Abstract
Background/Objectives:
Postprandial bloating is a common symptom in patients with functional gastrointestinal (GI) diseases. Whole meal bread (WMB) often aggravates such symptoms though the mechanisms are unclear. We used magnetic resonance imaging (MRI) to monitor the intragastric fate of a WMB meal (11% bran) compared with a rice pudding (RP) meal.
Subjects/Methods:
Twelve healthy volunteers completed this randomised crossover study. They fasted overnight and after an initial MRI scan consumed a glass of orange juice with a 2267 kJ WMB or an equicaloric RP meal. Subjects underwent serial MRI scans every 45 min up to 270 min to assess gastric volumes and small bowel water content, and completed a GI symptom questionnaire.
Results:
The MRI intragastric appearance of the two meals was markedly different. The WMB meal formed a homogeneous dark bolus with brighter liquid signal surrounding it. The RP meal separated into an upper liquid layer and a lower particulate layer allowing more rapid emptying of the liquid compared with solid phase (sieving). The WMB meal had longer gastric half-emptying times (132±8 min) compared with the RP meal (104±7 min), P<0.008. The WMB meal was associated with markedly reduced MRI-visible small bowel free mobile water content compared with the RP meal, P<0.0001.
Conclusions:
WMB bread forms a homogeneous bolus in the stomach, which inhibits gastric sieving and hence empties slower than the equicaloric rice meal. These properties may explain why wheat causes postprandial bloating and could be exploited to design foods that prolong satiation.
Keywords: magnetic resonance imaging, bran, stomach, small intestine, secretion
Introduction
Postprandial bloating, distension and discomfort are common symptoms in functional gastrointestinal (GI) disorders, including functional dyspepsia1 and the irritable bowel syndrome,2 though the precise mechanism remains obscure. Extensive studies have shown abnormal intestinal gas handling3 and alterations in abdominal wall tone in irritable bowel syndrome4 but how this relates to meal-provoked symptoms is unclear. Patients believe that bran aggravates symptoms,5 something which has been confirmed objectively in clinical studies.6, 7 Bran at a dose of 15 g in a 1516 kJ meal has been shown to delay gastric emptying in healthy subjects8 and bloated irritable bowel syndrome patients6 but to accelerate small bowel transit in healthy volunteers, possibly by increasing small bowel secretion.9, 10 Our previous studies also showed that its transit through the terminal ileum was delayed compared with liquid meals.6 Increased intestinal and pancreatic secretions due to bran have been shown in animal models11, 12 and increased small bowel water content (SBWC) due to the addition of coarse wheat bran to a rice meal has been observed in healthy humans using magnetic resonance imaging (MRI) methods.8 Interestingly, plastic particles caused a small bowel transit acceleration similar to bran suggesting that one of the mechanism is via mechanostimulation in the small bowel.10
The aim of this study was therefore to compare the upper GI processing of a whole meal bread (WMB) meal with an equicaloric non-bran-containing rice pudding (RP) meal. We hypothesised that the WMB meal, which usually contains 11% bran,13 when compared with the rice meal, will:
form a viscous bolus in the stomach and delay gastric-emptying time and
stimulate small bowel secretions increasing the water content of the small bowel.
Subjects and methods
Test meals
The total energy and macronutrient composition of the WMB and RP meals, as indicated by the manufacturers on the packaging, are given in Table 1. The two meals were designed to be isoenergetic and had only a 0.8% difference in total energy. Other meal characteristics such as appearance, temperature, volume and weight differed between meals. The components of both meals were purchased at a main supermarket as follows: thick sliced WMB, tinned creamed RP, pure orange juice from concentrate (all Sainsbury's own brand, Sainsbury's, London, UK) and Robertson's seedless raspberry jam (Hartley's, St Albans, Hertfordshire UK). The WMB meal was served at room temperature accompanied by the glass of orange juice. The participants were instructed to spread the jam evenly on the bread and to drink the juice with the meal, with no specific instructions given about how and when to drink it. The RP was first mixed with the jam and then heated covered in a microwave according to the packaging instructions, left to cool down to about 50 °C and then served as above.
