Abstract
Background:
Obsessive-compulsive disorder (OCD) is increasingly being viewed as a multidimensional heterogeneous disorder caused due to the dysfunction of several closely related, overlapping frontostriatal circuits. A study investigating the dimensional construct in treatment naïve, co-morbidity free patients with identical handedness is likely to provide the necessary homogeneity and power to elicit neural correlates of the various symptom dimensions, and overcome the limitations of previous studies.
Materials and Methods:
Nine DSM-IV OCD patients with predominant contamination-related obsessive-compulsive symptoms (age=29.8±7.1 years; five males: four females; years-of-education=13.9±1.6, YBOCS total score=28.8±4.7, DYBOCS Contamination dimension score=10.7±1.8) and nine healthy controls matched one to one with the patients for age, sex, and years of education (age=27.8±5.4, five males: four females; years-of-education=14.9±3.0), were examined during symptom provocation task performance in 3TMRI. Paired samples t test of brain activation differences (contamination relevant pictures – neutral pictures), limited to apriori regions of interest was done using SPM8 (uncorrected P<0.005).
Results:
Patients found significantly more pictures to be anxiety provoking in comparison to healthy controls. Patients were found to have deficient activation in the following areas in comparison with healthy controls: bilateral anterior prefrontal, dorsolateral prefrontal, orbitofrontal, anterior cingulate, insular and parietal cortices, precuneus, and caudate.
Conclusions:
Results underscore the importance of frontal, striatal, parietal, and occipital areas in the pathophysiology of OCD. Divergence of findings from previous studies might be attributed to the absence of confounding factors in the current study and may be due to production of intense anxiety in patients.
Keywords: Functional magnetic resonance imaging, hypoactivation, obsessive-compulsive disorder, symptom provocation, washers
INTRODUCTION
Obsessive-compulsive disorder (OCD), seen in 2-3% of the general population;[1] it is chronic and highly disabling condition characterized by the presence of obsessions in the form of intrusive and distressing thoughts, ideas, or images, and by the urge to perform repetitive or ritualistic behaviors known as compulsions.[2] OCD affects the personal, family, and occupational functioning of the individual and has severe impact on the family and is associated with poor quality of life.[3]
Studies exploring the neurobiology of OCD have consistently implicated the orbitofrontal cortex (OFC), the anterior cingulated cortex (ACC) and the basal ganglia.[4] This has led to the formulation of the traditional frontostriatal model for explaining the neural basis of this disorder.[5] Other areas that are often implicated in the pathophysiology of this disorder include the dorsolateral prefrontal cortex (DLPFC), components of the limbic system and thalamus. Lately, much importance has also been attached to the presence of parieto-cerebellar dysfunction,[4] and the neuroanatomical model has subsequently been expanded to include areas such as the parietal cortex, cerebellum, and several elements of the limbic circuitry.[4,5] Thus, the current understanding of this disorder posits the presence of a network level defect in the ventral frontostriatal circuits and the dorsal circuits involving the DLPFC, dorsal striatum, and the parieto-cerebellar regions.[4]
In the last few years, the dimensional concept of OCD has aroused much interest.[6] This perspective views OCD as a heterogeneous disorder caused due to the dysfunction of several closely related, overlapping fronto-striatal circuits. The phenomenology of OCD is thus broken down into separate dimensions, namely (1) aggressive obsessions, pathological doubts and related compulsions; (2) sexual and religious obsessions and related compulsions; (3) symmetry, ordering, counting, and arranging obsessions and compulsions; (4) contamination obsessions and cleaning compulsions; and (5) hoarding and collecting obsessions and compulsions. Research along this model has provided new insights; different symptom dimensions in OCD have been shown to have distinct neural correlates. A standardized tool – the Maudsley obsessive-compulsive stimuli set (MOCSS)– has been developed to provoke symptoms in patients with different dimensions of OCD.[7] Lately, the technique of virtual reality has been successfully used to provoke symptoms in OCD patients.[8]
Patients with OCD have characteristic bouts of symptoms precipitated by specific environmental or internal cues, outside of which, they are, usually, apparently normal. Hence, experimental protocols that rely on the symptom provocation paradigm, where the neural activity is studied during the artificial induction of the symptomatic state, seem to be more relevant to elicit neural patterns of activations related to the emergence of obsessive-compulsive symptoms than paradigms based on measuring functional activity during a “resting state.”[9] Functional imaging studies using the symptom provocation design have contributed significantly to the understanding of the underlying neurobiology of this complex disorder, pointing out the involvement of the fronto-striato-thalamo-parietal network in symptom generation.[10,11,12,13,14,15,16,17,18] However, there are inconsistent results, possibly due to heterogeneity of disease phenotype in OCD; most neuroimaging studies of OCD have grouped together patients with mixed symptoms thus potentially reducing their power and obscuring their findings.[19] Another issue of some import for a symptom provocation study is the cultural acceptability of the stimuli used. Even though the symptom dimensions and the nature of the motor acts is strikingly similar across populations,[20,21] the content of obsessions is an important exception, and is significantly influenced by the ethnic and cultural background.[22] Since symptom provocation paradigms focus on the generation of anxiety by simulating the content of the subject's obsessions, it is important that the tool conform to the cultural norms of the population under study.
