Abstract
GIPC1, GIPC2 and GIPC3 consist of GIPC homology 1 (GH1) domain, PDZ domain and GH2 domain. The regions around the GH1 and GH2 domains of GIPC1 are involved in dimerization and interaction with myosin VI (MYO6), respectively. The PDZ domain of GIPC1 is involved in interactions with transmembrane proteins [IGF1R, NTRK1, ADRB1, DRD2, TGFβR3 (transforming growth factorβ receptor type III), SDC4, SEMA4C, LRP1, NRP1, GLUT1, integrin α5 and VANGL2], cytosolic signaling regulators (APPL1 and RGS19) and viral proteins (HBc and HPV-18 E6). GIPC1 is an adaptor protein with dimerizing ability that loads PDZ ligands as cargoes for MYO6-dependent endosomal trafficking. GIPC1 is required for cell-surface expression of IGF1R and TGFβR3. GIPC1 is also required for integrin recycling during cell migration, angiogenesis and cytokinesis. On early endosomes, GIPC1 assembles receptor tyrosine kinases (RTKs) and APPL1 for activation of PI3K–AKT signaling, and G protein-coupled receptors (GPCRs) and RGS19 for attenuation of inhibitory Gα signaling. GIPC1 upregulation in breast, ovarian and pancreatic cancers promotes tumor proliferation and invasion, whereas GIPC1 downregulation in cervical cancer with human papillomavirus type 18 infection leads to resistance to cytostatic transforming growth factorβ signaling. GIPC2 is downregulated in acute lymphocytic leukemia owing to epigenetic silencing, while Gipc2 is upregulated in estrogen-induced mammary tumors. Somatic mutations of GIPC2 occur in malignant melanoma, and colorectal and ovarian cancers. Germ-line mutations of the GIPC3 or MYO6 gene cause nonsyndromic hearing loss. As GIPC proteins are involved in trafficking, signaling and recycling of RTKs, GPCRs, integrins and other transmembrane proteins, dysregulation of GIPCs results in human pathologies, such as cancer and hereditary deafness.
Keywords: actin dynamics, cancer antigen, endocytic transport, Frizzled, planar cell polarity, whole-genome sequencing
Introduction
GIPC1/GIPC (GAIP/RGS19-interacting protein),1 GIPC22 and GIPC33 are PDZ domain proteins that constitute the GIPC family.4 GIPC1 is also known as TIP2 (Tax-interacting protein 2),5 NIP [neuropilin 1 (NRP1)-interacting protein],6 GLUT1CBP [GLUT1 (SLC2A1) C-terminal binding protein],7 SEMCAP1 [Semaphorin 4C (SEMA4C)-interacting protein 1],8 Synectin [Syndecan 4 (SDC4)-interacting protein]9 and IIP1 [insulin-like growth factor-1 receptor (IGF1R)-interacting protein 1].10 GIPC3 is also known as DFNB15, DFNB72, DFNB95 and C19orf64.11, 12
Physiological roles of GIPC1 have been well characterized. The PDZ domain in the middle region of GIPC1 is involved in interaction with a variety of PDZ ligands, such as RGS19, NRP1, GLUT1, SEMA4C, SDC4 and IGF1R.1, 6, 7, 8, 9, 10 The N-terminal region of GIPC1 is involved in dimerization, whereas the C-terminal region of GIPC1 is involved in interaction with the retrograde motor protein, myosin VI (MYO6).13, 14, 15 On the basis of these protein–protein interactions, GIPC1 functions as an adaptor molecule for loading PDZ-target cargoes on the MYO6 motor protein (Figure 1a).
Figure 1.
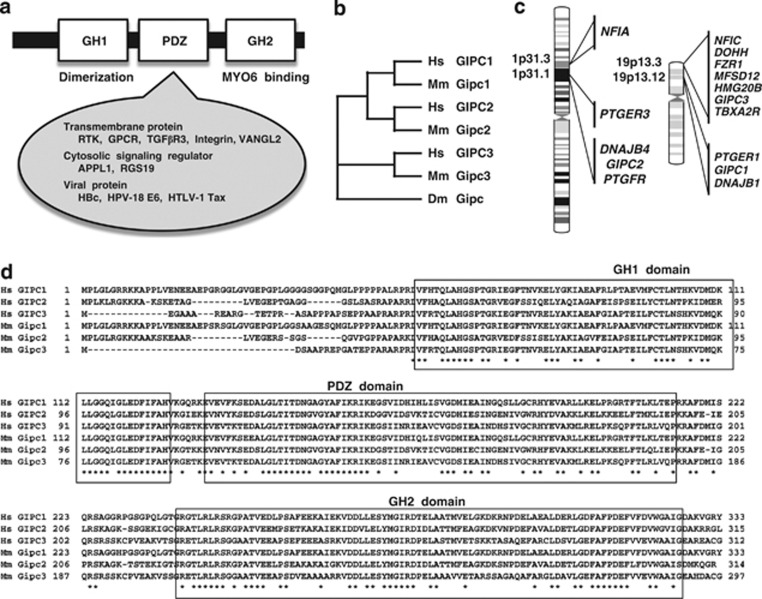
GIPC family. (a) Domain architecture of GIPC. GIPC family proteins consist of a GH1 domain, a PDZ domain and a GH2 domain. The regions around the GH1 and GH2 domains of GIPC1 are involved in dimerization and interaction with myosin VI (MYO6), respectively. The PDZ domain of GIPC1 is involved in interactions with transmembrane proteins, cytosolic signaling regulators and viral proteins. (b) Phylogenetic analyses of GIPC family proteins. GIPC1 and GIPC2 are more closely related. (c) Human chromosomal localization of GIPC family genes. (d) Alignment of human and mouse GIPC family proteins. The amino-acid position is shown on both sides of the alignment. Conserved amino acids are indicated by an asterisk below the alignment. The GH1 domain in the N-terminal part, PDZ domain in the middle part and GH2 domain in the C-terminal part are well conserved among GIPC family members. Dm, Drosophila; Hs, human; Mm, mouse.
GIPC family members are evolutionarily conserved with functional similarity.1, 2, 3, 4, 16, 17, 18, 19, 20, 21, 22, 23, 24 GIPCs are involved in the trafficking of various transmembrane proteins and regulate a variety of cellular processes, such as proliferation, planar cell polarity, cytokinesis and migration. Pathologies associated with the GIPCs, such as hearing loss11, 12 and cancer,25, 26, 27, 28, 29, 30, 31, 32, 33, 34 are emerging topics in the medical sciences. Here, the functional proteomics, evolutionary genetics, human genetics and cancer biology of the GIPC family members will be reviewed, with a focus on recent advances and future directions.