Table 1. Macronutrient composition of the meals.
| Amount (g) | Energy (kJ) | Protein (g) | Total carbohydrate (g) | Carbohydrate of which sugars (g) | Carbohydrate of which starch (g) | Fat (g) | Fibre (g) | Salt (g) | |
|---|---|---|---|---|---|---|---|---|---|
| WMB meal | |||||||||
| WMB | 190 | 1735 | 21.1 | 68.6 | 7.8 | 60.8 | 5.7 | 15.2 | 1.4 |
| Raspberry jam | 34 | 353 | 0.2 | 20.5 | 18.7 | 1.8 | 0.03 | 1.0 | 0.03 |
| Orange juice | 100 | 179 | 0.5 | 9.1 | 9.1 | 0 | 0.1 | 0.1 | trace |
| Total for WMB meal | 324 | 2267 | 21.8 | 98.2 | 35.6 | 62.6 | 5.8 | 16.3 | 1.4 |
| RP meal | |||||||||
| RP | 426 | 1717 | 13.2 | 72.0 | 43.5 | 26.8 | 7.2 | 0.9 | 0.2 |
| Raspberry jam | 34 | 353 | 0.2 | 20.5 | 18.7 | 1.8 | 0.03 | 1.0 | 0.03 |
| Orange juice | 100 | 179 | 0.5 | 9.1 | 9.1 | 0 | 0.1 | 0.1 | trace |
| Total for RP meal | 560 | 2249 | 13.9 | 101.6 | 71.3 | 28.6 | 7.4 | 2.0 | 0.2 |
Abbreviations: RP, rice pudding meal; WMB, whole meal bread meal.
Subjects
Twelve healthy subjects (eight male, four female, 22±1 years old and body mass index 23.1±0.8 kg/m2) were screened using a Rome II Bowel Symptoms Questionnaire and a MRI safety questionnaire, and completed the study. This protocol was approved by the University's Medical School Research Ethics Committee and all volunteers gave informed written consent before experiments.
Study design
This study was a randomised, open-label, paired crossover design. Volunteers were studied on two separate days 1 week apart. The subjects were asked to fast from 2000 h the previous evening and to avoid alcohol, caffeine, strenuous exercise and any medication that could affect GI function for 18 h before the experiment. They were only allowed a small glass of water on waking. They filled a questionnaire to ensure adherence to the study day restrictions. They underwent a baseline fasted scan at 0900 hours (defined at t=−45 min time point). At 0925 hours, they were asked to eat their study meal within a maximum time of 20 min so that at 0945 hours the subjects underwent a first immediate postprandial scan (defined as t=0 min). This was followed by a scan every 45 min up to 270 min. On completion of each scan, they filled a 100 mm Visual Analogue Scale (VAS) symptoms questionnaire scoring their feeling of fullness, bloating, distension, abdominal pain/discomfort and nausea. The VAS anchors were from ‘not' to ‘extremely').
Magnetic resonance imaging
MRI scanning was carried out supine on a 1.5T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands). Gastric emptying was assessed using a balanced gradient echo sequence acquiring 24 contiguous axial slices with reconstructed in-plane resolution 1.56 × 1.56 mm2, slice thickness 10 mm, echo time TE=1.2 ms, repetition time TR=2.4 ms, flip angle 45° and one breath hold of ∼10 s. This imaging sequence yields good contrast between the stomach contents and other abdominal organs. Samples containing mobile water appear brighter than viscous samples with reduced water content and mobility. The content of apparent freely mobile water in the small bowel (SBWC) was assessed as previously described and validated8, 14 using a single-shot fast spin echo sequence acquiring 24 contiguous coronal slices with reconstructed in-plane resolution 0.78 × 0.78 mm2, slice thickness 7 mm, TE=320 ms, TR= 8000 ms and one breath hold of 24 s. This sequence yields high-intensity signals from areas with freely mobile fluid and dark signals from poorly mobile or bound water and all other body tissues. At each time point, the positioning of the subject, setup and data collection took ∼5 min, after which the volunteers were kept sitting upright in a quiet lounge next to the scanner. After the in vivo study was completed, to further understand our results we scanned WMB, RP and gluten-free bread samples in vitro 90 min after hydrating with saliva by chewing and spitting the resulting chyme into a bowl to compare the resulting signal changes with the in vivo findings.
