hyperarousal. * P < .05; † P < .001.
Effect of MBX on serum cortisol
The repeated measures ANOVA on cortisol levels showed a significant difference over time between groups (P = .026). At postintervention week 8, basal serum cortisol concentrations were significantly higher in the MBX group than the CON group (P = .035; 95% CI, 8.8, 13.8). The t-test (Figure 2B) showed a significant increase in basal serum cortisol in the MBX group at postintervention (9.6 ± 4.1 vs 14.7 ± 5.7 μg/dL; 95% CI, 2.0, 8.1; P = .004). The CON group showed no significant change in serum cortisol concentration (12.9 ± 6.9 vs 13.8 ± 5.7 μg/dL; 95% CI, −2.2, 3.8; P = .549) (Table 2). There was no significant difference between serum cortisol concentration levels at baseline and week 8 in the BASE group (14.7 ± 5.4 vs 14.8 ± 8.9 μg/dL; P = .991). At the 16-wk follow-up, the changes of cortisol in the MBX group were maintained (14.7 ± 5.7 μg/dL at week 8 vs 13.5 ± 4.1 μg/dL at week 16; P = .599).
Effect of MBX on ACTH and DHEAS
No significant difference was found in plasma ACTH and serum DHEAS levels at week 8 in both groups.
Correlation of PTSD symptoms with hormone responses
In Figure 4, PCL-C data from baseline and week 8 are plotted as a function of serum cortisol for each of the participants in the MBX and CON groups (n = 43). The results show a significant relationship with a slope of −0.75 (P = .048). This indicates that for every 1 unit increase in cortisol there was a mean 0.75 point decrease in PCL-C score, demonstrating that as PTSD symptoms improved, cortisol levels normalized.
Figure 4.

PCL-C data from baseline and week 8 that are plotted as a function of serum cortisol for each of the participants in the MBX and CON groups (n = 43).
Discussion
To our knowledge, this is the first randomized controlled trial to examine the therapeutic benefits of MBX in nurses with PTSD symptoms using both biomarkers and self-reported symptom severity scores. This study demonstrates that participation in 8-week MBX is associated with a significant reduction of PTSD symptoms and an increase in serum cortisol in the MBX group compared to the CON group. Participation in an 8-week MBX program yielded a 41% reduction in PCL-C scores and a 67% increase in serum cortisol concentration, whereas the CON group showed a 4% decrease in PCL-C scores and a 17% increase in serum cortisol. The pre- and postintervention analysis shows that MBX significantly reduced PTSD symptom severity, whereas serum cortisol concentrations moved toward the range that is normal in healthy individuals. The effects were maintained for 8 weeks after the intervention. The improvement in cortisol suggested that normalization of cortisol levels may have occurred as a result of the intervention.
Considering that abnormally high secretion of CRH is associated with PTSD symptom severity (15), we speculate that the MBX-induced normalization of cortisol levels may have reduced CRH secretion in the hypothalamus, leading to decreases in PTSD symptom severity via negative feedback inhibition. This finding is consistent with previous studies demonstrating that administration of cortisol after trauma reduces the likelihood of developing PTSD (32) and decreases the retrieval of fear memories (33). It is not known, however, whether exercise-induced normalization in endogenous cortisol is directly comparable to exogenously administered cortisol with respect to PTSD symptoms. There were no significant changes in ACTH and DHEAS levels. This may be due to the small sample size of our study and the reliance on biomarker measurement taken at 1 point in time.
Further analyses show that MBX had comparable effects on all 3 symptom subtypes, resulting in improvement of both clinically and neurobiologically opposing symptom characteristics: undermodulated emotional dysregulation (re-experiencing and hyperarousal symptoms) and overmodulated emotional dysregulation (avoidance and numbness) (34). Lanius et al (34) categorized undermodulated symptoms as being mediated by the failure of prefrontal inhibition of limbic regions and overmodulated symptoms as resulting from midline prefrontal inhibition of the limbic regions. Our findings indicate that MBX may have potential as a PTSD intervention that addresses the normalization of both overmodulation and undermodulation of emotional dysregulation. Together, these data support our hypothesis that reduction in PTSD symptoms is positively associated with changes in cortisol levels as a result of participating in an 8-week MBX program.