GIPC Family
The GIPC1 gene at human chromosome 19p13.12, the GIPC2 gene at human chromosome 1p31.1 and the GIPC3 gene at human chromosome 19p13.3 constitute the human GIPC gene family.4 Phylogenetic analysis of human GIPC1 (NP_005707.1), GIPC2 (NP_060125.4), GIPC3 (NP_573568.1), mouse Gipc1 (NP_061241.1), Gipc2 (NP_058563.1), Gipc3 (NP_683753.1) and Drosophila Gipc (NP_652028.1) proteins reveals that GIPC1 is the paralog of GIPC2 (Figure 1b). The GIPC1 gene is located between the PTGER1 and DNAJB1 genes; the GIPC2 gene adjoins the DNAJB4 gene and lies close to the PTGER3 gene. The PTGER1–GIPC1–DNAJB1 and PTGER3–DNAJB4–GIPC2 loci are paralogous regions in the human genome (Figure 1c).
GIPC family proteins consist of a GIPC homology 1 (GH1) domain, a PDZ domain and a GH2 domain (Figure 1a). The GH1, PDZ and GH2 domains are well conserved among GIPC1, GIPC2 and GIPC3 orthologs (Figure 1d), as previously reported.4
Protein–protein interactions of GIPC1
Protein–protein interactions have been comprehensively characterized for GIPC1, the founding member of the GIPC family (Figure 1a).
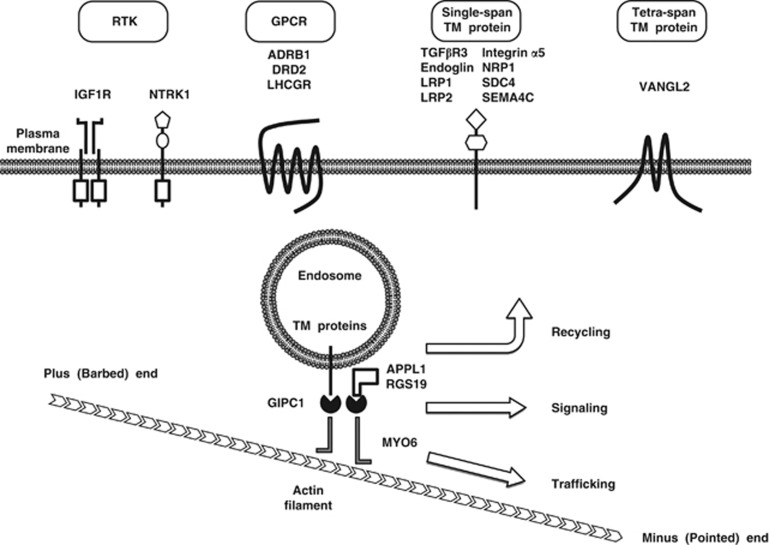
The PDZ domain of GIPC1 is involved in direct interactions with a variety of proteins, including adrenergic receptor β1 (ADRB1),35 APPL1,36, 37, 38 CD93 (C1QR1),39 dopamine receptor D2 (DRD2),40 endoglin,41 GLUT1,7 IGF1R,10, 27 integrin α5,42 integrin α6,42 luteinizing hormone/choriogonadotropin receptor (LHCGR),43 LRP1,44 LRP2 (megalin),44 NRP1,6, 45, 46 NTRK1 (TrkA),47 RGS19,1 SDC4,9 SEMA4C,8 transforming growth factorβ (TGFβ) receptor type III (TGFβR3),48, 49, 50 trophoblast glycoprotein (TPBG),51 TYRP152 and Vang-like 2 (VANGL2 or STB1).53 IGF1R and NTRK1 are receptor tyrosine kinases (RTKs) with a single transmembrane domain; ADRB1, DRD2 and LHCGR are G protein-coupled receptors (GPCRs) with seven transmembrane domains; CD93, endoglin, GLUT1, integrin α5, integrin α6, LRP1, LRP2, NRP1, SDC4, SEMA4C, TGFβR3, TPBG, TYRP1 and VANGL2 are also transmembrane proteins (Figure 2). APPL1 is a scaffold protein, interacting with GIPC1, NTRK1, Rab5, PIK3CA (the catalytic α subunit of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)) and AKT,36, 37, 38 while RGS19 is a regulator of G protein signaling, interacting with GIPC and G protein α inhibitory subunit 3.1 APPL1 and RGS19 are cytosolic proteins that regulate intracellular signaling. In addition to the cellular proteins mentioned above, the PDZ domain of GIPC1 directly interacts with viral proteins, such as HBc,54 E655 and Tax,5 which are derived from the hepatitis B virus, human papillomavirus type 18 (HPV-18) and human T-cell leukemia virus type 1, respectively. The majority of PDZ ligands for GIPC1 are transmembrane proteins, but a minority of them are cytosolic signaling regulators.
Figure 2.
GIPC1–MYO6 complex-dependent trafficking of endocytic vesicles. GIPC1 is an adaptor protein that can interact with trafficking cargoes, while MYO6 is a motor protein that can move along actin filaments from the plus (barbed) end near the plasma membrane to the minus (pointed) end in the cytoplasm. Representative cargoes of the GIPC1–MYO6 complex are classified into receptor tyrosine kinases (RTKs; IGF1R and NTRK1), G protein-coupled receptors (GPCRs; ADRB1, DRD2 and LHCGR), single-span transmembrane (TM) proteins (TGFβR3, endoglin, LRP1, LRP2, integrin α5, NRP1, SDC4 and SEMA4C), tetra-span TM protein (VANGL2) and cytosolic signaling regulators (APPL1 and RGS19). The GIPC1–MYO6 complex is directly involved in trafficking and signaling and is indirectly involved in the recycling of its cargoes.
The C-terminal region around the GH2 domain of GIPC1 can directly interact with MYO6, a member of the myosin family of motor proteins.13, 14, 15 MYO6 moves toward the minus (pointed) end of actin filaments located in the cytoplasm, whereas other myosin family proteins move toward the plus (barbed) end of actin filaments adjacent to the plasma membrane. MYO6 is a retrograde motor protein, involved in various cellular processes such as trafficking of early endosomes, cytokinesis and migration.56
The N-terminal region around the GH1 domain of GIPC1 is involved in dimerization.13 Because of its dimerization potential, GIPC1 is able to assemble PDZ-binding proteins as cargoes of the MYO6 motor protein in early endosomes (Figure 2).