Data analysis
Gastric volumes were measured by manually tracing a region of interest around the meal and around the gas within the stomach on each image slice using Analyze6 software (Mayo Foundation, Rochester, MN, USA) and summing across slices. At each time point, the sum of meal and gas volume gave the corresponding total gastric volume. Separate regions were also drawn for the liquid and particulate phases of the RP meal. The cross-sectional area of the duodenum half way through the D2 descending segment was also similarly measured on the balanced gradient echo sequence at t=135 min, a time point where SBWC was markedly different. The SBWC was measured using in-house software and methods previously validated.14 Briefly, bright water signal from organs other than the small bowel is segmented out manually, leaving only pixels containing water signal above a calculated threshold to integrate. The symptoms questionnaires VAS scores were mostly not normally distributed hence the area under the curve was calculated using the trapezoidal method. The correlation between the fullness VAS scores and total gastric volumes was assessed by calculating the corresponding postprandial changes from baseline for each individual and meal (n=24 data points in total).
Power and statistical analysis
The primary endpoint was the time to half empty the gastric contents. Based on previous RP meal data,8 a 33% change in half-emptying time (which we considered to be the minimal clinically significant difference) could be detected with α=0.05 and a power of 90% using 12 subjects in a paired crossover design.
The data are expressed as mean±s.e.m. Statistical analysis was carried out using Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). Normality of the data was checked using Shapiro–Wilk's test. Paired comparisons were performed using two-tailed Student's t-test or Wilcoxon's matched-pairs signed-rank test. Two-way repeated measures analysis of variance was used to assess the significance of differences. When the analysis of variance was significant, post hoc test assessments of the individual time points were performed using the Bonferroni correction to account for multiple comparisons. The correlation between postprandial changes from baseline in the fullness VAS scores and changes from baseline in total gastric volumes was assessed using Pearson's correlation r. Differences were considered significant at P<0.05.
Results
Stomach
The MRI appearance of the two study meals inside the stomach was markedly different (Figure 1). The WMB meal formed a large dark bolus with brighter signal around it in the stomach. Brighter signal from this MRI sequence generally arises from greater water content (as well as lipids like subcutaneous fat). The RP meal showed instead a darker layer at the bottom of the stomach consistent with sedimented particulate and a brighter (greater water content) layer at the top of the stomach.
Figure 1.
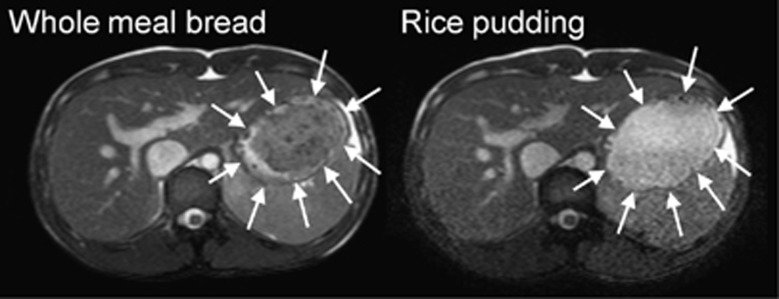
Representative example of axial MRI images of the abdomen of a healthy volunteer fed on one occasion WMB meal (left) and RP meal (right) on another occasion, taken at t=0. The closed arrowheads indicate the body of the stomach. In this balanced gradient echo MRI sequence, liquid appears bright and viscous solutions appear dark. The images show that the WMB meal formed a dark bolus occupying most of the distal stomach, surrounded by bright fluid at the edges, close to the stomach walls. The RP meal rapidly separated into an upper brighter fluid liquid and a lower particulate phase in the stomach.
