Abstract
Background:
Neurodevelopmental hypothesis of schizophrenia states abnormal pruning as one of the pathogenetic mechanism in schizophrenia. Though thalamic volume abnormalities have been documented, the shape differences of thalamus in antipsychotic-free schizophrenia in comparison with age- and sex-matched healthy volunteers need validation.
Materials and Methods:
We examined antipsychotic naïve schizophrenia patients (n=60) and age- and sex-matched healthy volunteers (n=44). The thalamic shape abnormalities were analyzed from their coded structural magnetic resonance imaging (MRI) data using three-dimensional automated image analysis software, FMRIB's (Oxford Center for the functional MRI of the brain) tools-FIRST (FMRIB's Integrated Registration and Segmentation Tool) by creating deformable mesh model. Correlation with the psychopathology scores was carried out using F-statistics.
Results:
Patients with schizophrenia showed significant inward deformations in the regions corresponding to anterior, ventromedial, mediodorsal, and pulvinar nuclei. There was a direct correlation between negative syndrome score and the deformation in the right mediodorsal and right pulvinar nuclei.
Conclusion:
The inward deformations of thalamus in antipsychotic naive schizophrenia patients correspond to those nuclei which have reciprocal connections with frontal, superior temporal, and anterior cingulate regions and support the neurodevelopmental hypothesis of schizophrenia.
Keywords: Schizophrenia, shape abnormality, thalamus
INTRODUCTION
Neuroimaging has been one of the main research modalities in understanding psychiatric illnesses, including schizophrenia. Multiple regions of brain and their connections have been implicated in the pathogenesis of schizophrenia.[1] Among multiple regions studied, thalamic volume deficits have been one of the consistently replicated findings.[2,3]
Various lines of evidence suggest involvement of thalamus in schizophrenia. Earlier studies examining thalamic volume using manual morphometric studies and automated analysis have reported thalamic volume abnormalities.[2,3,4] Anterior thalamic radiation which connects mediodorsal nucleus of thalamus with frontal cortex and anterior nucleus with anterior cingulate cortex has also shown to exhibit decrease fractional anisotropy in patients with schizophrenia.[5] Postmortem studies examining thalamus have shown reduced neuronal densities in patients with schizophrenia in comparison with healthy controls. Thalamus is also an important relay station in cortico-cerebello-thalamo-cortical circuit. Aberrations of the same have also been implicated in one of the important pathogenetic model – “cognitive dysmetria” in schizophrenia.[6]
Analysis of the shape of subcortical structures provides useful information about the location and pattern of structural changes in them. This helps in understanding the regional changes in the subcortical structures contributing to a larger volume changes as studied in volumetric studies. Previous studies looking into the shape of thalamus have revealed abnormalities in anterior, posterior poles, pulvinar, ventromedial, and dorsomedial nuclei.[7,8,9,10] However, the findings have been inconsistent. Most of these studies have smaller sample size and have included previously treated patients. The results also depend on the computational procedure used for image processing which primarily rely on the accuracy of segmentation of subcortical structures and their registration into atlas space. Consequent upon this, there is a need to examine the thalamic shape in larger number of patients who are never exposed to antipsychotics using a superior computational methodology.
In this study, we examined the anatomical shape differences of thalamus in patients with schizophrenia (n=60) and the correlations between psychopathology and thalamic shape abnormalities in comparison with age-, sex-, handedness-matched healthy subjects (n=44). We hypothesized that patients with schizophrenia will have significant thalamic shape abnormalities in comparison with age- and sex-matched healthy subjects and these thalamic shape abnormalities will show relevant correlates with psychopathology scores.
MATERIALS AND METHODS
Subjects
The study sample comprised 60 antipsychotic naïve schizophrenia patients and 44 healthy volunteers. Patients fulfilling the criteria for schizophrenia according to DSM-IV TR[11] were recruited from the clinical services of National Institute of Mental Health and Neurosciences. Patients were assessed using Structured Clinical Interview for DSM-IV (SCID)[12] and the diagnosis was confirmed by another qualified psychiatrist with semistructured interview. All patients were in the age group of 18-45 years and were right-handed. There was no history of any neurological or medical illness in any of the patients. None of them were exposed to any antipsychotics anytime in the past and all provided written informed consent. Sociodemographic details and the history were collected from the patients and corroborated by reliable informants. Psychopathology was assessed by using the Scale for Assessment of Negative Symptoms (SANS)[13] and Scale for Assessment of Positive Symptoms (SAPS).[14]
Healthy volunteers (n=44) consenting to participate in the study were recruited through word of mouth. SCID was applied to ascertain the absence of any psychiatric diagnosis. All subjects were right-handed and none of them had any history of medical illness or substance dependence.