Intracellular functions of GIPC1
Transmembrane proteins on the plasma membrane are internalized and packed into inside-out vesicles as a result of endocytosis.57 The endocytic vesicles are initially located in the periphery of the cytoplasm, beneath the plasma membrane, and are trafficked to early endosomes.58 As MYO6 is a motor protein moving along actin filaments from the barbed end near the plasma membrane to the pointed end in the cytoplasm, the GIPC1–MYO6 complex has a pivotal role in the trafficking of transmembrane proteins on endocytic vesicles (Figure 2). Most of transmembrane receptors on the early endosomes are returned to the plasma membrane directly or via recycling endosomes, although some of them are sorted for lysosomal degradation. GIPC1 is necessary for cell-surface expression of transmembrane receptors, such as IGF1R,27 LHCGR43 and TGFβR3.48
GIPC1 interacts with integrin α5 subunit,42 which is bound to integrin β subunit to form integrin heterodimers. Integrin α5β1 is trafficked to the early endosomes as a cargo of the GIPC1–MYO6 complex and is then sorted for recycling to the plasma membrane. As integrins are mechano-sensory receptors that bridge extracellular matrix and cytoplasmic adaptor proteins associated with actin filaments, integrin recycling to cell surface is required for the regulation of actin dynamisms.59, 60 Indeed, GIPC1 is required for the trafficking of internalized integrins during cell migration,19 angiogenesis61 and cytokinesis.15
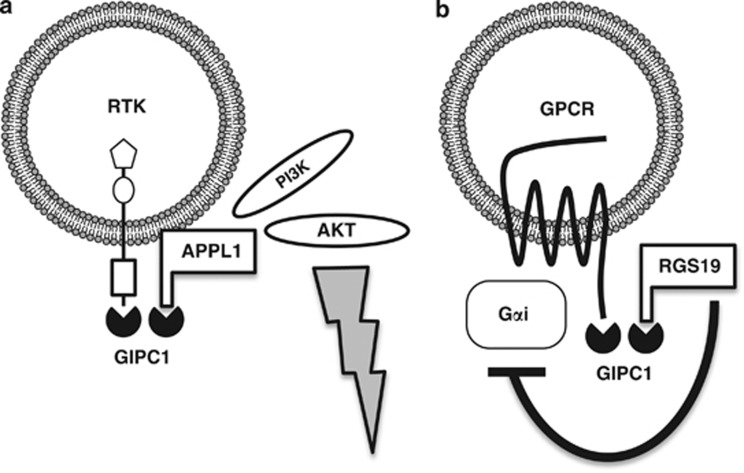
RTKs consist of an extracellular ligand-binding domain, a transmembrane domain and a cytoplasmic tyrosine kinase domain. RTKs are receptors for growth factors, such as epidermal growth factor, IGF1, nerve growth factor and fibroblast growth factor.62, 63, 64 The binding of ligands to RTKs induces their dimerization and autophosphorylation, which leads to the activation of RAS–ERK, PI3K–AKT, phospholipase Cγ (PLCγ) and other signaling cascades. RTKs are internalized as a result of ligand-induced dimerization. RTK signaling occurs from the plasma membrane as well as early endosomes.62, 63 GIPC1 binds to IGF1R and NTRK1 but not to insulin receptor,10, 47 and enhanced endosomal signaling occurs from NTRK1 but not from insulin receptor.63 As PI3K and AKT are recruited to the GIPC complex via interactions with APPL1, GIPC1 dimers induces clustering of RTK and APPL1 complex to early endosomes for the preferential activation of the PI3K–AKT signaling cascade rather than the RAS–ERK signaling cascade.36, 37, 38 GIPC1 is involved in the endosomal signaling from RTK to the PI3K–AKT signaling cascade (Figure 3a).
Figure 3.
Involvement of GIPC1 in endosomal signaling. (a) GIPC1-mediated early endosomal signaling from receptor tyrosine kinases (RTKs). IGF1R and NTRK1 are GIPC1-interacting RTKs, while APPL1 is a scaffold protein that interacts with GIPC1, RTK, Rab5, PI3K and AKT. GIPC1–RTK and GIPC1–APPL1 complexes are assembled on early endosomes through GIPC1 dimerization or RTK–APPL1 interaction. Internalized RTKs clustered with APPL1 preferentially activate the PI3K–AKT signaling cascade. (b) GIPC1-mediated early endosomal signaling from G protein-coupled receptors (GPCRs). Adrenergic receptor β1 (ADRB1) and dopamine receptor D2 (DRD2) are GIPC1-interacting GPCRs. ADRB1 transduces signals to Gαs and Gαi, while DRD2 transduces signals to Gαi. RGS19 is a GTPase activating protein that can inactivate Gαi. GIPC1–GPCR and GIPC1–RGS19 complexes are assembled on early endosomea through GIPC1 dimerization. RGS19 clustered with internalized GPCRs attenuates Gαi signaling.
GPCRs consist of extracellular ligand-binding regions, seven transmembrane domains and cytoplasmic G protein-binding regions. GPCRs are receptors for a variety of ligands, such as adrenaline (epinephrine), dopamine and WNT.65, 66 Ligand binding to GPCRs induces dissociation of heterotrimeric G proteins from GPCRs, leading to the activation of Gα- and Gβγ-mediated signaling cascades. The Gαs subunit activates adenylate cyclase to increase cyclic AMP concentrations. The inhibitory Gα (Gαi) subunit inhibits adenylate cyclase to decrease cyclic AMP concentrations. The Gαq subunit activates PLCβ. Gα12/13 subunit activates Rho GTPase. The Gβγ subunit activates PI3K, PLCβ and ion channels.67 GPCRs are internalized as a result of ligand-induced dissociation of the heterotrimeric G proteins and subsequent association with β-arrestin. GPCR signaling occurs from the plasma membrane as well as from early endosomes.68 As RGS19 functions as a GTPase-activating protein to inactivate Gαi, the dimerization of GIPC1 induces clustering of GPCR and RGS19 to the early endosomes for the attenuation of Gαi signaling.35, 40 GIPC1 is involved in the modulation of the endosomal GPCR signaling (Figure 3b).
