Abstract
The goal of this study was to test the hypothesis that the cardioprotective effects of the late phase of ischemic preconditioning (PC) can be mimicked by treatment with NO donors. In phase I (studies of myocardial stunning), conscious rabbits underwent a sequence of six 4-minute coronary occlusion/4-minute reperfusion cycles for 3 consecutive days (days 1, 2, and 3). In group I (controls, n=6), the total deficit of systolic wall thickening (WTh) after the sixth reperfusion was reduced by 54% on days 2 and 3 compared with day 1 (P<0.05), indicating a late PC effect against myocardial stunning. When rabbits were given the NO donors diethylenetriamine/NO (DETA/NO, 0.1 mg/kg IV, 4 times [group II, n=5]) or S-nitroso-N-acetylpenicillamine (SNAP, 2.5 µg • kg−1 • min−1 IV for 75 minutes [group III, n=5]) 24 hours before the first sequence of occlusion/reperfusion cycles, the deficit of WTh on day 1 was 60% (group II) and 54% (group III) less than that observed in controls (P<0.05 for both). In both groups II and III, there was no further improvement in the deficit of WTh on days 2 and 3 compared with day 1. The protective effect of DETA/NO was completely abrogated when this agent was given in conjunction with the ONOO− and •OH scavenger mercaptopropionyl glycine (MPG) (group IV, n=5). In phase II (studies of myocardial infarction), conscious rabbits underwent a 30-minute coronary occlusion followed by 3 days of reperfusion. When rabbits were preconditioned 24 hours earlier with six 4-minute occlusion/4-minute reperfusion cycles, infarct size was reduced by 43% (33.2±2.7% versus 58.3 ±4.1% of the region at risk in controls, P<0.05), indicating a late PC effect against myocardial infarction. When rabbits were pretreated with DETA/NO (group VII, n=8) or SNAP (group IX, n=7) 24 hours before the 30-minute occlusion, infarct size was reduced by a similar degree (29.3 ±3.6% and 32.0±3.3% of the region at risk, respectively; P<0.05 versus controls). The degree of protection could not be increased by doubling the dose of DETA/NO (group VIII, n=5). Coadministration of MPG completely abrogated the infarct-sparing action of DETA/NO (group X, n=7). Taken together, these results demonstrate that in conscious rabbits the administration of 2 structurally unrelated NO donors induces protection 24 hours later against both reversible (stunning) and irreversible (infarction) ischemia/reperfusion injury and that the magnitude of this protection is indistinguishable from that observed during the late phase of ischemic PC. The fact that the late phase of ischemic PC can be mimicked by NO donors provides direct evidence that NO in itself is sufficient to elicit this cardioprotective mechanism. The fact that NO donor-induced late PC was abrogated by MPG indicates that the mechanism whereby NO induces this phenomenon involves the generation of oxidant species, possibly ONOO− and/or OH. Since a relatively brief treatment with hemodynamically inactive doses of NO donors can induce long-lasting protective effects, these agents could be useful for preconditioning the heart in patients.
Keywords: myocardial ischemia, myocardial reperfusion, diethylenetriamine/nitric oxide, S-nitroso-N-acetylpenicillamine
Studies in rabbits have shown that ischemic PC induces a delayed cardioprotective effect that increases the tolerance of the heart both to myocardial stunning and to myo-cardial infarction1–7 (“late phase” of ischemic PC). It has recently been demonstrated that administration of NOS inhibitors (ie, L-arginine analogues) during the initial ischemic stimulus abrogates the late phase of ischemic PC, suggesting that the development of this cardioprotective phenomenon is triggered by the generation of NO (“NO hypothesis of late PC”).3,4 The evidence provided by these studies,3,4 however, is indirect. There are 2 limitations inherent in pharmacological manipulations of NOS activity with L-arginine analogues. First, a nonspecific action of these agents cannot be excluded. Second, increasing evidence suggests that NOS may produce •O2− rather than NO under conditions in which the local tissue levels of L-arginine or tetrahydrobiopterin drop.8–10 If this were the case during brief myocardial ischemia/reperfusion, the trigger for late PC could be an increased generation of •O2− by NOS, not an increased generation of NO, and the mechanism by which L-arginine analogues prevent late PC could be the prevention of •O2− formation rather than inhibition of NO synthesis. Assessing the role of NOS-generated •O2− in vivo is problematic, because direct measurements of •O2− in vivo are difficult and specific intracellular scavengers of •O2− that can be used in vivo are not available. Both of the aforementioned issues, however, could be addressed by determining whether NO donors can reproduce the protective effects of the late phase of ischemic PC in the absence of ischemia. If so, this would provide direct evidence that NO (instead of, or in conjunction with, •O2−) can indeed trigger late PC.
Besides these pathophysiological issues, there are other cogent reasons for testing the hypothesis that exogenous NO mimics the late phase of ischemic PC. Because of its sustained nature, late PC may have considerable clinical relevance as a cardioprotective mechanism that could be exploited therapeutically to minimize ischemic injury in patients with coronary artery disease.11 The identification of pharmacological interventions that are capable of mimicking the protective actions of late PC represents a critical step in this direction, since it would provide a framework for developing clinically applicable strategies aimed at maintaining a chronically preconditioned state in individuals at risk for myocardial infarction or other acute coronary events. Accordingly, in recent years the search for pharmacological triggers of late PC has been intense. A number of agents (eg, adenosine A1 receptor agonists,2 direct activators of protein kinase C,6,11 α-adrenergic agonists,12 endotoxin,13 cytokines,14 and monophosphoryl lipid A15) have been shown to elicit a late PC-like effect, but most of these compounds are impractical for clinical use, and none has yet been found suitable for clinical application. Therefore, the search for a clinically applicable intervention that can reproduce the cardioprotective effects of the late phase of ischemic PC continues.
NO donors, such as nitrates, have been widely used for the treatment of coronary artery disease for over a century. They are well tolerated and have relatively few side effects. In view of the mounting evidence suggesting that NO triggers the development of the late phase of ischemic PC,3,4 it seems logical to test whether pretreatment with NO donors, in the absence of ischemia, can reproduce the protective actions of late PC. If so, a new dimension would be added to the clinical role of NO-releasing agents.
The goals of the present study were to investigate (1) whether pretreatment with NO donors induces a delayed cardioprotective effect, (2) if so, whether such an effect is quantitatively equivalent to that induced by ischemic PC, and (3) whether it is mediated by ROS. To comprehensively analyze the spectrum of cardioprotection afforded by late PC, we tested the ability of NO donors to elicit delayed protection against both a mild reversible ischemic insult (myocardial stunning) and a severe irreversible insult (myocardial infarction). In an effort to exclude nonspecific effects of NO donors, 2 structurally unrelated agents, DETA/NO and SNAP, were tested in each experimental setting (stunning and infarction). To directly compare the effects of NO donors with those of ischemic PC, a well-characterized rabbit model was used in which a sequence of six 4-minute occlusion/4-minute reperfusion cycles elicits robust protection against both myocardial stunning3,5,6 and myocardial infarction.4,7 To examine the role of ROS as intermediary steps in the signal transduction pathway initiated by NO, we examined whether the thiol compound MPG, a scavenger of ONOO− and •OH,16,17 blocks NO donor–induced late PC. All studies were conducted in conscious animals in order to eliminate potential artifacts associated with open-chest preparations. 18–22
Materials and Methods
Experimental Preparation and Protocol
The experimental preparation has been described in detail previously. 3–7 Briefly, New Zealand White male rabbits (weight, 2.0 to 2.5 kg; age, 3 to 4 months) were instrumented under sterile conditions with a balloon occluder around a major branch of the left coronary artery, a 10-MHz pulsed Doppler ultrasonic crystal in the center of the region to be rendered ischemic, and bipolar ECG leads on the chest wall. The animals were allowed to recover for a minimum of 10 days after surgery. Throughout the experiments, rabbits were kept in a cage in a quiet dimly lit room. Left ventricular systolic WTh, range gate depth, and the ECG were recorded throughout the experiments on a thermal array chart recorder (Gould TA6000). The study consisted of 2 consecutive phases (phase I and phase II).
Phase I: Studies of Myocardial Stunning
The experimental protocol consisted of 3 consecutive days of coronary artery occlusions (days 1, 2, and 3, respectively). On each day, the rabbits were subjected to a sequence of six 4-minute coronary occlusion/4-minute reperfusion cycles (Figure 1). The performance of successful coronary occlusions was verified by observing the development of ST-segment elevation and changes in the QRS complex on the ECG and the appearance of paradoxical systolic wall thinning on the ultrasonic crystal recordings. No sedative or antiarrhythmic agents were given at any time.
Figure 1.
Experimental protocol for the studies of myocardial stunning (phase I). Four groups of rabbits underwent a sequence of six 4-minute coronary occlusion/4-minute reperfu-sion cycles (where O indicates occlusion and R indicates reperfusion) followed by a 5-hour observation period for 3 consecutive days (days 1, 2, and 3). Twenty-four hours before the first coronary occlusion (day 0), rabbits in group I (n=6, control group) received no pretreatment; rabbits in group II (n=5, DETA/NO group) received 4 intravenous bolus doses of DETA/NO (0.1 mg/kg each) every 25 minutes (total dose, 0.4 mg/kg); rabbits in group III (n=5, SNAP group) received an intravenous infusion of SNAP (2.5 µg • kg−1 • min−1) for 75 minutes (total, 187.5 µg/kg); and rabbits in group IV (n=5, DETA/ NO+MPG group) received the same dose of DETA/NO given to group II and an IV infusion of MPG (1.7 mg • kg•1 min−1) starting 1 hour before the first DETA/NO bolus and continuing until 3 hours after the fourth bolus.