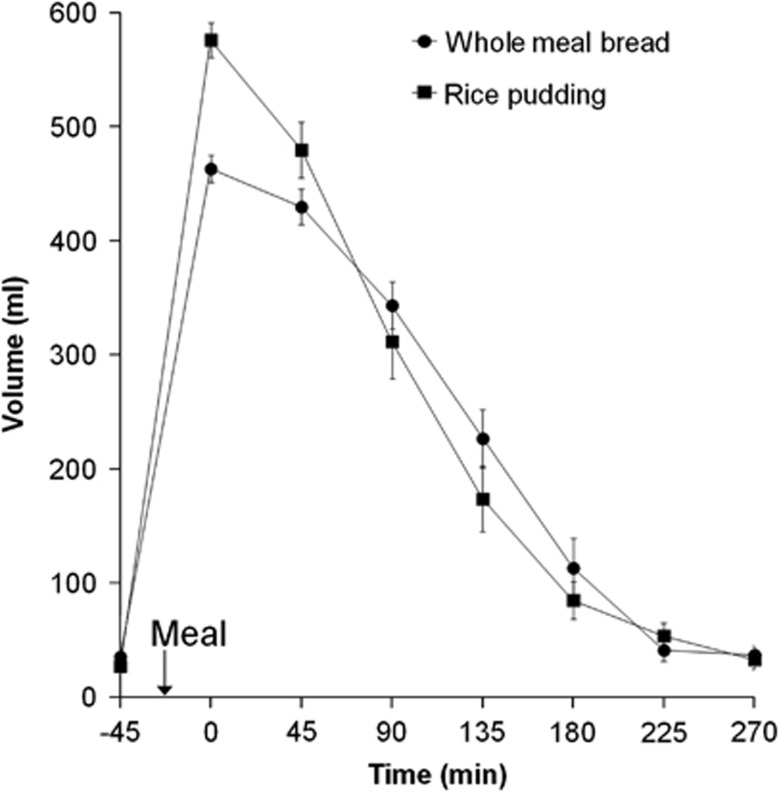
Gastric volumes (Figure 2) were initially higher for the RP meal in keeping with the greater weight and volume of the rice compared with the bread. The RP meal volume emptied from the stomach more rapidly than the WMB meal with time T50% to half empty (104±7 min) being shorter than for the WMB meal (132±8 min), two-tailed paired t-test P<0.008. It was possible to measure separately the intragastric volumes of liquid and particulate layers of the RP meal to assess sieving (Supplementary Figure 1). The data showed that the liquid layer volume had an initial lag phase, which probably reflects progressing sedimentation of the particulate before emptying of the liquid. After this, between 45 and 135 min the liquid layer volumes fell at a rate 65% faster than for the particulate layer indicating efficient sieving of the liquid from the stomach. The corresponding emptying slopes were 2.1±0.3 ml/min for the liquid layer and 1.3±0.2 ml/min for the particulate layer, the difference being just outside the significance threshold (P=0.052). Gastric volumes had mostly returned to baseline for both meals by t=270 min. The volumes of intragastric gas measured were variable ranging from 0 to 141 ml and with an overall mean of 26±1 ml with no differences between meals (P=0.5).
Figure 2.
Plot of the volume of the gastric contents for the healthy volunteers after they consumed the WMB meal and the RP meal. Values are mean±s.e.m., n=12. The arrow on the horizontal axis indicates the meal start time.