Research in understanding the neurobiology of different dimensions is still preliminary. A few studies that examined the neural correlates of dimensional model have suffered methodological limitations. One major confounding factor was that these studies included subjects who were on psychotropic medications during or till a few weeks before the scanning procedure,[7,19,23,24] which can potentially alter the findings,[9] especially in a symptom provocation paradigm. The presence of co-morbid axis I disorders, notably depression and other anxiety disorders was not excluded either. This limits the conclusions that can be drawn from the data collected. To the best of our knowledge, till date, no published fMRI study has used the dimensional approach to examine brain activation in a symptom provocation paradigm in treatment naïve, co-morbidity free OCD patients.
Therefore, a study investigating the dimensional construct in treatment naïve, co-morbidity free patients is needed. It is likely to provide the necessary homogeneity and power to elicit neural correlates of the various symptom dimensions in OCD. Further, use of a standardized symptom provocation tool – the MOCSS – adapted to suit the Indian context will lead to valid provocation of symptoms. In this study, we aim to study the neural correlates of symptom provocation in drug naïve or drug free patients with contamination dimension of OCD. Based on the earlier studies, we hypothesize that the neural activation in patients will be significantly different compared to controls in the anterior prefrontal cortex (aPFC), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), insula, parietal cortex, precuneus, thalamus, and basal ganglia.[5,9,19]
MATERIALS AND METHODS
Subjects
Nine patients fulfilling the criteria for diagnosis of OCD with according to DSM IV –TR with duration of illness longer than six months were recruited from among the patients attending OCD-clinic and adult psychiatry services of National Institute of Mental Health And Neurosciences (NIMHANS), Bangalore. The patients had to be right handed and either drug naïve or drug free for a minimum of six months for consideration for inclusion in the study. Five of the patients were drug naïve while four were drug free. All subjects identified contamination-related obsessions and associated compulsions are a prominent part of their illness. Nine healthy controls, matched one to one for age, sex, and years of education with the patients (P>0.05) were recruited by word of mouth. Exclusion criteria were: Mild OCD symptoms (yale-brown obsessive-compulsive scale (YBOCS) <16),[25,26] history of head injury or independent neurological illness or history of hereditary neurological illness, presence of co-morbid Axis-I disorder as assessed by Mini international neuropsychiatric interview-M.I.N.I. Plus,[27] score of more than eight on Hamilton depression rating scale –HDRS,[28] or more than 13 on Hamilton Rating Scale for Anxiety – HARS,[29] clinically significant hoarding (Score>14 in Hoarding rating scale-Interview),[30] MMSE score <24, pregnant or lactating, any contraindication for undergoing an Magnetic Resonance Imaging (MRI) procedure.