During the 8-week program, some participants reported that they experienced improved sleep, stress resilience, energy levels, and emotion regulation under stress, and a resumption of pleasurable activities that they had previously discontinued. At the end of the intervention, over half of the participants expressed a desire to continue. Given the hurdles to compliance with current PTSD treatment options, the high levels of voluntary adherence to the MBX intervention suggest that it may be an attractive self-care option.
A unique component of MBX interventions vs typical low- to moderate-intensity exercise is the inclusion of slow, deep breathing in synchronization with the exercise movements. Slow breathing is known to have a balancing effect on the autonomic nervous system via enhanced parasympathetic activation. Slow and deep breathing stimulates stretch-induced inhibitory signals and hyperpolarizes currents propagated in cells, leading to synchronization of neural elements in the heart, lungs, limbic system, and cortex (35). Slow breathing also enhances vagal activity, leading to reduced psychophysiological arousal and decreased sympathetic activity and stress responses (36), and it is associated with reduced PTSD symptom severity (37).
Changes in cortisol concentrations may be important in understanding the pathogenesis of PTSD, clinical assessment of physiological responses to stress, and treatment of PTSD (38). Recent studies have shown that lifetime PTSD is associated with lower cortisol levels (39), and changes in cortisol predict clinical response to PTSD treatment (32). Individuals with PTSD and lower urinary cortisol levels are less responsive to psychotherapy treatment (32). Cortisol concentrations predict gene expression in the HPA axis as well as the brain and immune cell function (40).
Yehuda et al (40) reported that FK506-binding protein (FKBP506), a modulator of glucocorticoid receptor sensitivity, is associated with PTSD symptom severity and cortisol levels. Specifically, FKBP506 expression is reduced in PTSD and is predicted by cortisol levels (40). The authors suggested that lower cortisol levels may contribute to a progression to chronic, treatment-resistant PTSD via attenuation of peripheral catabolism of cortisol and suppression of HPA axis responsiveness (32).
This study is limited by the small number of participants, who were predominantly female nurses with PTSD symptoms. As such, there may be limited generalizability of the outcomes to males or individuals with combat-related trauma. Absence of a PTSD diagnosis may also limit the validity of the study. Although the PCL-C has shown its validity in screening for PTSD with a good test–retest reliability and internal consistency (30), there may be issues with generalizability to individuals with clinically diagnosed PTSD. Of the 63 volunteers who were assessed for eligibility for the study, 22 (34.9%) were PTSD symptomatic. This number is 10.9% higher than a previous study (3) that found 24% of participating intensive care unit nurses tested positive for PTSD. The discrepancy may be due to the absence of PTSD diagnosis, an influence of a selection bias, or greater prevalence of PTSD in this population. Another limitation may be treatment allocation bias in which the phlebotomy nurses could not be entirely blinded to the group assignment of participants. However, the samples were analyzed at an independent laboratory that was blinded to treatment allocation and other study details. However, given the small number of PTSD-related studies conducted exclusively on civilian female populations, our findings may shed light on characteristics specific to a population that has received little attention. It should be noted that our findings are also limited by the absence of participants' menstrual cycle data and the possibility that some participants may have continued MBX practice between weeks 8 and 16. We also acknowledge that the absence of exclusion of patients with depression could have contributed to increased cortisol levels in some participants. Future studies may need to consider the impact of comorbidity of PTSD with depression.
Finally, it is possible that there was a ceiling effect, that time played a role in the symptom improvement, or that the Hawthorne effect contributed to the observed changes. As seen in Table 2, the CON group showed a small but not significant positive change in symptom severity and serum cortisol levels. A similar phenomenon may account for a portion of the positive changes in the MBX group. As expected, a small but statistically significant elevation in baseline cortisol was observed in the MBX group compared to the BASE group (P = .04); and although the elevation in baseline cortisol of the CON group compared to the MBX group was not statistically significant (P = .068), the possibility of a selection effect remains. Our repeated measures ANOVA of cortisol levels attempts to address this imbalance by analyzing the group difference, which may not have corrected the problem. When we examined the individual data, we identified 1 CON group participant whose cortisol result constituted outliers at both baseline and week 8 (29.3 and 24.2 μg/dL, respectively), skewing the means higher in both cases. Our investigation showed that the result was not due to an error of measurement or recording. The value was legitimate, and therefore we did not exclude it from our analysis.