Rabbits were assigned to four groups (Figure 1). Group I (control group) underwent the coronary artery occlusion/reperfusion protocol on days 1, 2, and 3 without any treatment. In group II (DETA/NO group), rabbits received 4 consecutive intravenous bolus doses of DETA/NO (0.1 mg/kg each) every 25 minutes (total dose, 0.4 mg/kg) 24 hours before the first sequence of coronary occlusion/reperfusion cycles. In group III (SNAP group), rabbits received a continuous intravenous infusion of SNAP (2.5 µg kg−1 min−1 for 75 minutes) 24 hours before the first sequence of coronary occlusion/reperfusion cycles. As a result of this protocol, both DETA/NO and SNAP were given over a 75-minute interval. In group IV (DETA/ NO+MPG group), rabbits received the same dose of DETA/NO as in group II; in addition, they were given a continuous intravenous infusion of MPG (1.7 mg kg−1 • min−1) beginning 1 hour before the first DETA/NO injection and continuing until 3 hours after the fourth DETA/NO injection. This dose of MPG was chosen because it has previously been shown to be effective (given together with superoxide dismutase and catalase) in abrogating late PC against stunning in pigs without causing any hemodynamic changes.23 DETA/NO (Alexis Corp) was dissolved in PBS (total volume infused, 4 mL); SNAP (Sigma Chemical Co) was dissolved in normal saline (total volume infused, ≈11 mL). Both DETA/NO and SNAP were dissolved immediately before the infusion; to remove oxygen from the solution, both the PBS and the normal saline solutions were bubbled with nitrogen for at least 30 minutes before dissolving DETA/NO or SNAP. MPG (Sigma) was dissolved in sterile water; because the pH of the solution was 1.0 to 2.0, 0.1 mmol/L NaOH was added to bring the pH to ≈7.5. All solutions were filtered through a 0.2-µm Millipore filter to ensure sterility.
Phase II: Studies of Myocardial Infarction
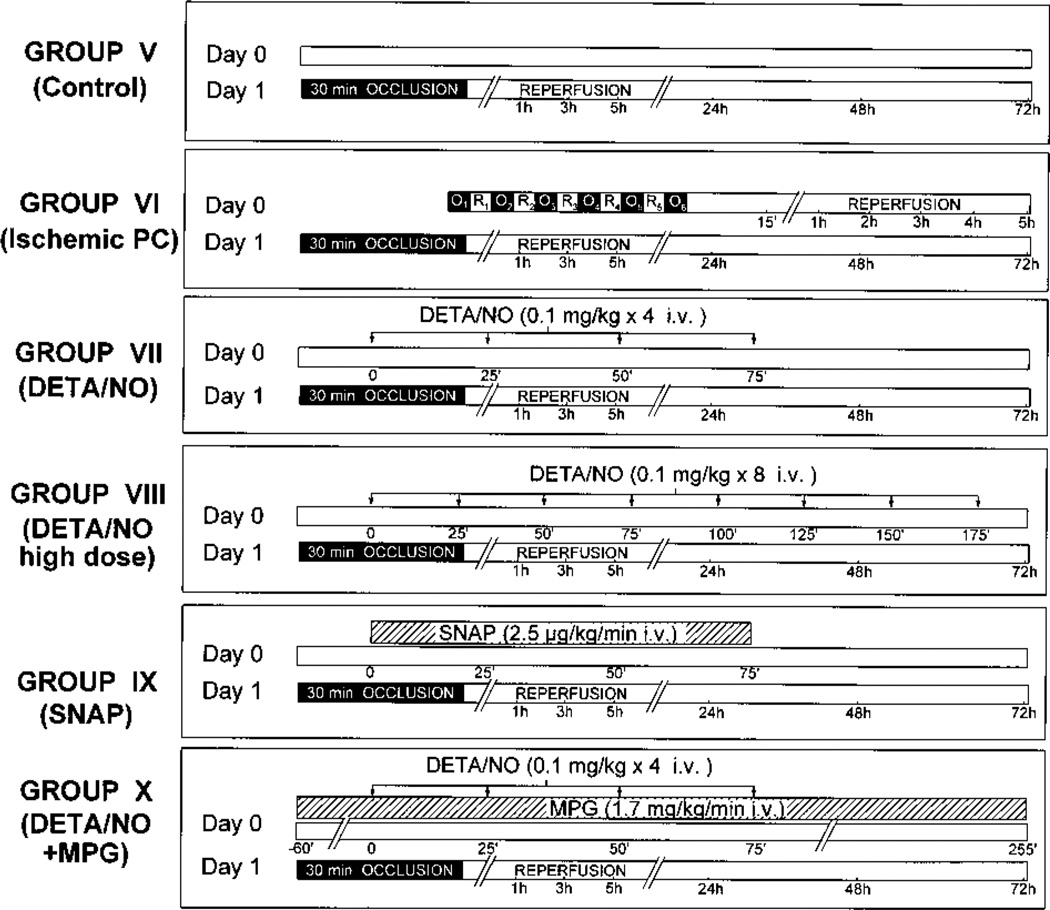
To examine the effect of NO donor pretreatment on myocardial infarction, rabbits were subjected to a 30-minute coronary artery occlusion followed by 3 days of reperfusion. Diazepam was administered 20 minutes before the onset of ischemia (4 mg/kg IP) to relieve the stress caused by the coronary occlusion. No antiarrhythmic agents were given at any time. Rabbits were assigned to 6 groups (Figure 2). Group V (control group) underwent the 30-minute occlusion with no PC protocol or drug pretreatment. Group VI (ischemic PC group) was preconditioned with a sequence of six 4-minute coronary occlusion/4-minute reperfusion cycles 24 hours before the 30-minute coronary occlusion. Group VII (DETA/NO group) was given 4 consecutive intravenous bolus doses of DETA/NO (0.1 mg/kg) every 25 minutes (total dose, 0.4 mg/kg) 24 hours before the 30-minute coronary occlusion (this is the same dose that was used in group II). To determine whether higher doses of DETA/NO would be more effective, group VIII (DETA/NO high-dose group) received 8 consecutive intravenous bolus doses of DETA/NO every 25 minutes (total dose, 0.8 mg/kg) 24 hours before the 30-minute coronary occlusion. Group IX (SNAP group) received a continuous intravenous infusion of SNAP (2.5 µg • kg−1 • min−1 for 75 minutes) 24 hours before the 30-minute coronary occlusion (this is the same dose that was used in group III). In Group X (DETA/ NO+MPG group), rabbits were given the same dose of DETA/NO as in group VII; in addition, they received a continuous infusion of MPG (1.7 mg • kg−1 • min−1) beginning 1 hour before the first DETA/NO injection and continuing until 3 hours after the fourth injection (this is the same treatment that was used in group IV).
Figure 2.
Experimental protocol for the studies of myocardial infarction (phase II). On day 1, 6 groups of rabbits were subjected to a 30-minute coronary occlusion followed by 3 days of reperfusion (where O indicates occlusion and R indicates reperfusion). Twenty-four hours earlier (day 0), rabbits in group V (n=10, control group) received neither ischemic PC nor drug pretreatment; rabbits in group VI (n=10, ischemic PC group) underwent six 4-minute coronary occlusion/4-minute reperfusion cycles; rabbits in group VII (n=8, DETA/NO group) received 4 intravenous bolus doses of DETA/NO (0.1 mg/kg each) every 25 minutes (total dose, 0.4 mg/kg); rabbits in group VIII (n=5, DETA/NO high-dose group) received 8 intravenous bolus doses of DETA/NO (0.1 mg/kg each) every 25 minutes (total dose, 0.8 mg/kg) instead of 4 bolus doses; rabbits in group IX (n=7, SNAP group) received an intravenous infusion of SNAP (2.5 µg kg−1 • min−1) for 75 minutes (total, 187.5 µg/kg); and rabbits in group X (n=7, DETA/NO+MPG group) received the same dose of DETA/NO given to group VII and an IV infusion of MPG (1.7 mg • kg−1 • min−1) starting 1 hour before the first DETA/NO bolus and continuing until 3 hours after the fourth bolus.
Measurement of Regional Myocardial Function
Regional myocardial function was assessed as systolic thickening fraction using the pulsed Doppler probe, as previously described.3 In the studies of myocardial stunning, the total deficit of systolic WTh (an integrative assessment of the overall severity of myocardial stunning) was calculated by measuring the area between the systolic WTh-versus-time line and the baseline (100% line) during the 5-hour recovery phase after the sixth reperfusion.3,5,6 In the studies of myocardial infarction, the total deficit of systolic WTh was calculated by the same method during the 72-hour recovery phase after the 30-minute occlusion.4,7 In all animals, measurements from at least 10 beats were averaged at baseline and from at least 5 beats at all subsequent time points.
Measurement of Region at Risk and Infarct Size
At the conclusion of the study, the rabbits were given heparin (1000 U IV), after which they were anesthetized with sodium pentobarbital (50 mg/kg IV) and euthanized with KCl. The heart was excised, and the size of the ischemic/reperfused region (region at risk) was determined by tying the coronary artery at the site of the previous occlusion and by perfusing the aortic root for 2 minutes with a 5% solution of Phthalo blue dye in normal saline at a pressure of 70 mm Hg using a Langendorff apparatus. The heart was then cut into 6 to 7 transverse slices, which were incubated for 10 minutes at 37°C in a 1% solution of triphenyltetrazolium chloride in phosphate buffer (pH 7.4). All atrial and right ventricular tissues were excised. In the studies of myocardial stunning (phase I), the region at risk (which was identified by the absence of blue dye) was separated from the rest of the left ventricle, and both components were weighed. In the studies of myocardial infarction (phase II), the slices were weighed, fixed in a 10% neutral buffered formaldehyde solution, and photographed (Nikon AF N6006). Transparencies were projected onto a paper screen at a 10-fold magnification, and the borders of the infarcted, ischemic/reperfused, and nonischemic regions were traced. The corresponding areas were measured by computerized planimetry (Adobe Photoshop, version 4.0), and from these measurements infarct size was calculated as a percentage of the region at risk.4,7
Statistical Analysis
Data are reported as mean±SEM. For intragroup comparisons, hemodynamic variables and WTh were analyzed by a 2-way repeated-measures ANOVA (time and day) followed by Student / tests for paired data with the Bonferroni correction. For intergroup comparisons, data were analyzed by either a 1-way or a 2-way repeated-measures (time and group) ANOVA, as appropriate, followed by unpaired Student / tests with the Bonferroni correction. The relationship between infarct size and risk region size was compared among groups with an ANCOVA using the size of the risk region as the covariate.4,7 The correlation between infarct size and risk region size was assessed by linear regression analysis using the least squares method. All statistical analyses were performed using the SAS software system.