Small bowel
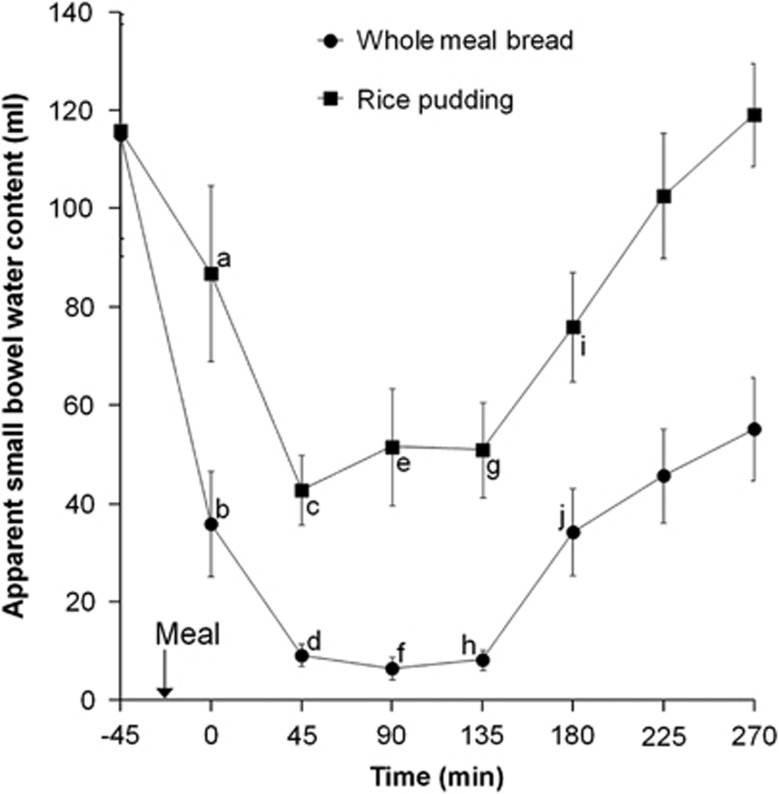
Both meals induced an initial drop in SBWC after feeding, which was more pronounced for the WMB meal (Figure 3). After 45 min, the RP meal induced a rapid rise in SBWC while for the WMB meal the SBWC remained close to zero up to 135 min and it showed only a delayed and limited rise. There was a highly significant meal effect on SBWC (P<0.0001, log-transformed analysis of variance). The effect of time was also significant (P<0.0001) as was the interaction meal × time (P<0.003).
Figure 3.
Plot of the apparent SBWC volumes with time for the healthy volunteers after they consumed the WMB meal and the RP meal. Values are mean±s.e.m., n=12. The arrow on the horizontal axis indicates the meal start time. Within a time point, labelled means without a common letter differ (a vs b and i vs j, P<0.01; c vs d and g vs h, P<0.001; e vs f, P<0.0001; Bonferroni corrected post hoc tests after demonstration of a significant main effect by meal by analysis of variance).
These marked differences persisted throughout the whole time course as shown also by the overall SBWC area under the curve, which was significantly higher for the RP meal (23 763±3776 ml min) than for the WMB meal (10 115±1401 ml min), Wilcoxon's matched-pairs signed-rank test P<0.0005. The cross-sectional area of the duodenum D2 segment at t=135 min was not different between RP (339±24 mm2) and WMB (374±44 mm2) meals (P=0.3).
Symptoms
The fullness scores (Table 2) showed the expected pattern with an immediate rise after eating the meal and declining with time, reaching again baseline values towards the end of the experiment at T=270 min, with no significant differences between meals (P=0.8). As predicted, these healthy subjects scored zero or very low symptoms with no postprandial differences between meals for bloating (P=0.1), distension (P=0.2), pain (P=0.4) and nausea (P=0.9). There was a strong correlation between the postprandial change from baseline in the volunteers' fullness VAS scores and the corresponding change from baseline in total (meal plus gas) gastric volumes, Pearson's r=0.54 with P=0.0055.
Table 2. Postprandial bowel symptoms.
| WMB meal (mm min) | RP meal (mm min) | |
|---|---|---|
| Fullness | 8573±1044 | 8864±1019 |
| Bloating | 2278±923 | 3347±114 |
| Distension | 1481±79 | 1913±87 |
| Pain | 92±49 | 93±47 |
| Nausea | 121±63 | 123±57 |
Abbreviations: RP, rice pudding; WMB, whole meal bread.
Data are areas under the curves (AUCs) for the 100 mm visual-analogue (VAS) bowel symptoms questionnaires measured between fasted baseline and T=270 min postprandially and are represented as mean±s.e.m.