Together, these facts indicate that GIPC1 regulates a variety of cellular processes, such as endosomal trafficking, signaling and recycling of RTKs, GPCRs, integrins and other transmembrane proteins.
Evolutionarily conservation of GIPCs
Kermit 1 and Kermit 2 are Xenopus orthologs of human GIPC2 and GIPC1, respectively. Kermit 1 interacts with WNT receptors, including Frizzled-3 (Fzd3) and Frizzled-7 (Fzd7), and is required for WNT signaling during neural crest development.16 Kermit 2 interacts with Igf1r similar to the interaction of human GIPC1 with IGF1R and is required for IGF1 signaling during eye development.17 Kermit 2 also interacts with the integrin α5 subunit to regulate endocytosis of α5β1 integrin, which is essential for the lining of the fibronectin matrix on the blastocoel roof during the gastrulation stage of embryogenesis.19
Zebrafish Gipc1 genetically interacts with Neuropilin-2 (Nrp2) and Vegfr3 (Flt4), and is involved in lymphangiogenic sprouting during thoracic duct formation.20 Nrp2 functions as a co-receptor for the Vegfr3 ligands, Vegf-c and Vegf-d. Nrp2 and Nrp1 are paralogs, sharing a common domain architecture, especially the C-terminal PDZ-binding site. As mouse Gipc1 directly interacts with the C-terminal tail of Nrp1,6 the interaction between Gipc1 and Nrp2 is thought to be required for lymphangiogenic signaling through the Nrp2–Vegfr3 receptor complex.
Drosophila gipc mRNA is upregulated during wing development21 and its overexpression causes planar cell polarity defects in the wing but not in the eye.22 Drosophila gipc does not genetically interact with core planar cell polarity components, such as frizzled, dishevelled, flamingo, strabismus (vang) and prickle; however, it does interact with jaguar, which is the Drosophila ortholog of human MYO6.
The Drosophila Gipc protein is expressed in the adult brain, especially in the dopaminergic neurons and glia.23 Drosophila gipc mutants show a reduction of dopaminergic neurons in the dorsomedial regions of the adult brain, which results in locomotor defects and reduced longevity. Although the interaction between Drosophila Gipc and the dopamine receptor remains unclear, interactions between rat Gipc and dopamine receptors suggest that Drosophila Gipc might also be involved in dopamine signaling.
Schistosoma japonicum is a pathogenic parasite that causes schistosomiasis. The expression level of S. japonicum gipc mRNA is relatively higher during the parasitic stages than during the cercarial stage, and S. japonicum Gipc interacts with the C-terminal tail of the glutamate receptor.24
GIPC proteins are conserved from mammals and other vertebrates to non-vertebrates, such as flies and worms, and some functions of GIPCs are also evolutionarily conserved.
GIPC3 mutations in familial hearing loss
Germ-line homozygous mutations of human GIPC3 are associated with familial hearing loss, such as the autosomal recessive nonsyndromic hearing impairment DFNB15, DFNB72 and DFNB95.11, 12, 69 Human GIPC3 is almost ubiquitously expressed in adult human tissues, and the expression level of GIPC3 is relatively high in the small intestine, lymph node, brain parietal lobe, fetal spleen and fetal thymus.3 G46R, M88I and G94D missense mutations are located within the GH1 domain; H170N and R189C missense mutations are located within the PDZ domain; T221I, G256D, L262R missense mutations, W301X nonsense mutation and A229GfsX10 frame-shift mutation are located within the GH2 domain. G46, M88, G94, H170, T221, G256, L262 and W301 amino-acid residues of GIPC3 are conserved in all human and mouse GIPC family members (Table 1).
Table 1. GIPC mutation spectra.
| Gene | Type | Mutation | Location | Disease | Conservation | Reference |
|---|---|---|---|---|---|---|
| GIPC1 | Somatic | F319L | GH2 domain | HNSCC | GIPC1, GIPC2 | 76 |
| GIPC2 | Somatic | F74Y | GH1 domain | Colorectal cancer | GIPC1, GIPC2, GIPC3 | 77 |
| Somatic | G102E | GH1 domain | Ovarian cancer | GIPC1, GIPC2, GIPC3 | 79 | |
| Somatic | D125N | PDZ domain | Malignant melanoma | GIPC1, GIPC2, GIPC3 | 78 | |
| Somatic | E216X | Colorectal cancer | 77 | |||
| Somatic | E288K | GH2 domain | Malignant melanoma | GIPC1, GIPC2 | 78 | |
| Somatic | R312Q | Colorectal cancer | 77 | |||
| GIPC3 | Germ line | G46R | GH1 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 12 |
| Somatic | E67K | GH1 domain | Breast cancer | GIPC1, GIPC3 | 80 | |
| Germ line | M88I | GH1 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 12 | |
| Germ line | G94D | GH1 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 12 | |
| Germ line | H170N | PDZ domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 69 | |
| Germ line | R189C | PDZ domain | Familial hearing loss | GIPC3 | 12 | |
| Germ line | T221I | GH2 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 12 | |
| Germ line | A229GfsX10 | GH2 domain | Familial hearing loss | 12 | ||
| Germ line | G256D | GH2 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 12 | |
| Germ line | L262R | GH2 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 11 | |
| Germ line | W301X | GH2 domain | Familial hearing loss | GIPC1, GIPC2, GIPC3 | 11 |
Abbreviations: GH2, GIPC homology 2; HNSCC, head and neck squamous cell carcinoma.