Results
A total of 94 rabbits were used in the present study (11 for the pilot studies, 6 for the studies of vasodilator response to DETA/NO, and 77 for the studies of late PC).
Pilot Studies
Pilot studies were conducted in 11 rabbits to identify the doses of DETA/NO and SNAP that have no effect on heart rate, arterial blood pressure, and systolic WTh. The concern was that hemodynamic perturbations caused by these agents (eg, a fall in blood pressure or an increase in heart rate) could contribute nonspecifically to induce a late PC effect unrelated to the direct actions of NO on the heart. Arterial pressure was measured by cannulating the ear dorsal artery with a 22-gauge angiocatheter under local anesthesia (benzocaine), as previously described.3
In 1 rabbit, a dose of 0.2 mg/kg of DETA/NO caused a sustained (20-minute) decrease in blood pressure (—14%), which was associated with a sustained (60-minute) increase in heart rate (12%). Therefore, we reduced the dose to 0.1 mg/kg, which did not cause any appreciable change in arterial pressure, heart rate, or WTh. In 4 rabbits, 2 intravenous bolus doses of 0.1 mg/kg of DETA/NO were given 25 minutes apart 24 hours before the 6 occlusion/reperfusion cycles; although 3 of the 4 rabbits were protected against stunning, the fourth rabbit was not. We therefore increased the number of bolus doses to 4, which proved to be effective in inducing protection against stunning 24 hours later (group II). The rationale for dividing the total dose of DETA/NO in multiple bolus doses given at 25-minute intervals was to administer an amount of drug sufficient to elicit a late PC effect without the hemodynamic changes that would have been associated with a single large bolus. With regard to SNAP, infusion rates of >10 µg • kg−1 • min−1 have been shown to induce a significant decrease in blood pressure (≈−20%) and a significant increase in heart rate (≈20%).24 In 3 rabbits, we infused SNAP at a rate of 5 µg • kg−1 • min−1 for 75 minutes; this dose also caused a significant increase in heart rate (≈15%). We therefore lowered the infusion rate of SNAP to 2.5 µg • kg−1 • min−1, which did not induce appreciable changes in heart rate, arterial pressure, or WTh. The duration of SNAP infusion (75 minutes) was chosen to correspond to the time interval during which the 4 bolus doses of DETA/NO were administered in group II. In summary, these pilot studies demonstrate that the doses of DETA/NO and SNAP selected in this protocol are the highest that can be given to conscious rabbits without causing hemodynamic perturbations.
Vasodilator Response to DETA/NO
Studies were conducted in 6 rabbits to rule out the possibility that MPG may scavenge NO. To avoid the spontaneous fluctuations in arterial pressure that are associated with the conscious state, the rabbits were anesthetized with sodium pentobarbital (≈20 mg/kg). MPG was given at the same rate and with the same protocol used in groups IV and X (1.7 mg • kg−1 • min−1 as a continuous intravenous infusion). Increasing bolus doses of DETA/NO were injected intravenously at 25-minute intervals before and during the infusion of MPG. The results are illustrated in Figure 3. The infusion of MPG had no appreciable effect on blood pressure (mean arterial pressure, 63 ±4 mmHg before MPG and 65 ±3 mmHg 60 minutes after the beginning of the continuous infusion of MPG). Before MPG, DETA/NO decreased mean arterial pressure dose-dependently ( 9.4±0.9%, —14.0±2.3%, −21.8±3.8%, and −26.3±3.5% at doses of 0.25, 0.5, 1.0, and 1.5 mg/kg, respectively) (Figure 3). The first bolus of DETA/NO (0.25 mg/kg) was given 60 minutes after the beginning of the infusion of MPG, and the fourth bolus (1.5 mg/kg) was given 135 minutes after the beginning of the MPG infusion (the timing of the DETA/NO bolus doses with respect to the MPG infusion was the same as that used in groups IV and X [Figures 1 and 2, respectively]). Both at 60 and 135 minutes into the infusion of MPG, the response to DETA/NO was similar to that observed before MPG (Figure 3). These results demonstrate that our dose of MPG did not affect DETA/NO-induced vasodilatation, indicating that MPG is unlikely to scavenge NO.
Figure 3.
Effect of MPG on the response of mean arterial pressure to intravenous bolus injections of DETA/NO. Increasing bolus doses of DETA/NO (0.25, 0.5, 1.0, and 1.5 mg/kg) were administered intravenously at 25-minute intervals before and 60 minutes after the beginning of a continuous infusion of MPG (1.7 mg • kg−1 • min−1). Thus, after the beginning of MPG infusion, the 0.25 mg/kg dose was given at 60 minutes into the infusion and the 1.5 mg/kg dose was given at 135 minutes into the infusion. This is the same time interval that was used for DETA/NO administration in groups IV and X. The response to DETA/NO during infusion of MPG was similar to that before MPG (n=6). Data are mean±SEM.
Phase I: Studies of Myocardial Stunning
Exclusions and Postmortem Analysis
Of the 21 rabbits instrumented for the studies of myocardial stunning, 6 were assigned to group I (control group), 5 to group II (DETA/NO group), 5 to group III (SNAP group), and 5 to group IV (DETA/NO+MPG group). All of the animals completed the protocol satisfactorily and were included in the data analysis. Postmortem analysis showed that the size of the occluded/reperfused vascular bed was similar in the 4 groups: 0.82±0.08 g (17.0±1.8% of LV weight) in group I, 0.92±0.16 g (18.0±1.5% of LV weight) in group II, 0.96±0.12 g (18.9±2.2% of LV weight) in group III, and 0.88±0.10 g (16.9±1.9% of LV weight) in group IV. Tissue staining with triphenyltetrazolium chloride confirmed the absence of infarction in all animals. In all rabbits, the ultrasonic crystal was found to be at least 3 mm from the boundaries of the ischemic/reperfused region.
Hemodynamic Variables
There were no significant changes in heart rate, arterial blood pressure, or systolic WTh at any time during or after the administration of DETA/NO, SNAP, or DETA/NO+MPG in groups II, III, and IV, respectively (representative measurements are given in Table 1). These results are in agreement with our pilot studies and confirm that the doses of NO donors selected in this study have no effects on hemodynamic variables or regional myocardial function in conscious rabbits. On days 1, 2, and 3, there were no appreciable differences in heart rate among the 4 groups, either during the sequence of coronary occlusion/reperfusion cycles or during the 5-hour reperfusion period (Table 2).
TABLE 1.
Hemodynamic Variables During and After Administration of DETA/NO, SNAP, or DETA/NO+MPG
| Baseline | End of Drug Administration* |
1 h After End of Drug Administration |
|
|---|---|---|---|
| Heart rate, bpm | |||
| Group II | 249±10 | 241 ±13 | 250±17 |
| Group III | 243±9 | 238 ±8 | 241 ±11 |
| Group IV | 243±8 | 250±15 | 238±11 |
| Group VII | 250±5 | 243 ±6 | 249±10 |
| Group VIII | 259±11 | 270±18 | 271 ±14 |
| Group IX | 251 ±14 | 256±19 | 260±17 |
| Group X | 240±6 | 242±10 | 241 ±9 |
| Mean arterial pressure, mm Hg | |||
| Group II | 75±5 | 71 ±4 | 75±4 |
| Group III | 72±3 | 71 ±2 | 68±3 |
| Group IV | 71±3 | 69±4 | 69±5 |
| Group VII | 73±2 | 71 ±2 | 73±3 |
| Group VIII | 83±7 | 81 ±7 | 81 ±7 |
| Group IX | 73±2 | 72±1 | 75±3 |
| Group X | 71±2 | 69±3 | 69±2 |
| Systolic wall thickening fraction, % | |||
| Group II | 39.9±2.2 | 40.8±1.9 | 40.7±2.5 |
| Group III | 36.3±2.1 | 37.9±1.9 | 37.8±2.0 |
| Group IV | 38.7±1.3 | 38.4±1.3 | 39.4±1.3 |
| Group VII | 35.3±4.4 | 34.7±4.5 | 35.2±4.9 |
| Group VIII | 37.4±1.6 | 37.8±2.2 | 37.0±1.1 |
| Group IX | 38.6±5.3 | 38.4±6.2 | 37.5±5.7 |
| Group X | 36.3±3.3 | 36.5±3.7 | 36.7±3.7 |
Groups II and VII received 4 bolus doses of 0.1 mg/kg DETA/NO at 25-minute intervals. Groups III and IX received a continuous infusion of SNAP at a rate of 2.5 µg • kg−1 • min−1 for 75 minutes. Group VIII received 8 bolus doses of 0.1 mg/kg DETA/NO at 25-minute intervals. Groups IV and X received DETA/NO according to the same protocol used in groups II and VII; in addition, they were given MPG as a continuous infusion at a rate of 1.7 µg • kg−1 • min−1 starting 60 minutes before the first DETA/NO bolus and continuing until 180 minutes after the fourth DETA/NO bolus. Values are mean±SEM.
In groups II, III, VII, and IX, these measurements were taken 75 minutes after beginning the infusion of SNAP (groups III and IX) or 75 minutes after the first bolus of DETA/NO (ie, immediately after the fourth bolus) (groups II and VII). In group VIII, these measurements were taken 175 minutes after the first bolus of DETA/NO (ie, immediately after the eighth bolus). In groups IV and X, these measurements were taken 315 minutes after beginning the infusion of MPG.
TABLE 2.