Discussion
Based on previous scintigraphic and MRI observations that both bran8, 10 and meal viscosity15, 16 delay gastric emptying, our first hypothesis was that WMB would delay gastric emptying compared with an equicaloric RP meal. This was confirmed. Despite the equicaloric RP meal weighing 73% more (largely owing to increased water content of RP) and having higher initial volume, it had a shorter half-emptying time than the WMB meal. Faster gastric emptying of a higher volume meal has been observed previously.17, 18 This is likely to be owing to increased gastro-duodenal pressure gradient associated with greater volume19 as well as, in this study, to differing intragastric behaviour of the rice versus WMB meals. The MRI images showed this clearly. The RP meal showed expected sedimentation of the particulate with a brighter, more fluid layer on the top. The WMB meal instead formed a darker more homogenous bolus with bright fluid shown at the edges close to the stomach walls. This appearance is consistent with observations reported previously on thick (just pourable) locust bean gum test meals15 whereby the secretions penetrated the outer layers of a gastric bolus in a process of elution and slow mixing. The darker MRI appearance of the intragastric WMB bolus suggests also lower content of mobile water. The WMB meal has lower moisture content than the RP meal, but it also contains gluten and 1.35–2.75% total arabinoxylans.20 These are non-starch polysaccharides that can bind water up to nine times in excess of their weight21 and greatly increase the viscosity of the solutions.22
The appearance of the mean SBWC time course for the RP meal closely resembles previous reports.8 The subjects' mean fasted baseline SBWC was 100 ml, which then showed a postprandial drop during an early ‘gastric phase' followed by an increase during a later ‘intestinal phase'. The early drop in SBWC is likely to be owing to the absorption of readily available nutrients (including sucrose and glucose) in the liquid phase, which empties selectively more rapidly than the solid phase of the meal.23, 24 These are efficiently co-transported with sodium and water following the resulting osmotic gradient across the small bowel mucosa. The gastro-ileal reflex would also contribute to reducing SBWC by emptying the ileal contents into the ascending colon. The following ‘intestinal phase' rise in SBWC is likely to be owing to increased pancreatico-biliary as well as enterocyte secretion25 stimulated by the subsequent arrival of particulate containing protein and fat into the small bowel.
Following this rationale and also based on previously observed secretory effects of bran,8, 11, 12 our second hypothesis was therefore that the WMB meal would induce increased SBWC compared with the RP meal. We observed exactly the opposite, with the small bowel virtually devoid of MRI-visible free mobile water up to t=135 min followed by an increase at slower rate and with smaller volumes than for the RP meal. The reason for this is unclear. These differences in SBWC are much more pronounced than differences in gastric emptying. The energy content of the two meals and the fat content did not differ substantially suggesting that the release of cholecystokinin and pancreatic secretion would also be similar (though this was untested). The most likely explanation is that the process of WMB taking up water both to increase viscosity and to bind arabinoxylans that started in the stomach continued in the small bowel. This would shorten the transverse relaxation time of the water molecules in the added secretions making them invisible to the long-echo-time MRI sequence used to measure SBWC. We carried out some in vitro chew-and-spit experiments to assess the MRI appearance of various bread ‘digesta'. These showed (using the same long-echo-time MRI sequence as for the in vivo studies) that the RP meal yielded 8 to 15 times more signal than WMB bread, the latter appearing very dark. White bread had double the signal compared with WMB while interestingly gluten-free bread had eight times more signal than WMB. Though only preliminary, these in vitro observations seem to support the hypothesis that WMB has a profound effect on the mobility of water molecules in the small bowel chyme, reducing the signal and hence the apparent water content. Independent assessment of small bowel geometric volume is difficult because the small bowel is so mobile. However, the first and second part of the duodenum (D1 and D2) is fixed to the retroperitoenum so we assessed the cross-sectional area of the D2 segment, and this was not different between meals. As there is virtually no gas in these segments, it implies that the volume of chyme that is largely water was not altered, and the decline in small bowel water is apparent rather than real.
To determine whether these MRI parameters correlate with the time course of the symptoms that many patients experience will require studies in patients as healthy volunteers rarely develop symptoms with these test meals. Total gastric volumes here correlated well with the sensation of postprandial fullness in keeping with previous MRI findings.15 The WMB meal volume at t=0 was ∼20% less than the RP meal while the overall fullness was just 4% less suggesting that had the meals been of identical volume, fullness would have been greater for the slower-emptying WMB meal.