In line with hereditary deafness caused by mutations of human GIPC3, germ-line homozygous G115R mutations of Gipc3 occur in Black Swiss mice, which manifest progressive sensorineural hearing loss.11 Mouse Gipc3 mRNA is relatively highly expressed in the brain, lung and testes.70 G115 of mouse Gipc3, which corresponds to G130 of human GIPC3, is located within the PDZ domain. In Gipc3 mutant mice, the expression levels of the Gipc3 protein are decreased in the sensory hair cells of Corti's organ and their afferent neurons in the cochlear spiral ganglion, which is accompanied by disorientation and degradation of the stereocilia bundle of sensory hair cells and degradation of sensory neurons in the spiral ganglion, respectively.11
Cancer biology of GIPC family members
GIPC1 is upregulated in human cancers, such as breast cancer,25, 28, 29, 32 ovarian cancer26, 28, 34 and pancreatic cancer.25, 27, 31 GIPC1 is a cancer-associated auto-antigen, as anti-GIPC1 human monoclonal antibody was established from a breast cancer patient.29 Immunohistochemical analyses using the anti-GIPC1 human monoclonal antibody revealed that the GIPC1 protein is overexpressed in primary breast cancer and ovarian cancer.28 GIPC1 overexpression in ovarian cancer is associated with amplification and overexpression of the ADRM1 gene, encoding a transmembrane protein that interacts with the UCH37 deubiquitinating enzyme.34 GIPC1, which is involved in IGF1R stabilization, promotes proliferation and survival of pancreatic cancer cells and breast cancer cells.27, 31, 32 GIPC1 knockdown in cancer cells inhibits proliferation and promotes apoptosis. GIPC1 knockdown also results in G2 cell-cycle arrest and deceased motility in MDA-MB231 cells,33 because GIPC1 is involved in cytokinesis15 and cell migration.19 CR1023 (N-myristoyl-PSQSSSEA) is a cell-permeable octapeptide corresponding to the IGF1R-binding interface within the PDZ domain of GIPC1 that is able to inhibit the proliferation of IGF1R-dependent cancer cells by downregulating cell-surface IGF1R.31, 32 CR1166 (N-myristoyl-PSQSK(ɛN-4-bromobenzoyl)SK(ɛN-4-bromobenzoyl)A) is the most active CR1023 derivative that downregulates both IGF1R and EGFR.71
GIPC1 is downregulated in cervical cancer associated with HPV-18 infection.55 The E6 oncoprotein derived from HPV-18 induces poly-ubiquitination and proteasomal degradation of GIPC1 using mechanisms similar to those observed for p53, Scribbled (SCRIB), MUPP1, MAGI1, MAGI2 and MAGI3. As GIPC1 enhances the cell-surface expression of TGFβR3 and the cellular responsiveness to TGFβ,48 E6-mediated GIPC1 downregulation results in decreased sensitivity to cytostatic TGFβ signaling.55
GIPC2 is upregulated in gastric cancer,72 whereas it is downregulated in kidney cancer,2 acute lymphocytic leukemia (ALL)30 and adrenocortical carcinoma.73 The CpG island within the promoter region of the GIPC2 gene is hyper-methylated in all 23 leukemia cell lines, including MOLT4, Jurkat, Peer, T-ALL1, CEM, J-TAG, B-JAB, RS4, ALL1, Raji, REH and Ramos cells of lymphoid origin, as well as K562, BV173, HL60, NB4, THP1, U937, ML1, OCI, HEL, MOLM13 and KBM5R cells of myeloid origin.30 The GIPC2 promoter is also hyper-methylated in 29 of the 31 cases of ALL. As GIPC2 repression in MOLT4, Jurkat, CEM, RS4 and Raji cells is restored after 5-aza-2′-deoxycytidine treatment, GIPC2 downregulation in primary ALL cases is predicted to be the result of epigenetic silencing-associated promoter hyper-methylation.30
Mouse Gipc2 is upregulated in estrogen-induced mammary lesions of Caveolin-1 knockout mice, which resembles human ductal carcinoma in situ.74 Mouse Gipc2 is specifically co-expressed with Esr1, which encodes estrogen receptor (ER), and Keratin 18 (Krt18) in the ER+ luminal cells of the virgin mammary gland.75 These facts suggest the involvement of GIPC2 in human breast cancer of the ER+ luminal type.
Somatic mutations of GIPC family genes have been identified based on whole-exome or whole-genome sequencing. F319L missense mutation of GIPC1 occurs in head and neck squamous cell carcinoma.76 F74Y and R312Q missense mutations and E216X nonsense mutation of GIPC2 occur in colorectal cancer.77 D125N and E288K missense mutations of GIPC2 occur in malignant melanoma.78 G102E missense mutation of GIPC2 occurs in ovarian cancer.79 E67K missense mutation of GIPC3 occurs in breast cancer.80 The F74Y, G102E and D125N missense mutations might alter GIPC2 functions, because the F74, G102 and D125 amino-acid residues of GIPC2 are conserved in all human and mouse GIPC family members (Table 1). The E216X nonsense mutation of GIPC2 in colorectal cancer is a deleterious mutation that results in a loss of the MYO6-binding GH2 domain.
Conclusion
GIPC proteins function as adaptor molecules that assemble RTKs, GPCRs, integrins, transmembrane proteins and cytoplasmic signaling regulators as cargoes of MYO6-dependent endocytic transport. Germ-line mutations of the GIPC3 gene occur in nonsyndromic hearing loss. Somatic mutations of GIPC family genes occur in several types of human cancers, such as head and neck squamous cell carcinoma, colorectal cancer, malignant melanoma, ovarian cancer and breast cancer. GIPC1 can be oncogenic or tumor suppressive in a context-dependent manner. As GIPCs are involved in trafficking, signaling and recycling of receptors and adhesion molecules, GIPC dysregulation results in a spectrum of human diseases, such as cancer and hereditary deafness.
Perspectives
Germ-line mutations of the human GIPC3 gene occur in autosomal recessive nonsyndromic hearing loss, such as DFNB15, DFNB72 and DFNB95,11, 12, 69 while those of human MYO6 gene occur in other types of familial hearing loss, such as DFNA22 and DFNB37.81, 82 Mutations of mouse Gipc3 and Myo6 are also associated with hereditary hearing loss.11, 83 GIPC1 interacts with MYO6 using the GH2 domain,13, 14, 15 which is well conserved between GIPC1 and GIPC3 orthologs (Figure 1d). Taken together, these facts suggest that GIPC3 might directly interact with MYO6 to regulate sensorineural signaling in the cochlea of the inner ear.
It is interesting to ponder what the cargo of the putative GIPC3–MYO6 complex may be. Disorientation of the stereocilia bundle of sensory hair cells in Gipc3 mutant mice11 is similar to a ‘Frizzled' phenotype in the wing hair of Drosophila, which is caused by mutations of planar cell polarity genes, such as frizzled and vang.22, 84, 85, 86, 87 Xenopus Gipc2 directly interacts with Fzd3 and Fzd7, which are Xenopus homologs of Drosophila Frizzled.16 Mouse Gipc1 directly interacts with Vangl2, which is a mouse homolog of Drosophila Vang.53, 86, 88 As the PDZ domain is also well conserved among GIPC family members, Frizzled and Vangl family members might be cargoes of the GIPC3–MYO6 complex. To confirm this, PZD ligands for GIPC3 in sensory hair cells should be elucidated.