Heart Rate During Coronary Occlusion and Reperfusion
| Heart Rate, bpm |
|||
|---|---|---|---|
| Baseline | Occlusion* | Reperfusion (1 h) |
|
| Phase I | |||
| Group I (control) | |||
| Day 1 | 257±13 | 253±14 | 251 ±11 |
| Day 2 | 265±13 | 246±9 | 244±11 |
| Day 3 | 262±6 | 246±6 | 243±6 |
| Group II (DETA/NO) | |||
| Day 1 | 255±7 | 238±10 | 232±17 |
| Day 2 | 263±12 | 236±10 | 221±12 |
| Day 3 | 253±14 | 232±11 | 227±15 |
| Group III (SNAP) | |||
| Day 1 | 243±14 | 239±13 | 232±13 |
| Day 2 | 244±10 | 232±8 | 222 ±6 |
| Day 3 | 247±8 | 230±7 | 218±8 |
| Group IV (DETA/NO+MPG) | |||
| Day 1 | 232±10 | 236±7 | 228±8 |
| Day 2 | 241 ±12 | 238±10 | 234±13 |
| Day 3 | 239±14 | 239±11 | 230±8 |
| Phase II | |||
| Group V (control) | 254±6 | 279±12 | 254±11 |
| Group VI (ischemic PC) | 246±5 | 277±3 | 266±8 |
| Group VII (DETA/NO) | 239±4 | 258±5 | 254±8 |
| Group VIII (DETA/NO high dose) | 245±13 | 262±11 | 254±11 |
| Group IX (SNAP) | 250±8 | 260±9 | 257±9 |
| Group X (DETA/NO + MPG) | 240±6 | 256±5 | 271 ±7 |
In the studies of myocardial stunning (phase I, groups I to IV), all rabbits underwent a sequence of 6 cycles of 4-minute coronary occlusion/4-minute reperfusion followed by a 5-hour observation period on days 1, 2, and 3. Heart rate was measured 5 minutes before occlusion (baseline), 3 minutes into the third occlusion, and at selected times after the sixth reperfusion (for the sake of brevity, only the 1-hour measurements are reported). In the studies of myocardial infarction (phase II, groups V to X), all groups underwent a 30-minute coronary occlusion followed by 72 hours of reperfusion. Heart rate was measured 5 minutes before occlusion (baseline), at 15 minutes into the 30-minute coronary occlusion, and at selected times after reperfusion (for the sake of brevity, only the 1-hour measurements are reported). Values are mean±SEM.
Third occlusion for phase I, and fifteen-minute occlusion for phase II.
Regional Myocardial Function
Baseline systolic thickening fraction on days 1, 2, and 3 averaged 37.4±5.5%, 35.5±4.4%, and 36.4±4.6%, respectively, in group I; 40.8±3.5%, 41.9±3.6%, and 43.2±4.3% in group II; 38.0±2.7%, 39.1 ±2.7%, and 36.3±2.2% in group III; and 41.3±1.2%, 41.3±1.4%, and 41.7±1.3% in group IV. There were no significant differences among groups I, II, III, and IV on the same day, or among different days within the same group. Furthermore, within the same group there were no significant differences among days 1, 2, and 3 with respect to the extent of paradoxical systolic thinning during the 6 occlusions (Figures 4 to 7).
Figure 4.
Systolic thickening fraction in the ischemic/reperfused region in group I (control group) 5 minutes before the first occlusion (baseline), 3 minutes into each coronary occlusion (O), 3 minutes into each reperfusion (R), and at selected times during the 5-hour reperfusion interval following the sixth occlusion. Measurements taken on day 1 are represented by the dashed line with open circles, measurements taken on day 2 are represented by the continuous line with solid circles, and measurements taken on day 3 are represented by the interrupted line with solid triangles (n=6 for all 3 days). Thickening fraction is expressed as a percentage of baseline values. Data are mean±SEM.
Figure 7.
Systolic thickening fraction in the ischemic/reperfused region in group IV (DETA/NO+MPG group) 5 minutes before the first occlusion (baseline), 3 minutes into each coronary occlusion (O), 3 minutes into each reperfusion (R), and at selected times during the 5-hour reperfusion interval following the sixth occlusion. Measurements taken on day 1 are represented by the dashed line with open circles, measurements taken on day 2 are represented by the continuous line with solid circles, and measurements taken on day 3 are represented by the interrupted line with solid triangles (n=5 for all 3 days). To facilitate comparisons, the data pertaining to day 1 of group I (control) are also shown (thick interrupted line without symbols, n=6). Thickening fraction is expressed as a percentage of baseline values. Data are mean±SEM.
Group I (Control Group)
On day 1, the thickening fraction remained significantly (P<0.05) depressed for 4 hours after the sixth reperfusion and recovered by 5 hours (Figure 4), indicating that the sequence of six 4-minute occlusion/4-minute reperfusion cycles resulted in severe myocardial stunning that lasted, on average, 4 hours. On days 2 and 3, however, the recovery of WTh after the 6 occlusion/reperfusion cycles was markedly improved compared with WTh recovery on day 1 (Figure 4). The total deficit of WTh after the sixth reperfusion was 54% less on both days 2 and 3 compared with day 1 (P<0.01) (Figure 8). Thus, as expected,3,5,6 myocardial stunning was attenuated markedly, and to a similar extent, on days 2 and 3 compared with day 1.
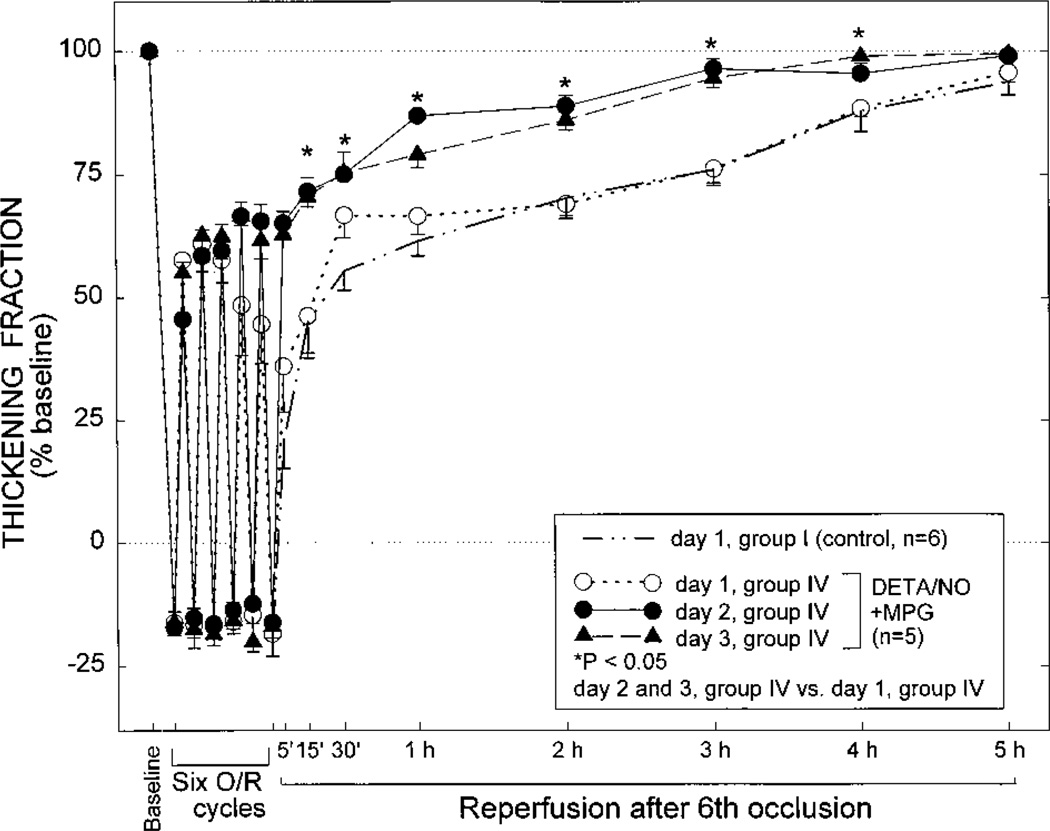
Figure 8.
Total deficit of WTh after the sixth reperfusion on days 1,2, and 3 in the control (n=6), DETA/NO (n=5), SNAP (n=5), and DETA/ NO+MPG groups (groups I, II, III, and IV, respectively). The values of total deficit of WTh in individual rabbits are illustrated in the left panel; the mean±SEM values of total deficit of WTh are depicted in the right panel. The total deficit of WTh was measured in arbitrary units, as described in the text.