Written informed consent was taken from all subjects before assessment and recruitment. Approval from the ethics committee of the institute was obtained.
Scanning protocol
MRI was done with a 1.5 T scanner (Magnetom “Vision,” Siemens, Erhlangen, Germany). T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence was performed (TR=9.7 ms, TE=4 ms, nutation angle=12°, and slice thickness: 1 mm with no interslice gap) yielding 160 sagittal slices.
Image analysis
Image analyses were carried out using tools from FSL (FMRIB Software Library) (http://www.fmrib.ox.ac.uk/fsl).[15] Using deformable mesh model, surface mesh is created by FIRST for each subcortical structure. The mesh is composed of a set of triangles and the apex of adjoining triangles is called a vertex. The vertices can be compared between groups and within groups as the number of vertices are fixed for each structure. Vertex correspondence is crucial for the FIRST methodology, as it facilitates the investigation of localized shape differences through the examination of group differences in the spatial location of each vertex. Although the vertices retain correspondence, the surfaces reside in the native image space and thus have an arbitrary orientation/position. Therefore, the surfaces must all be aligned to a common space prior to investigating any group differences. The mean surface from the FIRST models (in MNI152 space) is used as the target to which surfaces from the individual subjects are aligned. Pose (rotation and translation) is removed by minimizing the sum-of-squares difference between the corresponding vertices of a subject's surface and the mean surface (target). Group comparisons of vertices were carried out using F-statistics.[16,17]
Statistical analysis
Statistical analyses were carried out using Statistical Package for Social Sciences version 17.0. Clinical data were analyzed using analysis of variance, analysis of covariance, and Chi-square test. The data of all 60 patients and 44 subjects were used for examining group differences in thalamic shape. However, the data of only 42 patients were available to carry out the correlational analysis for parameters like gender differences, age at onset, psychopathology scores, and duration of illness.
RESULTS
Sociodemographic profile
Analysis of sociodemographic profile showed that the mean age for patient group was 29.9±8.0 years and the mean age for the control group was 27.9±7.8 (P=0.21) suggesting that there was no statistical difference in age between the groups [Table 1]. Data of the psychopathology scores were available for 42 out of 66 patients. The average SAPS score was 21.8±7.7 and the SANS score for the group was 22.6±8.3. The mean age at onset of the illness for this group was 26.7±7.4 years.
Table 1.
Sociodemographic details of the study group

Shape analysis
Analysis of the shape of thalamus between the groups revealed a significant inward deformation in the areas corresponding to anterior nucleus, mediodorsal nucleus, and the pulvinar nuclei bilaterally and inward deformations in the ventral nuclei, more prominently on the right side. This significance between the groups was observed even after correcting for the false discovery rate (FDR) and after controlling for the potential confounding effects of age and sex (FDR corrected F=5.0; P<0.05) [Figure 1].
Figure 1.
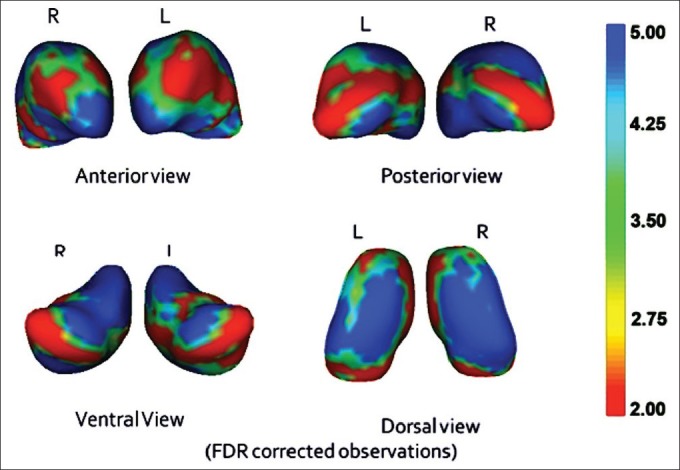
The differences in regions of thalamus between schizophrenia patients and healthy controls (significant inward deformation in blue)
Post hoc analysis showed significant correlation; increasing inward deformations with increasing negative syndrome score in the right pulvinar and the right mediodorsal nuclei (P<0.05). However, there were no significant shape correlations with respect to positive symptoms, age at onset and duration of illness [Figure 2].
Figure 2.