Rat Gipc1 directly interacts with dopamine receptors, such as DRD2 and DRD3,40, 89 and Drosophila Gipc is expressed in dopaminergic neurons.23 Germ-line mutations of the human DRD2 gene occur in myoclonus dystonia,90 while allelic variation of the human DRD3 gene is associated with schizophrenia91 and essential tremor.92 In addition, as juveniles Gipc3 mutant mice hyper-react to acoustic stimulation by running around in an uncontrolled manner and having seizures, but they become resistant to loud noise at 6 weeks old, owing to the progression of hearing impairment.11 Germ-line mutations of GIPC family genes in neurological diseases, such as myoclonus dystonia, schizophrenia, essential tremor and juvenile epilepsy, might be discovered based on whole-genome or whole-exome sequencing.
GIPC1 upregulation in breast and pancreatic cancers leads to tumor proliferation through IGF1R stabilization and tumor invasion through integrin recycling, whereas GIPC1 downregulation in cervical cancer results in decreased sensitivity to cytostatic signaling through TGFβR3 destabilization. CR1023 and its derivatives, which block the interaction between GIPC1 and IGF1R, inhibit proliferation of IGF1R-dependent tumors. However, these GIPC1 inhibitors might promote proliferation of TGFβ-sensitive tumors. Patients should be rigorously selected for clinical application of GIPC1 inhibitors, including CR1023 and its derivatives. Specific inhibitors of GIPC1 targets, such as the small-molecule IGF1R inhibitor and anti-IGF1R human antibody, might be preferable as therapeutic choices for cancer patients.
Acknowledgments
This work was supported in part by a grant-in-aid for the National Cancer Center Research and Development Fund.
The author declares no conflict of interest.
References
- De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG. GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci USA. 1998;95:12340–12345. doi: 10.1073/pnas.95.21.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikoshi H, Katoh M. Molecular cloning and characterization of human GIPC2, a novel gene homologous to human GIPC1 and Xenopus Kermit. Int J Oncol. 2002;20:571–576. [PubMed] [Google Scholar]
- Saitoh T, Mine T, Katoh M. Molecular cloning and characterization of human GIPC3, a novel gene homologous to human GIPC1 and GIPC2. Int J Oncol. 2002;20:577–582. [PubMed] [Google Scholar]
- Katoh M. GIPC gene family. Int J Mol Med. 2002;9:585–589. [PubMed] [Google Scholar]
- Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene. 1998;16:643–654. doi: 10.1038/sj.onc.1201567. [DOI] [PubMed] [Google Scholar]
- Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn R, Jensen M, Reed B. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 1999;10:819–832. doi: 10.1091/mbc.10.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Kalb RG, Strittmatter SM. A PDZ protein regulates the distribution of the transmembrane semaphorin, M-SemF. J Biol Chem. 1999;274:14137–14146. doi: 10.1074/jbc.274.20.14137. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li M, Chen W, Simons M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J Cell Physiol. 2000;184:373–379. doi: 10.1002/1097-4652(200009)184:3<373::AID-JCP12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ligensa T, Krauss S, Demuth D, Schumacher R, Camonis J, Jaques G, et al. A PDZ domain protein interacts with the C-terminal tail of the insulin-like growth factor-1 receptor but not with the insulin receptor. J Biol Chem. 2001;276:33419–33427. doi: 10.1074/jbc.M104509200. [DOI] [PubMed] [Google Scholar]
- Charizopoulou N, Lelli A, Schraders M, Ray K, Hildebrand MS, Ramesh A, et al. Gipc3 mutations associated with audiogenic seizures and sensorineural hearing loss in mouse and human. Nat Commun. 2011;2:201. doi: 10.1038/ncomms1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Gul K, Morell RJ, Lee K, Ahmed ZM, Riazuddin S, et al. Mutations of GIPC3 cause nonsyndromic hearing loss DFNB72 but not DFNB81 that also maps to chromosome 19p. Hum Genet. 2011;130:759–765. doi: 10.1007/s00439-011-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BC, Cefalu C, Bellaire BH, Cardelli JA, Louis T, Salamon J, et al. GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol Biol Cell. 2005;16:4183–4201. doi: 10.1091/mbc.E04-11-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci USA. 2006;103:12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden SD, Puri C, Au JS, Kendrick-Jones J, Buss F. Myosin VI is required for targeted membrane transport during cytokinesis. Mol Biol Cell. 2007;18:4750–4761. doi: 10.1091/mbc.E07-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C, Deardorff MA, Saint-Jeannet JP, Yang J, Arzoumanian A, Klein PS. Kermit, a frizzled interacting protein, regulates frizzled 3 signaling in neural crest development. Development. 2001;128:3665–3674. doi: 10.1242/dev.128.19.3665. [DOI] [PubMed] [Google Scholar]
- Wu J, O'Donnell M, Gitler AD, Klein PS. Kermit 2/XGIPC, an IGF1 receptor interacting protein, is required for IGF signaling in Xenopus eye development. Development. 2006;133:3651–3660. doi: 10.1242/dev.02547. [DOI] [PubMed] [Google Scholar]
- Vamathevan JJ, Hasan S, Emes RD, Amrine-Madsen H, Rajagopalan D, Topp SD, et al. The role of positive selection in determining the molecular cause of species differences in disease. BMC Evol Biol. 2008;8:273. doi: 10.1186/1471-2148-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer E, Suckert C, Al-Attar H, Marsden M. Integrin α5β1 function is regulated by XGIPC/kermit2 mediated endocytosis during Xenopus laevis gastrulation. PLoS ONE. 2010;5:e10665. doi: 10.1371/journal.pone.0010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans K, Claes F, Vandevelde W, Zheng W, Geudens I, Orsenigo F, et al. Role of synectin in lymphatic development in zebrafish and frogs. Blood. 2010;116:3356–3366. doi: 10.1182/blood-2009-11-254557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N, Zhu C, Lee H, Adler PN. Gene expression during Drosophila wing morphogenesis and differentiation. Genetics. 2005;171:625–638. doi: 10.1534/genetics.105.043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane A, Mlodzik M. The Drosophila GIPC homologue can modulate myosin based processes and planar cell polarity but is not essential for development. PLoS ONE. 2010;5:e11228. doi: 10.1371/journal.pone.0011228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Ko S, Kim-Ha J. dGIPC is required for the locomotive activity and longevity in Drosophila. Biochem Biophys Res Commun. 2010;402:565–570. doi: 10.1016/j.bbrc.2010.10.095. [DOI] [PubMed] [Google Scholar]