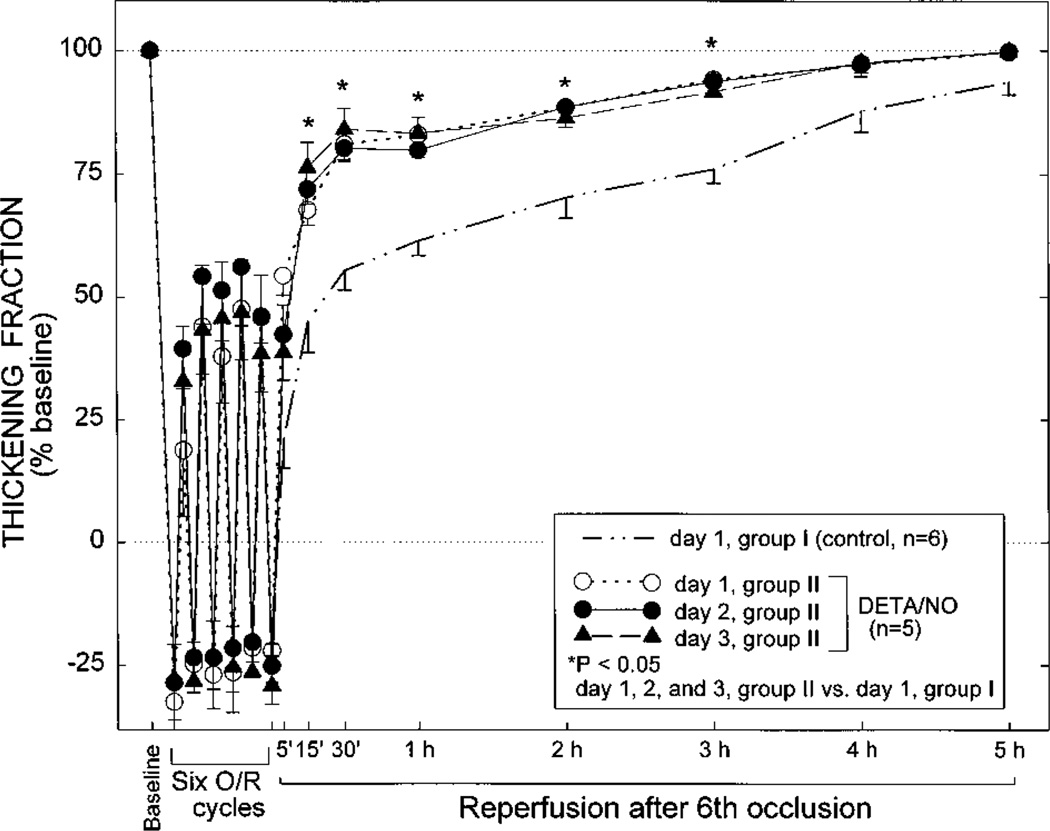
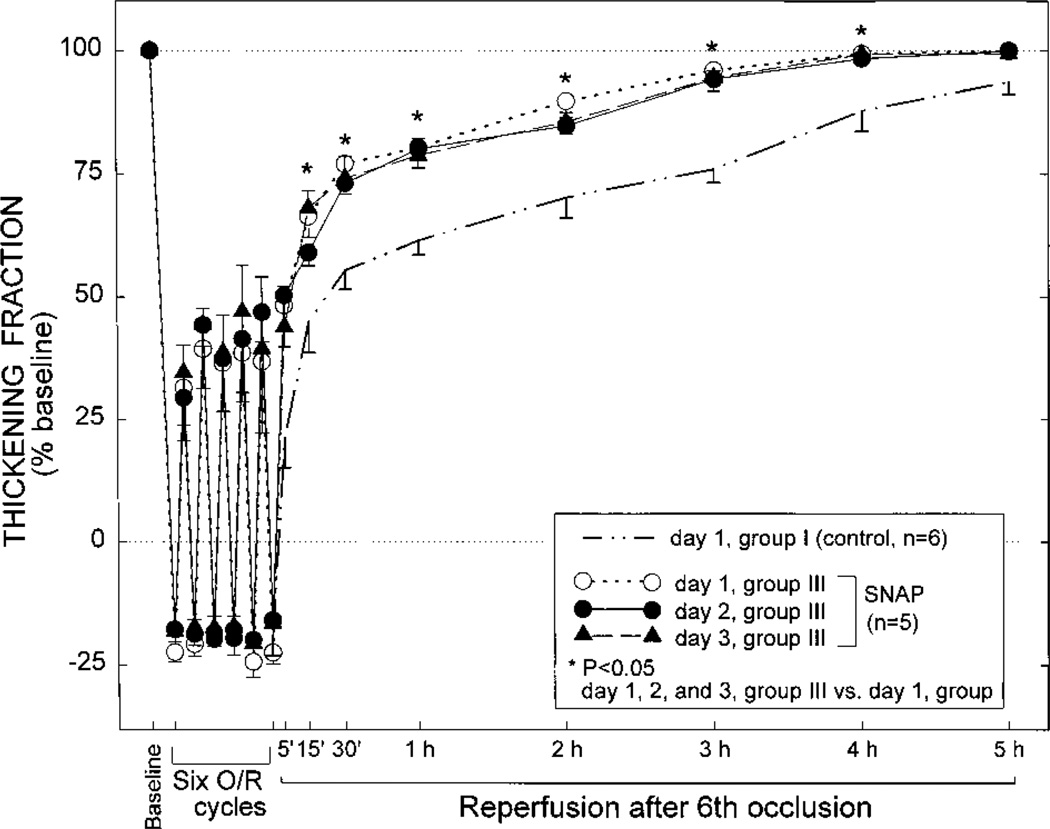
Groups II (DETA/NO Group) and III (SNAP Group)
Although on day 1 the extent of paradoxical wall thinning in groups II and III was similar to that noted in control rabbits, the recovery of WTh after the sixth reperfusion was markedly faster than in the control group, and this improvement was sustained throughout the entire reperfusion interval (Figures 5 and 6). The total deficit of WTh in groups II and III was 60% less and 54% less, respectively, than that observed in control rabbits on day 1 (P<0.05) and similar to that observed in control rabbits on days 2 and 3 (Figure 8). On days 2 and 3, there was no further improvement in either the recovery of WTh (Figures 5 and 6) or the total deficit of WTh (Figure 8) compared with day 1. Thus, administration of either DETA/NO or SNAP 24 hours before the sequence of six 4-minute occlusion/reperfusion cycles resulted in an attenuation of myocardial stunning on day 1 that was essentially equivalent to that effected by ischemic PC.
Figure 5.
Systolic thickening fraction in the ischemic/reperfused region in group II (DETA/NO group) 5 minutes before the first occlusion (baseline), 3 minutes into each coronary occlusion (O), 3 minutes into each reperfusion (R), and at selected times during the 5-hour reperfusion interval following the sixth occlusion. Measurements taken on day 1 are represented by the dashed line with open circles, measurements taken on day 2 are represented by the continuous line with solid circles, and measurements taken on day 3 are represented by the interrupted line with solid triangles (n=5 for all 3 days). To facilitate comparisons, the data pertaining to day 1 of group I (control group) are also shown (thick interrupted line without symbols, n=6). Thickening fraction is expressed as a percentage of baseline values. Data are mean±SEM.
Figure 6.
Systolic thickening fraction in the ischemic/reperfused region in group III (SNAP group) 5 minutes before the first occlusion (baseline), 3 minutes into each coronary occlusion (O), 3 minutes into each reperfusion (R), and at selected times during the 5-hour reperfusion interval following the sixth occlusion. Measurements taken on day 1 are represented by the dashed line with open circles, measurements taken on day 2 are represented by the continuous line with solid circles, and measurements taken on day 3 are represented by the interrupted line with solid triangles (n=5 for all 3 days). To facilitate comparisons, the data pertaining to day 1 of group I (control) are also shown (thick interrupted line without symbols, n=6). Thickening fraction is expressed as a percentage of baseline values. Data are mean±SEM.
Group IV (DETA/NO+MPG Group)
The combination of DETA/NO and MPG was studied to elucidate the mechanism of the PC effect induced by DETA/NO and, specifically, to determine whether it was mediated by the formation of MPG-sensitive oxidants (•OH and ONOO−). On day 1, both the recovery of WTh (Figure 7) and the total deficit of WTh (Figure 8) were virtually indistinguishable from those noted in the control group, indicating the absence of PC against stunning. Thus, the PC effect induced by DETA/NO was completely abrogated by the concomitant administration of MPG. A PC effect became apparent on days 2 and 3, as documented by the enhanced recovery of WTh (Figure 7) and the reduced deficit of WTh (Figure 8) compared with those values on day 1.
Phase II: Studies of Myocardial Infarction
Exclusions and Arrhythmias
Of the 56 rabbits instrumented for the studies of myocardial infarction, 13 were assigned to group V (control group), 12 to group VI (ischemic PC group), 9 to group VII (DETA/NO group), 6 to group VIII (DETA/NO high-dose group), 8 to group IX (SNAP group), and 8 to group X (DETA/NO+MPG group). Six rabbits died of ventricular fibrillation during coronary occlusion (2 in group V, 2 in group VI, 1 in group VIII, and 1 in group IX), and 1 died after reperfusion (in group X). Two rabbits (1 in group V and 1 in group VII) were excluded because of technical problems during the postmortem analysis. Therefore, the total number of rabbits that completed the experimental protocol was 10 in group V, 10 in group VI, 8 in group VII, 5 in group VIII, 7 in group IX, and 7 in group X. No rabbit included in the final analysis was subjected to defibrillation.
In the control group, 15% of the rabbits (2 of 13) developed ventricular fibrillation during the 30-minute occlusion, and 36% (4 of 11) developed ventricular tachycardia after reperfusion (no control rabbit exhibited ventricular fibrillation after reperfusion). The incidence of ventricular fibrillation during the 30-minute occlusion and ventricular tachycardia after reperfusion did not differ significantly between the control and treated groups (data not shown).
Hemodynamic Variables
In agreement with our pilot studies, no significant changes in heart rate, arterial blood pressure, or systolic WTh were observed at any time during or after the administration of DETA/NO, high-dose DETA/NO, SNAP, or DETA/ NO+MPG in groups VII, VIII, IX, and X, respectively (representative measurements are given in Table 1). Furthermore, there were no appreciable differences in heart rate among groups V, VI, VII, VIII, IX, and X during the 30-minute coronary occlusion or during the 72-hour reperfusion period (representative measurements are shown in Table 2). Baseline systolic thickening fraction was also similar among the 6 groups (38.7±4.5%, 38.8±2.9%, 35.3±4.4%, 37.4±1.6%, 38.6±5.3%, and 36.3±3.3% in groups V, VI, VII, VIII, IX, and X, respectively).