In summary, in this study of human endocrine and exercise physiology, we have demonstrated that after participation in an 8-week mind-body exercise intervention, participants experienced normalization in basal serum cortisol concentration with concurrent reduction in PTSD symptom severity. Considering that early intervention is critical in ameliorating the development of PTSD (10) and that PTSD symptoms are strongly correlated with the degree of distress immediately after trauma (10), mind-body interventions such as MBX may provide an effective nonpharmacological treatment for individuals with PTSD symptoms. Long-term studies to examine the neuroendocrine responses of cortisol and CRH, using blood oxygen level-dependent brain imaging and assessing long-term outcomes, should be undertaken.
Acknowledgments
We thank Michael Briggs and the staff at the University of New Mexico (UNM) Clinical and Translational Science Center and Kathy Lopez-Bushnell, Mary Blessing, and Sheena Ferguson at the UNM Hospital for assisting in conducting the study. We acknowledge Sara Newman and Carla Roybal for coordinating the study, Diana Gonzales-Pacheco for nutritional support, Mariana Gallegos and Jacqueline Torrez for phlebotomy, Patsy Lucero for scheduling, Julia Middendorf for assisting with the exercise class, and Gwenyth R. Wallen, RN, PhD, at the National Institutes of Health Clinical Center for editorial assistance. We also thank the nurses at the UNM Hospital who volunteered to participate in the study.
This work was supported by DHHS/NIH/NCATS UL1RR031977-01 and 5KL2RR031976-02, UNM Clinical and Translational Science Center.
ClinicalTrials.gov Identifier: NCT01462045.
Disclosure Summary: None of the authors have any duality of interest to declare regarding this study.
Footnotes
- CI
- confidence interval
- CON
- control
- DHEAS
- dehydroepiandrosterone sulfate
- HPA
- hypothalamic-pituitary-adrenal
- MBX
- mindfulness-based stretching and deep breathing exercise
- PCL-C
- PTSD Checklist–Civilian version
- PTSD
- post-traumatic stress disorder.
References
- 1. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-R). Revised 4th ed Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- 2. Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mealer ML, Shelton A, Berg B, Rothbaum B, Moss M. Increased prevalence of post-traumatic stress disorder symptoms in critical care nurses. Am J Respir Crit Care Med. 2007;175:693–697 [DOI] [PubMed] [Google Scholar]
- 4. Gates DM, Gillespie GL. Secondary traumatic stress in nurses who care for traumatized women. J Obstet Gynecol Neonatal Nurs. 2008;37:243–249 [DOI] [PubMed] [Google Scholar]
- 5. Laposa JM, Alden LE. Posttraumatic stress disorder in the emergency room: exploration of a cognitive model. Behav Res Ther. 2003;41:49–65 [DOI] [PubMed] [Google Scholar]
- 6. Potter P, Deshields T, Divanbeigi J, et al. Compassion fatigue and burnout: prevalence among oncology nurses. Clin J Oncol Nurs. 2010;14:E56–E62 [DOI] [PubMed] [Google Scholar]
- 7. Acker KH. Do critical care nurses face burnout, PTSD, or is it something else?: getting help for the helpers. AACN Clin Issues Crit Care Nurs. 1993;4:558–565 [PubMed] [Google Scholar]
- 8. Grodin MA, Piwowarczyk L, Fulker D, Bazazi AR, Saper RB. Treating survivors of torture and refugee trauma: a preliminary case series using qigong and t'ai chi. J Altern Complement Med. 2008;14:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Libby DJ, Pilver CE, Desai R. Complementary and alternative medicine use among individuals with posttraumatic stress disorder. Psychol Trauma. 2013;5:277–285 [Google Scholar]
- 10. Descilo T, Vedamurtachar A, Gerbarg PL, et al. Effects of a yoga breath intervention alone and in combination with an exposure therapy for post-traumatic stress disorder and depression in survivors of the 2004 South-East Asia tsunami. Acta Psychiatr Scand. 2010;121:289–300 [DOI] [PubMed] [Google Scholar]
- 11. Telles S, Naveen KV, Dash M. Yoga reduces symptoms of distress in tsunami survivors in the Andaman islands. Evid Based Complement Alternat Med. 2007;4:503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89 [DOI] [PubMed] [Google Scholar]
- 13. McEwen BS. The neurobiology and neuroendocrinology of stress. Implications for post-traumatic stress disorder from a basic science perspective. Psychiatr Clin North Am. 2002;25:469–494 [DOI] [PubMed] [Google Scholar]