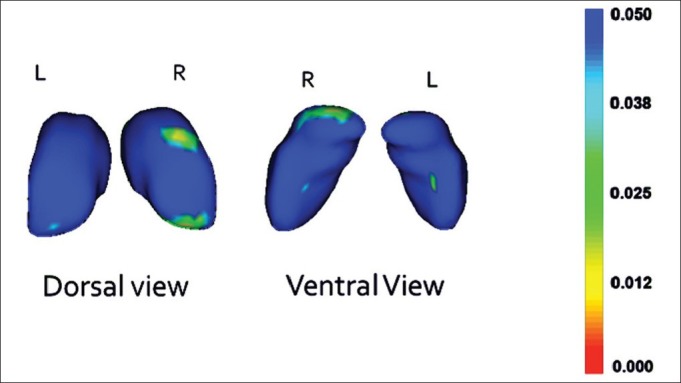
The regions of thalamus having inward deformity in correlation with the negative syndrome score in patients with schizophrenia (Significant areas in green and yellow)
DISCUSSION
This study examined the thalamic shape differences between schizophrenia patients and age-, sex-, and handedness-matched healthy controls. The shape of thalamus is significantly different with inward deformations (FDR corrected) at the anterior, ventromedial, the mediodorsal, and pulvinar regions of the thalamus. A significant direct correlation was found between negative syndrome score and the shape abnormalities in anterior dorsolateral and the posterior aspects of right thalamus corresponding to right pulvinar and the right mediodorsal nuclei. This suggests that more the negative syndrome score, more will be the inward deformation in these regions. These findings are in tune with the previous studies which report involvement of anterior nucleus, mediodorsal nucleus, and pulvinar nucleus volume deficits in schizophrenia.[7,8,9,10]
Thalamus has been implicated in having a central role in cerebral development.[18] Defects in thalamic neurogenesis have been previously hypothesized to have influenced the neuroanatomical abnormalities of cerebral cortex in schizophrenia.[19] The frontal lobe has an extensive interconnection with the medial dorsal nucleus, while the temporal lobe is relatively more strongly interconnected with the pulvinar nucleus.[20] Postmortem studies looking into these nuclei of thalamus have revealed reduced neuronal density and number.[21,22,23] Studies looking into the relationship of course and outcome of schizophrenia with different brain regions have reported that the poorer outcome is seen in patients who exhibit deficits in frontal, superior temporal, and thalamic nuclei, which imply that these areas along with their interconnections have prominent role in negative symptoms and in turn poorer outcome.[24,25]
Thalamus is a central relay station – relaying information to the cortical regions and receiving inputs back. The medial dorsal nucleus, the anteroventral, and the anteromedial nuclei of thalamus are have reciprocal connections with Dorsolateral Prefrontal Cortex (DLPFC), Orbito-Frontal Cortex (OFC), and the insular cortices, all of which have been implicated in schizophrenia.[26] Thalamus acts as a central gate in connections between these regions. Thalamus has also been implicated in modulating the neural maturation of other cortical structures, and irradiation of the brain in second trimester has shown decreased neuronal density and volume in thalamus, and this has direct correlation with frontal lobe deficits – neuropathologically very similar to schizophrenia.[19]
Neurodevelopment hypothesis of schizophrenia explains synaptic pruning as process in which reorganization of cortical brain connection occurs through a programmed synaptic restructuring.[27] Schizophrenic symptoms may be due to aberrant migration or impaired pruning in the thalamocortical circuits and excessive pruning in thalamus might leads on to decreased thalamic volume and in turn frontal cortical regions leading on to negative symptoms. The volume deficits of thalamus have been attributed to pathological pruning, and the shapes deformed in the thalamus – mediodorsal, anterior, ventromedial, and pulvinar nuclei might be the areas where the process is active, supporting the neurodevelopmental theory of schizophrenia.
Methodological issues
To the best of our knowledge, this is the first study to incorporate FSL – FIRST vertex analysis methodology to study the shape abnormalities in antipsychotic naïve schizophrenia patients. Some of the methodological advantages include antipsychotic-naïve status of the patients during the assessments, SCID interview to establish the diagnosis of the patients, independent confirmation of the diagnosis by an experienced psychiatrist, sex-, education-, handedness and socioeconomic status matched controls, and use of FSL – FIRST vertex analysis – which improves the segmentation and registration of the vertices in turn giving a better common space alignment and accurate results.
Potential implications and future directions
This study takes a step further in understanding the specific areas deformed which lead on to volume deficits in subcortical structures in schizophrenia. This helps in focussing further neuropathological and chemical imaging studies focusing on these regions. Further studies involving neuroendocrine, neurotropic as well as immunological correlates of thalamus in schizophrenia might further our understanding of role of thalamus in pathogenesis of schizophrenia.