Region at Risk and Infarct Size
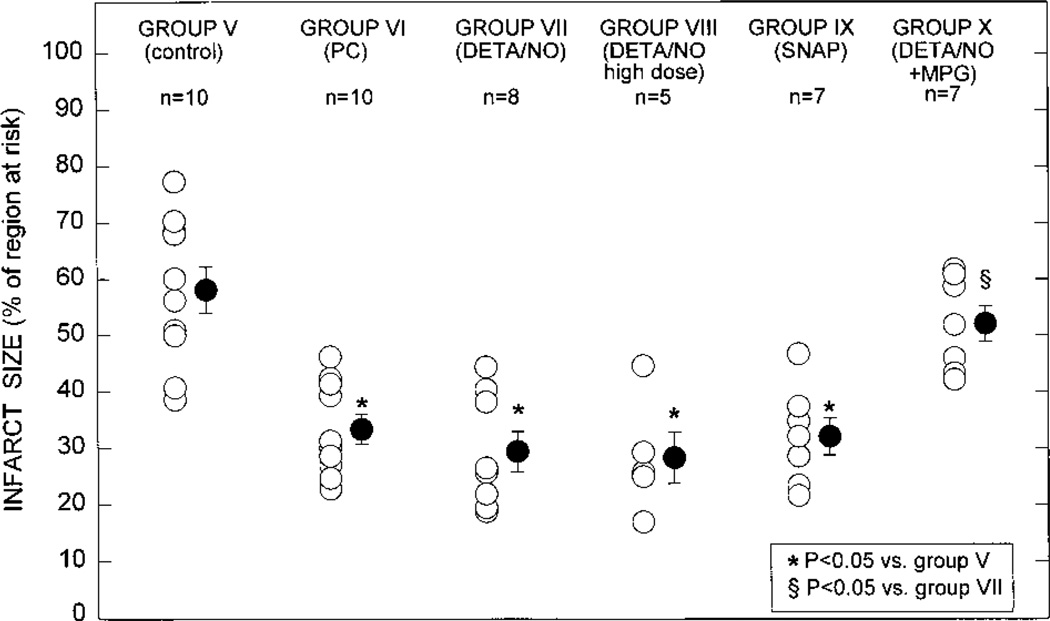
There were no significant differences among groups V, VI, VII, VIII, IX, and X with respect to the weight of the region at risk (0.68±0.08 g [15.1 ±1.3% of LV weight], 0.69±0.08 g [15.5±1.7% of LV weight], 0.68±0.07 g [15.0±1.5% of LV weight], 0.66±0.06 g [15.3± 1.5% of LV weight], 0.61±0.02 g [14.9±0.8% of LV weight], and 0.72±0.07 g [16.0±1.4% of LV weight], respectively). The average infarct size was 43% smaller in group VI (ischemic PC group) compared with group V (control group) (33.2±2.7% versus 58.3±4.1% of the region at risk, respectively; P<0.05 [Figure 9]), indicating a late PC effect against myocardial infarction. A quantitatively similar PC effect was observed in groups VII (DETA/NO group) and IX (SNAP group): the average infarct size in these groups (29.3±3.6% and 32.0±3.3% of the region at risk, respectively) was similar to that measured in group VI and significantly (P<0.05) smaller than that measured in group V (Figure 9), indicating that pretreatment with these NO donors 24 hours before the 30-minute occlusion resulted in a protective effect that was equivalent to that induced by ischemic PC in group VI. Although group VIII was given 0.8 mg/kg of DETA/NO instead of the 0.4 mg/kg given to group VII, infarct size in these rabbits was not smaller than in group VII (Figure 9), indicating that the protection could not be enhanced by doubling the dose of the NO donor. In group X, infarct size was indistinguishable from that measured in the control group and significantly (P<0.05) larger than that measured in group VII (Figure 9), indicating that MPG completely blocked the infarct-sparing effect of DETA/NO pretreatment.
Figure 9.
Myocardial infarct size in groups V (n=10, control group), VI (n=10, ischemic PC group), VII (n=8, DETA/NO group), VIII (n=5, DETA/NO high dose group), IX (n=7, SNAP group), and X (n=7, DETA/NO+MPG group). Infarct size is expressed as a percentage of the region at risk of infarction. Open circles represent individual rabbits; solid circles, mean±SEM. *P<0.05 vs group V (control group); §P<0.05 vs group VII (DETA/NO group).
Since the effects of DETA/NO and SNAP on infarct size in groups VII, VIII, and IX were indistinguishable (Figure 9), the rabbits in these 3 groups were pooled together (NO donor group) for the analysis of the infarct-risk region relationship (Figure 10) and of the recovery of WTh (Figure 11). In all groups, the size of the infarction was positively and linearly related to the size of the region at risk (r=0.93 in the control group, r=0.79 in the ischemic PC group, and r=0.66 in NO donor-pretreated rabbits [groups VII, VIII, and IX pooled together], and r=0.90 in the DETA/NO+MPG group) (Figure 10). As expected,4,7 the regression line was shifted to the right in the ischemic PC group compared with the control group (P<0.05 by ANCOVA) (Figure 10). In the 3 groups pretreated with NO donors, the regression line was virtually indistinguishable from that of the ischemic PC group and was significantly shifted to the right compared with that of the control group (P<0.05 by ANCOVA) (Figure 10), indicating that for any given size of the region at risk, the resulting infarct size was reduced by pretreatment with DETA/NO or SNAP and that the magnitude of this effect was similar to that induced by ischemic PC. In contrast, in the DETA/NO+MPG group (group X), the regression line did not differ from that observed in the control group (Figure 10).
Figure 10.
Relationship between size of the region at risk and size of myocardial infarction. The figure illustrates both individual values and the regression lines obtained by linear regression analysis for the control group (group V, n=10), the ischemic PC group (group VI, n=10), the NO donor-pretreated rabbits (groups VII, VIII, and IX combined, n=20), and the DETA/ NO+MPG group (group X, n=7). In all groups, infarct size was positively and linearly related to risk region size. The linear regression equations were as follows: control group, y=0.74x-0.09 (r=0.93); PC group, y=0.33x (r=0.79); NO donor group, y=0.39x-0.06 (r=0.66); and DETA/NO+MPG group, y=0.66x-0.09 (r=0.90). ANCOVA demonstrated that the regression lines for the ischemic PC and NO donor groups were significantly different from that for the control group (P<0.05 for each comparison), indicating that for any given risk region size, infarct size was smaller in the ischemic PC and NO donor-pretreated rabbits compared with control rabbits; in contrast, the line for the DETA/NO+MPG group was similar to that for the control group.
Figure 11.
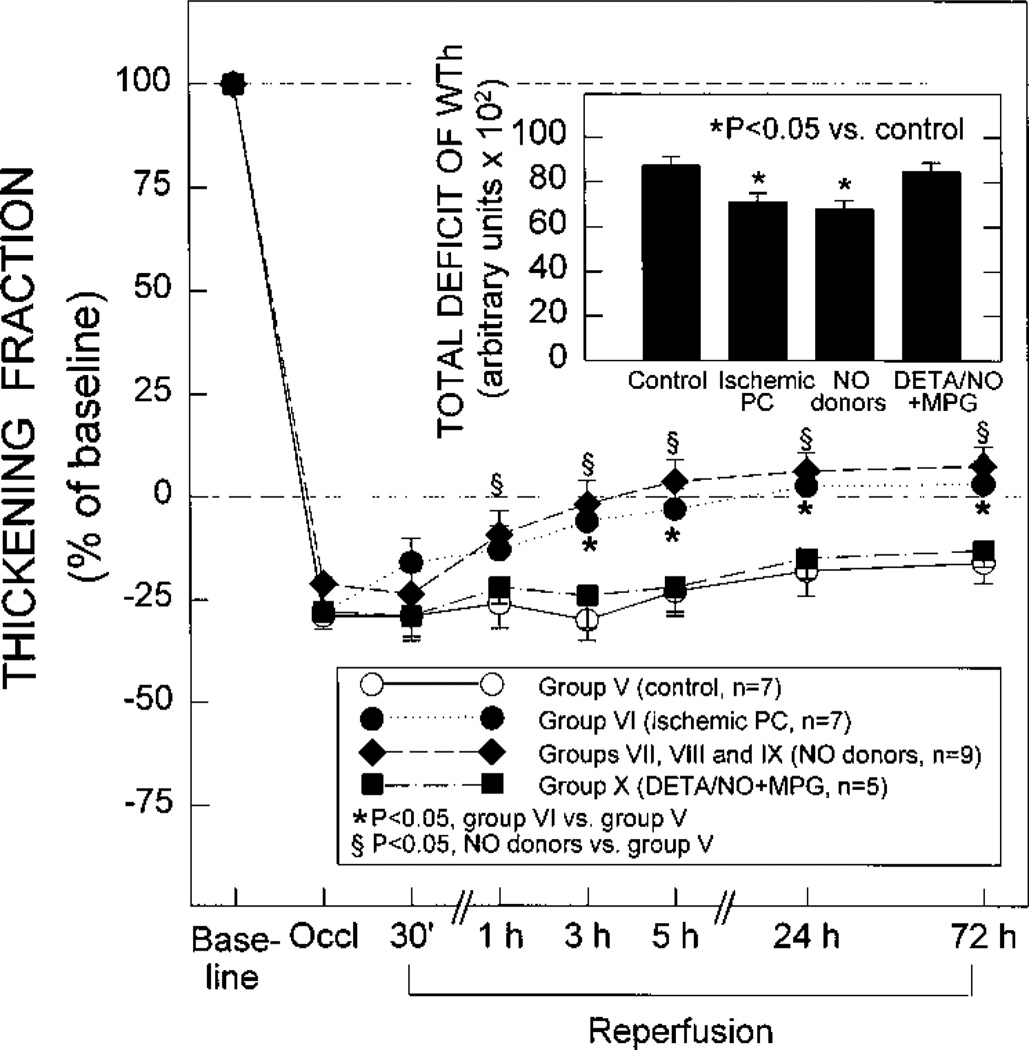
Systolic thickening fraction in the ischemic/reper-fused region in the control rabbits (group V, n=7), in the ischemic PC rabbits (group VI, n=7), in NO donor-pretreated rabbits (groups VII, VIII, and IX combined, n=9), and in the DETA/ NO+MPG rabbits (group X, n=5) 5 minutes before the 30-minute occlusion (baseline), 15 minutes into the 30-minute coronary occlusion (Occl), and at selected times during the 72-hour reperfusion interval. Because of Doppler probe malfunction, complete measurements of thickening fraction could not be obtained in all of the rabbits in groups V to X. Thickening fraction is expressed as a percentage of baseline values. The total deficit of WTh after infarction is depicted in the inset. The total deficit of WTh was calculated by measuring the area between the systolic WTh-vs-time line and the baseline (100% line) during the 3-day reperfusion period after the 30-minute occlusion (see text). Data are mean±SEM. *P<0.05 between group V and group VI; §P<0.05 between group V and NO donor-pretreated group. In the inset, *P<0.05 vs the control group (group V).
Regional Myocardial Function
Because of Doppler probe malfunction, complete measurements of WTh for 3 days after reperfusion could be obtained in only 7 of 10 rabbits in group V, 7 of 10 rabbits in group VI, 3 of 8 rabbits in group VII, 2 of 5 rabbits in group VIII, 4 of 7 rabbits in group IX, and 5 of 7 rabbits in group X. After release of the 30-minute occlusion, control rabbits (group V) exhibited essentially no recovery of WTh, even at 3 days (Figure 11). In rabbits preconditioned with ischemia (group VI), the recovery of WTh was significantly (P<0.05) improved compared with control rabbits at 3 hours, 5 hours, 24 hours, and 72 hours after reperfusion (Figure 11). The total deficit of WTh during the 72 hours of reperfusion was 18% less in group VI versus group V (P<0.05) (Figure 11). In the rabbits pretreated with NO donors (groups VII, VIII, and IX combined, n=9), the recovery of WTh was similar to that noted in the rabbits preconditioned with ischemia and significantly (P<0.05) improved compared with control rabbits at 1 hour, 3 hours, 5 hours, 24 hours, and 72 hours after reperfusion (Figure 11). The total deficit of WTh during the 72-hour reperfusion period was 23% less in NO donor-pretreated rabbits versus control rabbits (P<0.05) (Figure 11). Thus, pretreatment with NO donors resulted in enhanced recovery of myocardial contractile function, which became evident soon after reperfusion (1 hour) and was sustained throughout the 72-hour reperfusion interval; the magnitude of this effect was similar to that observed after ischemic PC in group VI. In the DETA/NO+MPG group, both the recovery of WTh and the total deficit of WTh were similar to those observed in the control group (Figure 11), indicating that the salutary actions of NO donor pretreatment on the recovery of myocardial function were abrogated by the concomitant administration of MPG. Since the effects of ischemic PC, NO donors, and MPG on WTh paralleled those on infarct size (Figure 9), the WTh data provide an independent confirmation of the results obtained with tetrazolium staining.