- 14. Yehuda R, Golier J. Is there a rationale for cortisol-based treatments for PTSD? Expert Rev Neurother. 2009;9:1113–1115 [DOI] [PubMed] [Google Scholar]
- 15. Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–114 [DOI] [PubMed] [Google Scholar]
- 16. Twamley EW, Hami S, Stein MB. Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Res. 2004;126:265–274 [DOI] [PubMed] [Google Scholar]
- 17. Boscarino JA. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J Consult Clin Psychol. 1996;64:191–201 [DOI] [PubMed] [Google Scholar]
- 18. Mason JW, Wang S, Yehuda R, et al. Marked lability in urinary cortisol levels in subgroups of combat veterans with posttraumatic stress disorder during an intensive exposure treatment program. Psychosom Med. 2002;64:238–246 [DOI] [PubMed] [Google Scholar]
- 19. Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27:245–247 [DOI] [PubMed] [Google Scholar]
- 20. Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115 [DOI] [PubMed] [Google Scholar]
- 21. Aerni A, Traber R, Hock C, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490 [DOI] [PubMed] [Google Scholar]
- 22. Brandenberger G, Follenius M. Influence of timing and intensity of muscular exercise on temporal patterns of plasma cortisol levels. J Clin Endocrinol Metab. 1975;40:845–849 [DOI] [PubMed] [Google Scholar]
- 23. Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith MA, Davidson J, Ritchie JC, et al. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol Psychiatry. 1989;26:349–355 [DOI] [PubMed] [Google Scholar]
- 25. Rasmusson AM, Vasek J, Lipschitz DS, et al. An increased capacity for adrenal DHEA release is associated with decreased avoidance and negative mood symptoms in women with PTSD. Neuropsychopharmacology. 2004;29:1546–1557 [DOI] [PubMed] [Google Scholar]
- 26. Johnson LG, Kraemer RR, Haltom R, Kraemer GR, Gaines HE, Castracane VD. Effects of estrogen replacement therapy on dehydroepiandrosterone, dehydroepiandrosterone sulfate, and cortisol responses to exercise in postmenopausal women. Fertil Steril. 1997;68:836–843 [DOI] [PubMed] [Google Scholar]
- 27. Neuhauser D, Diaz M. Shuffle the deck, flip that coin: randomization comes to medicine. Qual Saf Health Care. 2004;13:315–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown KW, Ryan RM, Creswell JD. Mindfulness: theoretical foundations and evidence for its salutary effects. Psychol Inq. 2007;18:211–237 [Google Scholar]
- 29. Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43 [DOI] [PubMed] [Google Scholar]
- 30. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther. 1996;34:669–673 [DOI] [PubMed] [Google Scholar]
- 31. Weathers F, Litz B, Herman D, Huska J, Keane T. 1993 The PTSD Checklist (PCL): reliability, validity, and diagnostic utility. In: Proceedings from the 9th Annual Conference of the International Society for Traumatic Stress Studies; October 24–27, 1993; San Antonio, TX [Google Scholar]
- 32. Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the World Trade Center attacks on September 11, 2001. Psychoneuroendocrinology. 2009;34:1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, DE Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann NY Acad Sci. 2006;1071:46–53 [DOI] [PubMed] [Google Scholar]
- 34. Lanius RA, Vermetten E, Loewenstein RJ, et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167:640–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006;67:566–571 [DOI] [PubMed] [Google Scholar]
- 36. Dishman RK, Nakamura Y, Garcia ME, Thompson RW, Dunn AL, Blair SN. Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. Int J Psychophysiol. 2000;37:121–133 [DOI] [PubMed] [Google Scholar]
- 37. Cohen H, Kotler M, Matar MA, et al. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma-related reminder. Biol Psychiatry. 1998;44:1054–1059 [DOI] [PubMed] [Google Scholar]
- 38. Matousek RH, Dobkin PL, Pruessner J. Cortisol as a marker for improvement in mindfulness-based stress reduction. Complement Ther Clin Pract. 2010;16:13–19 [DOI] [PubMed] [Google Scholar]
- 39. Yehuda R, Halligan SL, Bierer LM. Cortisol levels in adult offspring of Holocaust survivors: relation to PTSD symptom severity in the parent and child. Psychoneuroendocrinology. 2002;27:171–180 [DOI] [PubMed] [Google Scholar]
- 40. Yehuda R, Cai G, Golier JA, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry. 2009;66:708–711 [DOI] [PubMed] [Google Scholar]