- Mu Y, Huang H, Liu S, Cai P, Gao Y. Molecular characterization and ligand binding specificity of the PDZ domain-containing protein GIPC3 from Schistosoma japonicum. Parasit Vectors. 2012;5:227. doi: 10.1186/1756-3305-5-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikoshi H, Katoh M. Expression of human GIPC1 in normal tissues, cancer cell lines, and primary tumors. Int J Mol Med. 2002;9:509–513. [PubMed] [Google Scholar]
- Heinzelmann-Schwarz VA, Gardiner-Garden M, Henshall SM, Scurry J, Scolyer RA, Davies MJ, et al. Overexpression of the cell adhesion molecules DDR1, Claudin 3, and Ep-CAM in metaplastic ovarian epithelium and ovarian cancer. Clin Cancer Res. 2004;10:4427–4436. doi: 10.1158/1078-0432.CCR-04-0073. [DOI] [PubMed] [Google Scholar]
- Muders MH, Dutta SK, Wang L, Lau JS, Bhattacharya R, Smyrk TC, et al. Expression and regulatory role of GAIP-interacting protein GIPC in pancreatic adenocarcinoma. Cancer Res. 2006;66:10264–10268. doi: 10.1158/0008-5472.CAN-06-2321. [DOI] [PubMed] [Google Scholar]
- Yavelsky V, Rohkin S, Shaco-Levy R, Tzikinovsky A, Amir T, Kohn H, et al. Native human autoantibodies targeting GIPC1 identify differential expression in malignant tumors of the breast and ovary. BMC Cancer. 2008;8:247. doi: 10.1186/1471-2407-8-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudchenko S, Scanlan M, Kalantarov G, Yavelsky V, Levy C, Estabrook A, et al. A human monoclonal autoantibody to breast cancer identifies the PDZ domain containing protein GIPC1 as a novel breast cancer-associated antigen. BMC Cancer. 2008;8:248. doi: 10.1186/1471-2407-8-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang SQ, Tong WG, Yang H, Lin W, Lee MK, Fang ZH, et al. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia. Leukemia. 2008;22:1529–1538. doi: 10.1038/leu.2008.130. [DOI] [PubMed] [Google Scholar]
- Muders MH, Vohra PK, Dutta SK, Wang E, Ikeda Y, Wang L, et al. Targeting GIPC/synectin in pancreatic cancer inhibits tumor growth. Clin Cancer Res. 2009;15:4095–4103. doi: 10.1158/1078-0432.CCR-08-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Paek AR, Kim SY, You HJ. GIPC mediates the generation of reactive oxygen species and the regulation of cancer cell proliferation by insulin-like growth factor-1/IGF-1R signaling. Cancer Lett. 2010;294:254–263. doi: 10.1016/j.canlet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Chittenden TW, Pak J, Rubio R, Cheng H, Holton K, Prendergast N, et al. Therapeutic implications of GIPC1 silencing in cancer. PLoS ONE. 2010;5:e15581. doi: 10.1371/journal.pone.0015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejzo MS, Ginther C, Dering J, Anderson L, Venkatesan N, Konecny G, et al. Knockdown of ovarian cancer amplification target ADRM1 leads to downregulation of GIPC1 and upregulation of RECK. Genes Chromosomes Cancer. 2011;50:434–441. doi: 10.1002/gcc.20868. [DOI] [PubMed] [Google Scholar]
- Hu LA, Chen W, Martin NP, Whalen EJ, Premont RT, Lefkowitz RJ. GIPC interacts with the β1-adrenergic receptor and regulates β1-adrenergic receptor-mediated ERK activation. J Biol Chem. 2003;278:26295–26301. doi: 10.1074/jbc.M212352200. [DOI] [PubMed] [Google Scholar]
- Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, Grimes ML, et al. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol. 2006;26:8928–8941. doi: 10.1128/MCB.00228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, et al. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol. 2006;26:8942–8952. doi: 10.1128/MCB.00305-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Bohlson SS, Zhang M, Ortiz CE, Tenner AJ. CD93 interacts with the PDZ domain-containing adaptor protein GIPC: implications in the modulation of phagocytosis. J Leukoc Biol. 2005;77:80–89. doi: 10.1189/jlb.0504305. [DOI] [PubMed] [Google Scholar]
- Jeanneteau F, Guillin O, Diaz J, Griffon N, Sokoloff P. GIPC recruits GAIP (RGS19) to attenuate dopamine D2 receptor signaling. Mol Biol Cell. 2004;15:4926–4937. doi: 10.1091/mbc.E04-04-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ray B, How T, Blobe GC. Endoglin promotes transforming growth factor β-mediated Smad 1/5/8 signaling and inhibits endothelial cell migration through its association with GIPC. J Biol Chem. 2008;283:32527–32533. doi: 10.1074/jbc.M803059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani TT, Mercurio AM. PDZ interaction sites in integrin α subunits: TIP-2/GIPC binds to a type I recognition sequence in α6A/α5 and a novel sequence in α6B. J Biol Chem. 2001;276:36535–36542. doi: 10.1074/jbc.M105785200. [DOI] [PubMed] [Google Scholar]
- Hirakawa T, Galet C, Kishi M, Ascoli M. GIPC binds to the human lutropin receptor (hLHR) through an unusual PDZ domain binding motif, and it regulates the sorting of the internalized human choriogonadotropin and the density of cell surface hLHR. J Biol Chem. 2003;278:49348–49357. doi: 10.1074/jbc.M306557200. [DOI] [PubMed] [Google Scholar]
- Gotthardt G, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, et al. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275:25616–25624. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- Prud'homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Yano H, Lee F, Chao MV, Farquhar MG. GIPC and GAIP form a complex with TrkA: a putative link between G protein and receptor tyrosine kinase pathways. Mol Biol Cell. 2001;12:615–627. doi: 10.1091/mbc.12.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobe GC, Liu X, Fang SJ, How T, Lodish HF. A novel mechanism for regulating transforming growth factor β (TGF-β) signaling: functional modulation of type III TGF-β receptor expression through interaction with the PDZ domain protein, GIPC. J Biol Chem. 2001;276:39608–39617. doi: 10.1074/jbc.M106831200. [DOI] [PubMed] [Google Scholar]