Discussion
Before the NO hypothesis of late PC can be conclusively accepted, it is necessary to verify that the effects of exogenous NO mimic those of the late phase of ischemic PC. The present study demonstrates that administration of 2 structurally different NO donors, in the absence of ischemia, induces a significant protection against myocardial ischemia/reperfusion injury 24 hours later, which is indistinguishable from that observed during the late phase of ischemic PC. This NO-induced late PC was effective in reducing not only the severity of myocardial stunning after a sequence of six 4-minute occlusion/4-minute reperfusion cycles (phase I) but also the extent of cell death (myocardial infarction) after a 30-minute coronary occlusion (phase II). The magnitude of these beneficial actions was essentially equivalent to that observed after ischemic PC. The reduction in infarct size was accompanied by enhanced recovery of contractile function in the surviving myocardium, and again, the magnitude of this salutary effect was equivalent to that observed after ischemic PC. The ability of DETA/NO and SNAP to induce a late PC effect cannot be ascribed to myocardial ischemia secondary to a decrease in arterial pressure or an increase in heart rate, because neither NO donor produced any appreciable hemodynamic change; accordingly, the late PC effect must have been the result of a direct action of NO on the heart. Finally, the ability of NO donors to induce delayed protection against both stunning and infarction was completely abrogated by the concomitant administration of the antioxidant MPG, indicating that the mechanism whereby NO induces late PC involves the generation of oxidant species, such as ONOO− and/or •OH.
Previous studies have demonstrated beneficial actions of NO precursors or NO donors in various models of acute ischemia in which the agents were present during ischemia and/or reperfusion.25–29 To our knowledge, this is the first study to indicate (1) that treatment with NO donors elicits a cardioprotective effect at a distance of 24 hours and (2) that NO donors induce this late PC effect via formation of oxidant species. This is also the first study to determine that a form of therapy widely used in patients elicits a late PC effect. Prior findings indicating that the development of the late phase of ischemic PC was blocked by NOS inhibitors had provided indirect evidence that this phenomenon is triggered by NO3,4 The present finding that the effects of the late phase of ischemic PC can be mimicked by the administration of NO donors provides direct evidence that NO in itself is sufficient to elicit this cardioprotective mechanism without the concurrent perturbations associated with ischemia/reperfusion.
Methodological Considerations
All of the studies reported herein were performed in conscious animals in an effort to rigorously test the potential cardioprotective actions of NO donors under conditions that are as physiological as possible. Open-chest animal preparations are associated with a number of factors (such as anesthesia, surgical trauma, fluctuations in temperature, elevated catecholamine levels, abnormal hemodynamics, and exaggerated formation of ROS) that may interfere with myocardial stunning,18,20 with myocardial infarction,21 and/or with ischemic PC.19,22 Moreover, since the focus of the present study was to examine the role of NO in triggering late PC, we felt that it was important to eliminate experimental conditions that may modulate the activity of NOS. For example, the trauma and the inflammatory reaction associated with a thoracotomy may promote the release of cytokines, which in turn could induce iNOS activity. In this regard, Hoshida et al30 found in dogs that the myocardial content of manganese superoxide dismutase in the nonischemic (control) region increased significantly 24 hours after a thoracotomy, possibly as a result of the release of cytokines in the initial hours after surgery. Also, surgical exposure of the brain has been found to induce iNOS in cerebral tissue even in the absence of ischemia.31 Since transcription of the iNOS gene is controlled in part by antioxidant-sensitive transcription factors (eg, nuclear factor-κB), the excessive formation of ROS in open-chest animals18 could also contribute to artifactual iNOS induction in these models.
To exclude the possibility that DETA/NO or SNAP could elicit PC by causing myocardial ischemia as a result of hypotension and/or tachycardia, we used doses that had no discernible hemodynamic effects. To obtain another index of infarct size limitation that is independent of tetrazolium staining, in phase II we also measured the recovery of WTh, which essentially paralleled the changes in infarct size (Figure 11). To minimize the possibility that the cardioprotection observed in NO donor-pretreated rabbits could be due to nonspecific actions, we examined 2 structurally unrelated agents. DETA/NO is a relatively long-acting NO donor that spontaneously and nonenzymatically releases NO with predictable first-order kinetics.32,33 SNAP is a widely used NO donor that lacks tolerance-producing effects.24 The finding that the effects of DETA/NO and SNAP were essentially identical, both in the setting of myocardial stunning (Figures 5 and 6) and in the setting of myocardial infarction (Figures 9 to 11), makes it very unlikely that the protection was caused by moieties of the DETA/NO or SNAP molecule other than NO itself. We elected to use DETA/NO and SNAP to avoid some of the problems associated with other NO donors. For example, 3-morpholinosydnonimine generates both NO and •O2~, which would make it impossible to examine the role of NO in itself in triggering late PC. Sodium nitroprusside is a widely used NO donor, but the toxicity associated with the formation of cyanide could confound the results. Furthermore, unlike other NO donors, neither DETA/NO nor SNAP is dependent on the enzymatic release of NO24,32,33
NO as a Trigger of Late PC
Previous studies have demonstrated that administration of NOS inhibitors during an ischemic stress blocks the development of late PC against both myocardial stunning3 and myocardial infarction.4 Although these results implicate NO as the trigger of the late phase of ischemic PC, the evidence is indirect. Nonspecific actions of L-NA (the NOS inhibitor used in these studies3,4) cannot be ruled out. Furthermore, it is now apparent that NOS generates not only NO but also •O2− and that the relative proportions of these 2 radicals depend on the availability of substrate (L-arginine) and cofactors (such as tetrahydrobiopterin).8–10 For example, recent studies have shown that when skeletal muscle is subjected to acute ischemia, the main product of NOS is •O2− rather than NO9 If the same occurs during acute myocardial ischemia, NOS may trigger the development of late PC by generating •O2−, not by generating NO. In this hypothetical scenario, the ability of L-NA to block late PC3,4 would reflect the inhibition of NOS-mediated formation of •O2− rather than NO. To elucidate this issue, we administered NO donors in the absence of ischemia (and, consequently, in the absence of any changes in •O2− formation associated with ischemia). Our finding that both DETA/NO and SNAP induced a late PC effect indistinguishable from that observed after ischemic PC indicates that NO in itself can trigger this phenomenon, without the need for a concomitant increase in •O2− formation. Thus, increased availability of NO is sufficient to activate cellular adaptive mechanisms that result in increased resistance of the heart to ischemia/reperfusion injury 24 hours later.
Mechanism of NO-Induced Late PC
There are several potential mechanisms whereby increased biosynthesis of NO could elicit the development of a cardioprotective effect 24 hours later (reviewed in Reference 3). In the present study, we postulated that NO induces late PC via formation of NO-derived ROS (eg, ONOO−). To test this hypothesis, we administered DETA/NO in conjunction with MPG, a cell-permeant antioxidant that reacts avidly with both ONOO− and •OH by virtue of its thiol group.16,17 Since ONOO− can decompose to form OH or OH-like species,16 we reasoned that the administration of MPG would be useful to interrogate the ROS pathway as a mechanism for NO-induced late PC. Our results demonstrate that MPG completely eliminated the late PC effect induced by DETA/NO, both in the setting of myocardial stunning (Figure 7) and in the setting of myocardial infarction (Figures 9 to 11). It seems highly unlikely that the abrogation of late PC by MPG could have been mediated by a direct reaction of MPG with either DETA/NO or NO, since MPG had no effect on DETA/NO-induced vasodilation (Figure 3). Accordingly, these results strongly suggest that NO elicits late PC via formation of ROS, most likely ONOO− and/or •OH.
This conclusion would explain the fact that the late phase of ischemic PC can be blocked both by inhibiting NOS3,4 and by scavenging ROS.23,34 This concept is also consistent with the notion that the development of late PC involves the activation of protein kinase C,6,35,36 since it is well established that ROS can stimulate this family of enzymes.37 It should be noted that MPG also prevents the development of late PC against stunning after six 4-minute occlusion/4-minute reper-fusion cycles in our conscious rabbit model.34 The ability of MPG to block both ischemia-induced late PC and NO-induced late PC supports a common mechanism for these 2 phenomena. We therefore propose that the mechanism of late PC after an ischemic stimulus involves increased formation of NO, which reacts with •O2− to form ONOO− and •OH and that ONOO− and/or •OH activates a PKC-mediated signal transduction cascade that culminates in the development of a protective effect 24 hours later.
Clinical Implications
Although the late phase of ischemic PC has been shown to provide significant and sustained protection against myocardial stunning and infarction, no widely applicable pharmacological treatment has thus far been developed that can reproduce this endogenous cardioprotective effect in patients with coronary artery disease. Most agents that elicit a late PC-like protection are not clinically applicable, for various reasons. In contrast, NO donors (ie, nitrates) are widely used clinically. Our finding that a relatively brief treatment with NO donors can induce a long-lasting cardioprotective effect raises the intriguing possibility that these agents, in addition to their well-known beneficial hemodynamic actions, may also precondition the heart against subsequent ischemic injury occurring at a distance of hours or days. If this concept is confirmed in patients and if the ability of nitrates to induce late PC is not hampered by the development of tolerance, investigations would be warranted to identify appropriate dosages and treatment modalities that could be used to maintain a protracted preconditioned state.