The advantage of using MRI in this study was its non-invasive nature allowing serial studies and providing details of multiple GI function parameters within the same experimental session.26 The short scan time allowed interleaving 3–4 volunteers per day thus reducing cost considerably. The subjects spent only a limited amount of time in the supine position hence possible effects on digestion would have been limited.27
This study had some limitations. The physical appearance and the flavour of the two test meals were clearly different. Chewing was not standardised and could have induced different amounts of salivation. The differences in meal volume may have obscured some symptoms but, given the importance of calorie load in the GI response to food, we considered it more important to have the meals equicaloric than of equal volume. Future studies focusing on symptoms should control for volume by adding more fluid to the WMB before or during ingestion. Increased viscosity in the small intestine may delay both hydrolysis and absorption of sugars, which is generally faster for white rice than whole meal bread28 but whether this would alter the osmolality of the contents is unclear.
In conclusion, this study provided novel MRI insights on the GI fate of WMB bread, which forms a bolus in the stomach, empties slower than a non-bran-containing equicaloric meal and maintains a postprandial form in the small intestine that is poor in mobile water (the WMB ‘dry small bowel'). The exact physical chemistry and physiological mechanism for this warrants further investigation, particularly in patients with postprandial bloating and pain.
Acknowledgments
This work was supported by NDDC divisional academic research funding. We are also grateful for support from the NIHR Biomedical Research Unit in Gastrointestinal and Liver Diseases at Nottingham University.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website (http://www.nature.com/ejcn)
Supplementary Material
References
- Tack J, Talley NJ, Camilleri M, Holtmann G, Hu PJ, Malagelada JR, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada J-R. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology. 2009;136:1544–1551. doi: 10.1053/j.gastro.2009.01.067. [DOI] [PubMed] [Google Scholar]
- Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet. 1994;344:39–40. doi: 10.1016/s0140-6736(94)91055-3. [DOI] [PubMed] [Google Scholar]
- Hebden JM, Blackshaw E, D'Amato M, Perkins AC, Spiller RC. Abnormalities of GI transit in bloated irritable bowel syndrome: effect of bran on transit and symptoms. Am J Gastroenterol. 2002;97:2315–2320. doi: 10.1111/j.1572-0241.2002.05985.x. [DOI] [PubMed] [Google Scholar]
- Snook J, Shepherd HA. Bran supplementation in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:511–514. doi: 10.1111/j.1365-2036.1994.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Marciani L, Cox EF, Hoad CL, Pritchard S, Totman JJ, Foley S, et al. Postprandial changes in small bowel water content in healthy subjects and patients with irritable bowel syndrome. Gastroenterology. 2010;138:469–477. doi: 10.1053/j.gastro.2009.10.055. [DOI] [PubMed] [Google Scholar]
- Vincent R, Roberts A, Frier M, Perkins AC, Macdonald IA, Spiller RC. Effect of bran particle size on gastric emptying and small bowel transit in humans: a scintigraphic study. Gut. 1995;37:216–219. doi: 10.1136/gut.37.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A, Vincent RM, Perkins AC, Spiller RC. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut. 1997;40:223–227. doi: 10.1136/gut.40.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen HN, Knudsen KEB. Effects of wheat flour and oat mill fractions on jejunal flow, starch degradation and absorption of glucose over an isolated loop of jejunum in pigs. Br J Nutr. 1994;72:299–313. doi: 10.1079/bjn19940031. [DOI] [PubMed] [Google Scholar]
- Langlois A, Corring T, Fevrier C. Effects of wheat bran on exocrine pancreas secretion in the pig. Reprod Nutr Dev. 1987;27:929–939. doi: 10.1051/rnd:19870705. [DOI] [PubMed] [Google Scholar]