- Finger EC, Turley RS, Dong M, How T, Fields TA, Blobe GC. TβRIII suppresses non-small cell lung cancer invasiveness and tumorigenicity. Carcinogenesis. 2008;29:528–535. doi: 10.1093/carcin/bgm289. [DOI] [PubMed] [Google Scholar]
- Townsend TA, Robinson JY, How T, DeLaughter DM, Blobe GC, Barnett JV. Endocardial cell epithelial-mesenchymal transformation requires Type III TGFβ receptor interaction with GIPC. Cell Signal. 2012;24:247–256. doi: 10.1016/j.cellsig.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awan A, Lucic MR, Shaw DM, Sheppard F, Westwater C, Lyons SA, et al. 5T4 interacts with TIP-2/GIPC, a PDZ protein, with implications for metastasis. Biochem Biophys Res Commun. 2002;290:1030–1036. doi: 10.1006/bbrc.2001.6288. [DOI] [PubMed] [Google Scholar]
- Liu TF, Kandala G, Setaluri V. PDZ domain protein GIPC interacts with the cytoplasmic tail of melanosomal membrane protein gp75 (tyrosinase-related protein-1) J Biol Chem. 2001;276:35768–35777. doi: 10.1074/jbc.M103585200. [DOI] [PubMed] [Google Scholar]
- Giese AP, Ezan J, Wang L, Lasvaux L, Lembo F, Mazzocco C, et al. Gipc1 has a dual role in Vangl2 trafficking and hair bundle integrity in the inner ear. Development. 2012;139:3775–3785. doi: 10.1242/dev.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razanskas R, Sasnauskas K. Interaction of hepatitis B virus core protein with human GIPC1. Arch Virol. 2010;155:247–250. doi: 10.1007/s00705-009-0561-z. [DOI] [PubMed] [Google Scholar]
- Favre-Bonvin A, Reynaud C, Kretz-Remy C, Jalinot P. Human papillomavirus type 18 E6 protein binds the cellular PDZ protein TIP-2/GIPC, which is involved in transforming growth factor beta signaling and triggers its degradation by the proteasome. J Virol. 2005;79:4229–4237. doi: 10.1128/JVI.79.7.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss F, Kendrick-Jones J. How are the cellular functions of myosin VI regulated within the cell. Biochem Biophys Res Commun. 2008;369:165–175. doi: 10.1016/j.bbrc.2007.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- Torres VA, Stupack DG. Rab5 in the regulation of cell motility and invasion. Curr Protein Pept Sci. 2011;12:43–51. doi: 10.2174/138920311795659461. [DOI] [PubMed] [Google Scholar]
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, et al. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2011;12:104–117. doi: 10.1038/nrm3048. [DOI] [PubMed] [Google Scholar]
- Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- Katoh M. FGFR2 abnormalities underlie a spectrum of bone, skin, and cancer pathologies. J Invest Dermatol. 2009;129:1861–1867. doi: 10.1038/jid.2009.97. [DOI] [PubMed] [Google Scholar]
- Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Lappano R, Maggiolini M. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov. 2011;10:47–60. doi: 10.1038/nrd3320. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature. 2013;495:534–538. doi: 10.1038/nature12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirmaci A, Edwards YJ, Akay H, Tekin M. Challenges in whole exome sequencing: an example from hereditary deafness. PLoS ONE. 2012;7:e32000. doi: 10.1371/journal.pone.0032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T, Mine T, Katoh M. Molecular cloning and characterization of mouse Gipc3. Int J Mol Med. 2002;9:251–256. [PubMed] [Google Scholar]
- Patra CR, Rupasinghe CN, Dutta SK, Bhattacharya S, Wang E, Spaller MR, et al. Chemically modified peptides targeting the PDZ domain of GIPC as a therapeutic approach for cancer. ACS Chem Biol. 2012;7:770–779. doi: 10.1021/cb200536r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikoshi H, Katoh M. Up-regulation of GIPC2 in human gastric cancer. Int J Oncol. 2002;20:1183–1187. [PubMed] [Google Scholar]
- Fernandez-Ranvier GG, Weng J, Yeh R, Khanafshar E, Suh I, Barker C, et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg. 2008;143:841–846. doi: 10.1001/archsurg.143.9.841. [DOI] [PubMed] [Google Scholar]
- Mercier I, Casimiro MC, Zhou J, Wang C, Plymire C, Bryant KG, et al. Genetic ablation of caveolin-1 drives estrogen-hypersensitivity and the development of DCIS-like mammary lesions. Am J Pathol. 2009;174:1172–1190. doi: 10.2353/ajpath.2009.080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick H, Regan JL, Magnay FA, Grigoriadis A, Mitsopoulos C, Zvelebil M, et al. Transcriptome analysis of mammary epithelial subpopulations identifies novel determinants of lineage commitment and cell fate. BMC Genomics. 2008;9:591. doi: 10.1186/1471-2164-9-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, Berchuck A, Birrer M, Chien J, Cramer D, Dao F, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Melchionda S, Ahituv N, Bisceglia L, Sobe T, Glaser F, Rabionet R, et al. MYO6, the human homologue of the gene responsible for deafness in Snell's waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, et al. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, et al. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation. Trends Genet. 2002;18:564–571. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Katoh M. WNT/PCP signaling pathway and human cancer. Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. doi: 10.1016/j.semcdb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Katoh M. Strabismus (STB)/Vang-like (VANGL) gene family. Int J Mol Med. 2002;10:11–15. [PubMed] [Google Scholar]
- Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004;15:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Gurvich N, Sena-Esteves M, Bressman S, Brin MF, Ebersole BJ, et al. Evaluation of the role of the D2 dopamine receptor in myoclonus dystonia. Ann Neurol. 2000;47:369–373. [PubMed] [Google Scholar]
- Crocq MA, Mant R, Asherson P, Williams J, Hode Y, Mayerova A, et al. Association between schizophrenia and homozygosity at the dopamine D3 receptor gene. J Med Genet. 1992;29:858–860. doi: 10.1136/jmg.29.12.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotte G, Lagarde JP, Funalot B, Sokoloff P. Linkage with the Ser9Gly DRD3 polymorphism in essential tremor families. Clin Genet. 2006;69:437–440. doi: 10.1111/j.1399-0004.2006.00600.x. [DOI] [PubMed] [Google Scholar]