CONCLUSION
There is a significant difference in the shape of thalamus corresponding to anterior, mediodorsal, ventromedial, and pulvinar regions between schizophrenia patients and age-, sex-, and handedness-matched healthy controls. There is a significant correlation between negative syndrome and the shape abnormalities in mediodorsal and the posterior aspects of right thalamus. These findings support the neurodevelopmental model of schizophrenia.
ACKNOWLEDGMENT
The authors thank the patients and their relatives for their valuable time given for study assessments. This study was supported by Indo-US research Grant. Sunil V. Kalmady is supported by the Wellcome Trust/DBT India Alliance.
Footnotes
Source of Support: Indo-US research Grant
Conflict of Interest: None.
REFERENCES
- 1.Levitt JJ, Bobrow L, Lucia D, Srinivasan P. A selective review of volumetric and morphometric imaging in schizophrenia. Curr Top Behav Neurosci. 2010;4:243–81. doi: 10.1007/7854_2010_53. [DOI] [PubMed] [Google Scholar]
- 2.Rao NP, Kalmady S, Arasappa R, Venkatasubramanian G. Clinical correlates of thalamus volume deficits in anti-psychotic-naive schizophrenia patients: A 3-Tesla MRI study. Indian J Psychiatry. 2012;52:229–35. doi: 10.4103/0019-5545.70975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 4.Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–91. doi: 10.1016/j.pnpbp.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Res. 30;183:144–50. doi: 10.1016/j.pscychresns.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–20. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 7.Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. Thalamic morphology in schizophrenia and schizoaffective disorder. J Psychiatr Res. 2011;45:378–85. doi: 10.1016/j.jpsychires.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci. 2007;27:13835–42. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coscia DM, Narr KL, Robinson DG, Hamilton LS, Sevy S, Burdick KE, et al. Volumetric and shape analysis of the thalamus in first-episode schizophrenia. Hum Brain Mapp. 2009;30:1236–45. doi: 10.1002/hbm.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu A, Zhong J, Graham S, Chia MY, Sim K. Combined analyses of thalamic volume, shape and white matter integrity in first-episode schizophrenia. Neuroimage. 2009;47:1163–71. doi: 10.1016/j.neuroimage.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 11.4th ed. Washington DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JB. User's Guide. New York: American Psychiatric Press; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- 13.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): Conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;1:49–58. [PubMed] [Google Scholar]
- 14.Andreasen NC. University of Iowa; 1984. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- 15.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 16.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, et al. Combining shape and connectivity analysis: An MRI study of thalamic degeneration in Alzheimer's disease. Neuroimage. 2010;49:1–8. doi: 10.1016/j.neuroimage.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Bendito G, Molnar Z. Thalamocortical development: How are we going to get there? Nat Rev Neurosci. 2003;4:276–89. doi: 10.1038/nrn1075. [DOI] [PubMed] [Google Scholar]
- 19.Selemon LD, Wang L, Nebel MB, Csernansky JG, Goldman-Rakic PS, Rakic P. Direct and indirect effects of fetal irradiation on cortical gray and white matter volume in the macaque. Biol Psychiatry. 2005;57:83–90. doi: 10.1016/j.biopsych.2004.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bachevalier J, Meunier M, Lu MX, Ungerleider LG. Thalamic and temporal cortex input to medial prefrontal cortex in rhesus monkeys. Exp Brain Res. 1997;115:430–44. doi: 10.1007/pl00005713. [DOI] [PubMed] [Google Scholar]
- 21.Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Danos P, Schmidt A, Baumann B, Bernstein HG, Northoff G, Stauch R, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: A replication study. Psychiatry Res. 2005;140:281–9. doi: 10.1016/j.pscychresns.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Young KA, Manaye KF, Liang C, Hicks PB, German DC. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry. 2000;47:944–53. doi: 10.1016/s0006-3223(00)00826-x. [DOI] [PubMed] [Google Scholar]
- 24.Preuss UW, Zetzsche T, Jager M, Groll C, Frodl T, Bottlender R, et al. Thalamic volume in first-episode and chronic schizophrenic subjects: A volumetric MRI study. Schizophr Res. 2005;73:91–101. doi: 10.1016/j.schres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Brickman AM, Buchsbaum MS, Shihabuddin L, Byne W, Newmark RE, Brand J, et al. Thalamus size and outcome in schizophrenia. Schizophr Res. 2004;71:473–84. doi: 10.1016/j.schres.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- 27.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–65. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]