- Hoseney RC.Principles of Cereal Science and Technology2nd ednAmerican Association of Cereal Chemists Inc.: St Paul, Minnesota; 1998. p136 [Google Scholar]
- Hoad CL, Marciani L, Foley S, Totman JJ, Wright J, Bush D, et al. Non-invasive quantification of small bowel water content by MRI: a validation study. Phys Med Biol. 2007;52:6909–6922. doi: 10.1088/0031-9155/52/23/009. [DOI] [PubMed] [Google Scholar]
- Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol. 2001;280:G1227–G1233. doi: 10.1152/ajpgi.2001.280.6.G1227. [DOI] [PubMed] [Google Scholar]
- Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, et al. Gastric response to increased meal viscosity assessed by echo- planar magnetic resonance imaging in humans. J Nutr. 2000;130:122–127. doi: 10.1093/jn/130.1.122. [DOI] [PubMed] [Google Scholar]
- Hunt JN, Macdonald I. The influence of volume on gastric emptying. J Physiol (Lond) 1954;126:459–474. doi: 10.1113/jphysiol.1954.sp005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehauf H, Steingoetter A, Fox MR, Kwiatek MA, Boesiger P, Schwizer W, et al. Characterization of gastric volume responses and liquid emptying in functional dyspepsia and health by MRI or barostat and simultaneous C-13-acetate breath test. Neurogastroenterol Motil. 2009;21:697–E637. doi: 10.1111/j.1365-2982.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- Kwiatek MA, Menne D, Steingoetter A, Goetze O, Forras-Kaufman Z, Kaufman E, et al. Effect of meal volume and calorie load on postprandial gastric function and emptying: studies under physiological conditions by combined fiber-optic pressure measurement and MRI. Am J Physiol. 2009;297:G894–G901. doi: 10.1152/ajpgi.00117.2009. [DOI] [PubMed] [Google Scholar]
- Gebruers K, Dornez E, Boros D, Fras A, Dynkowska W, Bedo Z, et al. Variation in the content of dietary fiber and components thereof in wheats in the HEALTHGRAIN diversity screen. J Agric Food Chem. 2008;56:9740–9749. doi: 10.1021/jf800975w. [DOI] [PubMed] [Google Scholar]
- Courtin CM, Delcour JA. Arabinoxylans and endoxylanases in wheat flour bread-making. J Cereal Sci. 2002;35:225–243. [Google Scholar]
- Mathlouthi N, Saulnier L, Quemener B, Larbier M. Xylanase, beta-glucanase, and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and beta-glucanase used alone or in combination. J Agric Food Chem. 2002;50:5121–5127. doi: 10.1021/jf011507b. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Thomson JB, Cohen MB, Shadchehr A, Mandiola SA. Sieving of solid food by the canine stomach and sieving after gastric surgery. Gastroenterology. 1979;76:804–813. [PubMed] [Google Scholar]
- Collins PJ, Houghton LA, Read NW, Horowitz M, Chatterton BE, Heddle R, et al. Role of the proximal and distal stomach in mixed solid and liquid meal emptying. Gut. 1991;32:615–619. doi: 10.1136/gut.32.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JM, Albuquerque FC, Stoner MC, Harris RP. Stroking human jejunal mucosa induces 5-HT release and Cl- secretion via afferent neurons and 5-HT4 receptors. Am J Physiol. 1999;277:G515–G520. doi: 10.1152/ajpgi.1999.277.3.G515. [DOI] [PubMed] [Google Scholar]
- Marciani L. Assessment of gastrointestinal motor functions by MRI: a comprehensive review. Neurogastroenterol Motil. 2011;23:399–407. doi: 10.1111/j.1365-2982.2011.01670.x. [DOI] [PubMed] [Google Scholar]
- Steingoetter A, Fox M, Treier R, Weishaupt D, Marincek B, Boesiger P, et al. Effects of posture on the physiology of gastric emptying: a magnetic resonance imaging study. Scand J Gastroenterol. 2006;41:1155–1164. doi: 10.1080/00365520600610451. [DOI] [PubMed] [Google Scholar]
- Shobana S, Kumari SRU, Malleshi NG, Ali SZ. Glycemic response of rice, wheat and finger millet based diabetic food formulations in normoglycemic subjects. Int J Food Sci Nutr. 2007;58:363–372. doi: 10.1080/09637480701252229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.