Conclusions
Because the protection afforded by the late phase of ischemic PC is sustained (3 to 4 days),11 pharmacological agents capable of mimicking this phenomenon (“late PC mimetics”) could have significant therapeutic value. The present study demonstrates that 2 unrelated NO donors produce effective protection against both reversible and irreversible injury during myocardial ischemia/reperfusion in the absence of any hemodynamic changes and that the magnitude of this protection is equivalent to that afforded by ischemic PC. Accordingly, administration of appropriate doses of NO donors could be a useful clinical approach to the protection of the ischemic myocardium. In addition, the present study provides new insights into the mechanism of ischemic PC by indicating that NO in itself can induce late PC and that it acts via formation of secondary oxidant species.
Acknowledgments
This study was supported in part by National Institutes of Health grants R01 HL-43151 and HL-55757 (Dr Bolli), by American Heart Association, Kentucky Affiliate, Inc, grants KY-96-GB-32 (Dr Qiu) and KY-96-GB-31(Dr Tang), and by the Medical Research Grant Program of the Jewish Hospital Foundation, Louisville, Ky. We gratefully acknowledge Christiane Trauss, Wen-Jian Wu, and Gregg Shirk for expert technical assistance and Trudy Keith for expert secretarial assistance.
Selected Abbreviations and Acronyms
- DETA
diethylenetriamine
- iNOS
inducible NOS
- L-NA
Nω-nitro-L-arginine
- LV
left ventricular
- MPG
mercaptopropionyl glycine
- NOS
NO synthase
- PC
preconditioning
- ROS
reactive oxygen species
- SNAP
S-nitroso-N-acetylpenicillamine
- WTh
wall thickening
References
- 1.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 2.Baxter GF, Marber MS, Patel VC, Yellon DM. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Bhatti ZA, Tang X-L, Qiu Y, Zhang Q, Guo Y, Jadoon AK. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Qiu Y, Rizvi A, Tang X-L, Manchikalapudi S, Takano H, Jadoon AK, Wu W-J, Bolli R. Nitric oxide triggers late preconditioning against myocardial infarction in conscious rabbits. Am J Physiol. 1997;273:H2931–H2936. doi: 10.1152/ajpheart.1997.273.6.H2931. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Manchikalapudi S, Tang X-L, Takano H, Qiu Y, Guo Y, Zhang Q, Jadoon AK. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase: evidence that nitric oxide acts both as a trigger and as mediator of the late phase of preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y, Ping P, Tang X-L, Manchikalapudi S, Rizvi A, Zung J, Takano H, Wu W-J, Teschner S, Bolli R. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that є is the isoform involved. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takano H, Manchikalapudi S, Tang X-L, Qiu Y, Rizvi A, Jadoon AK, Zhang Q, Bolli R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. In press doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huk I, Nanobashvili J, Neumayer C, Punz A, Mueller M, Afkhampour K, Mittlboeck M, Losert U, Polterauer P, Roth E, Patton S, Malinski T. L-Arginine treatment alters the kinetics of nitric oxide and superoxide release and induces ischemia/reperfusion injury in skeletal muscle. Circulation. 1997;96:667–675. doi: 10.1161/01.cir.96.2.667. [DOI] [PubMed] [Google Scholar]
- 10.Wever RMF, Luscher TF, Consentino F, Rabelink TJ. Atherosclerosis and the two faces of endothelial nitric oxide synthase. Circulation. 1998;97:108–112. doi: 10.1161/01.cir.97.1.108. [DOI] [PubMed] [Google Scholar]
- 11.Marber MS, Yellon DM. Myocardial adaptation, stress proteins, and the second window of protection. Ann NY Acad Sci. 1996;793:123–141. doi: 10.1111/j.1749-6632.1996.tb33510.x. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Cleveland JC, Jr., Rowland RT, Mitchell MB, Brown JM, Banerjee A, Harken AH. Norepinephrine-induced sustained myocardial adaptation to ischemia is dependent on alpha 1-adrenoceptors and protein synthesis. JMol Cell Cardiol. 1996;28:2017–2025. doi: 10.1006/jmcc.1996.0194. [DOI] [PubMed] [Google Scholar]
- 13.Brown JM, Grosso MA, Terada LS, Whitman GJ, Banerjee A, White CW, Harken AH, Repine JE. Endotoxin pretreatment increases endogenous myocardial catalase activity and decreases ischemia-reperfusion injury of isolated rat hearts. Proc Natl Acad Sci U S A. 1989;86:2516–2520. doi: 10.1073/pnas.86.7.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JM, White CW, Terada LS, Grosso MA, Shanley PF, Mulvin DW, Banerjee A, Whitman GJ, Harken AH, Repine JE. Interleukin-1 pre-treatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 1990;87:5026–5030. doi: 10.1073/pnas.87.13.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott GT. Monophosphoryl lipid A induces delayed preconditioning against cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 1998;30:3–17. doi: 10.1006/jmcc.1997.0586. [DOI] [PubMed] [Google Scholar]
- 16.Crow JP, Beckman JS. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo . Adv Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 17.Sun J-Z, Kaur H, Halliwell B, Li X-Y, Bolli R. Use of aromatic hydroxy-lation of phenylalanine to measure production of hydroxyl radicals after myocardial ischemia in vivo: direct evidence for a pathogenetic role of the hydroxyl radical in myocardial stunning. Circ Res. 1993;73:534–549. doi: 10.1161/01.res.73.3.534. [DOI] [PubMed] [Google Scholar]
- 18.Li X-Y, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993;92:1025–1041. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haessler R, Kuzume K, Chien GL, Wolff RA, Davis RF, Van Winkle DM. Anaesthetics alter the magnitude of infarct limitation by ischaemic preconditioning. Cardiovasc Res. 1994;28:1574–1580. doi: 10.1093/cvr/28.10.1574. [DOI] [PubMed] [Google Scholar]
- 20.Bolli R. Common methodological problems and artifacts associated with studies of myocardial stunning in vivo . Basic Res Cardiol. 1995;90:257–262. doi: 10.1007/BF00797893. [DOI] [PubMed] [Google Scholar]
- 21.Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–H1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz LM, Jennings RB, Reimer KA. Premedication with the opioid analgesic butorphanol raises the threshold for ischemic preconditioning in dogs. Basic Res Cardiol. 1997;92:106–114. doi: 10.1007/BF00805571. [DOI] [PubMed] [Google Scholar]
- 23.Sun J-Z, Tang X-L, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–576. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer JE, Han B-J, Chern WH, Lee FW. Lack of tolerance to a 24-hour infusion of S-nitroso N-acetylpenicillamine (SNAP) in conscious rabbits. J Pharmacol Exp Ther. 1992;260:286–293. [PubMed] [Google Scholar]
- 25.Siegfried MR, Erhardt J, Rider T, Ma X-L, Lefer AM. Cardioprotection of organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther. 1992;260:668–675. [PubMed] [Google Scholar]
- 26.Nakanishi K, Vinten-Johansen J, Lefer DJ, Zhao Z, Fowler WC, McGee DS, Johnston WE. Intracoronary L-arginine during reperfusion improves endothelial function and reduces infarct size. Am J Physiol. 1992;263:H1650–H1658. doi: 10.1152/ajpheart.1992.263.6.H1650. [DOI] [PubMed] [Google Scholar]
- 27.Weyrich AS, Ma XL, Lefer AM. The role of L-arginine in ameliorating reperfusion injury after myocardial ischemia in the cat. Circulation. 1992;86:279–288. doi: 10.1161/01.cir.86.1.279. [DOI] [PubMed] [Google Scholar]
- 28.Williams MW, Taft CS, Ramnauth S, Zhao ZQ, Vinten-Johansen J. Endogenous nitric oxide (NO) protects against ischaemia-reperfusion injury in the rabbit. Cardiovasc Res. 1995;30:79–86. [PubMed] [Google Scholar]
- 29.Engelman DT, Watanabe M, Maulik N, Engelman RM, Rousou JA, Flack JE, Deaton DW, Das DK. Critical timing of nitric oxide supplementation in cardioplegic arrest and reperfusion. Circulation. 1996;94(suppl II):II-407–II-411. [PubMed] [Google Scholar]
- 30.Hoshida S, Kuzuya T, Fuji H, Yamashita N, Oe H, Hori M, Suzuki K, Taniguchi N, Tada M. Sublethal ischemia alters myocardial antioxidant activity in canine heart. Am J Physiol. 1993;264:H33–H39. doi: 10.1152/ajpheart.1993.264.1.H33. [DOI] [PubMed] [Google Scholar]
- 31.Iadecola C, Zhang F, Xu X, Casey R, Ross ME. Inducible nitric oxide synthase gene expression in brain following focal cerebral ischemia. J Cereb Blood Flow Metab. 1995;15:378–384. doi: 10.1038/jcbfm.1995.47. [DOI] [PubMed] [Google Scholar]
- 32.Maragos CM, Morley D, Wink DA, Dunams TM, Saavedra JE, Hoffman A, Bove AA, Isaac L, Hrabie JA, Keefer LK. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide: vasorelaxant effects. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 33.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol. 1995;25:674–678. [PubMed] [Google Scholar]
- 34.Tang X-L, Rizvi A, Qiu Y, Takano H, Zang Q, Guo Y, Bolli R. Evidence that the hydroxyl radical triggers late preconditioning against myocardial stunning in conscious rabbits [abstract] Circulation. 1997;96(suppl I):I-255. [Google Scholar]
- 35.Baxter GF, Goma FM, Yellon DM. Involvement of protein kinase C in the delayed cytoprotection following sublethal ischaemia in rabbit myocardium. Br J Pharmacol. 1995;115:222–224. doi: 10.1111/j.1476-5381.1995.tb15866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ping P, Zhang J, Qiu Y, Tang X-L, Manchikalapudi S, Cao X, Bolli R. Ischemic preconditioning induces selective translocation of protein kinase C isoforms є and η in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 37.Downey JM, Cohen MV, Ytrehus K, Liu Y. Cellular mechanisms in ischemic preconditioning: the role of adenosine and protein kinase C. Ann N Y Acad Sci. 1994;723:82–98. [PubMed] [Google Scholar]