The patients’ mean illness duration was 6.9 years (SD, 7.2 years; range, 1-20 years). The OCD severity was moderate to severe (YBOCS total: Mean, 28.8; SD, 4.7; obsessions: Mean, 14.2; SD, 2.6; compulsions: Mean, 14.6; SD, 2.3). Dimensional YBOCS was applied for all subjects.[31] The mean total score in the contamination dimension for the subjects was 10.7 (SD, 1.8). The protocol was approved by the Ethics Committee of the NIMHANS, Bangalore, India and all participants were recruited after obtaining written informed consent.
Measures
Severity and types of OCD symptoms were assessed with the yale-brown obsessive-compulsive scale and the symptom checklist.[25,26] The symptom dimension scores were obtained with the dimensional YBOCS (three items for each dimension, score range 3-15).[31] The presenting author and another author (UB) rated five OCD subjects in turn, with simultaneous symptom rating for inter-rater reliability (IRR). The IRR for YBOCS scale scores as well as dimensional YBOCS scores was found to be excellent as ascertained by intra class correlation coefficient >0.8.
Stimuli
Pictures depicting contamination/washing, aggressive/doubts/checking, and symmetry were chosen from the MOCSS. MOCCS is a stimulus set containing 300 pictures in all, 50 each in contamination, checking, symmetry, and hoarding dimensions, 50 general aversive and 50 neutral pictures. The neutral and general aversive pictures have been selected from the International Affective Picture System (IAPS),[32] while the OCD relevant stimuli has been chosen from a larger pool of pictures depicting common OCD triggers by experienced clinicians. The stimulus set has demonstrated good validity and has been shown to reliably provoke symptoms in patients with OCD.[7] Thirty pictures were chosen for each dimension in the current study. Three clinicians with experience in OCD were shown the pictures to ensure cross cultural validation of images. In case an image was perceived as culturally inappropriate, it was selected for replacement with a similar picture from Indian background to increase cultural validity. For example, the image of a western toilet was replaced with an Indian one. Likewise the picture of a device meant to assist pedestrians with crossing the road was replaced with the picture of an elevator call button since the former is not commonly seen in India. A total of 37 images were selected in this way. Before the change was made, all the pictures and their proposed replacements were shown to an unrelated group of 18 patients and five healthy controls. Out of the 37 proposed replacements, 21 were found to be more culturally appropriate and evoked equivalent or more anxiety than those in the original set. These 21 pictures were replaced with their Indianized version. Further, all replacements were done in consultation with the developers of the original instrument. Fourteen pictures in the contamination dimension, five pictures in the checking dimension and two pictures in the symmetry dimension from the original set were changed. Thirty color pictures of scenes rated as aversive or disgusting by normal subjects (e.g., insects, mutilated bodies, and decaying food) and 120 pictures of neutral scenes (e.g., furniture, nature scenes, and household items) were selected from IAPS.[32] These stimuli were carefully chosen to avoid resembling common triggers of OCD symptoms. While many of these pictures formed a part of MOCCS, new pictures were added to avoid repetition of any image during the course of the paradigm. Similar principles as used during the design of the MOCSS were implemented during the broadening of the stimuli set. All new images taken from the IAPS were group matched for various measures of visual and affective content of the pictures such as dominance, arousal, and complexity with the pictures in the original set.
Symptom provocation paradigm
All subjects participated in a 91/2 min task involving the presentation of pictures by means of a display monitor viewed through a reflecting mirror mounted on the head coil while undergoing MRI. Subjects viewed forty12 s alternating blocks of emotional (washing, checking, or symmetry related or normally aversive) and neutral pictures. The paradigm had four equal “parts”: Washing relevant, checking relevant, symmetry relevant, and general aversive. The order in which these parts were conducted was fully counterbalanced, except that the general aversive part was always conducted last and all scans started with the neutral block first. The entire paradigm consisted of 240 pictures – all unique with no repetition of any stimulus. In each part, a total of 30 emotional pictures (depending on the part) in five blocks of six pictures each and 30 neutral pictures (in five blocks of six pictures each) were shown to subjects. Pictures were shown on the screen for 2 s each. Within this time, subjects had to indicate by means of button presses whether they felt anxious after seeing the picture or not. The beginning of each block of pictures was preceded by relevant instruction slide [Figure 1].
Figure 1.
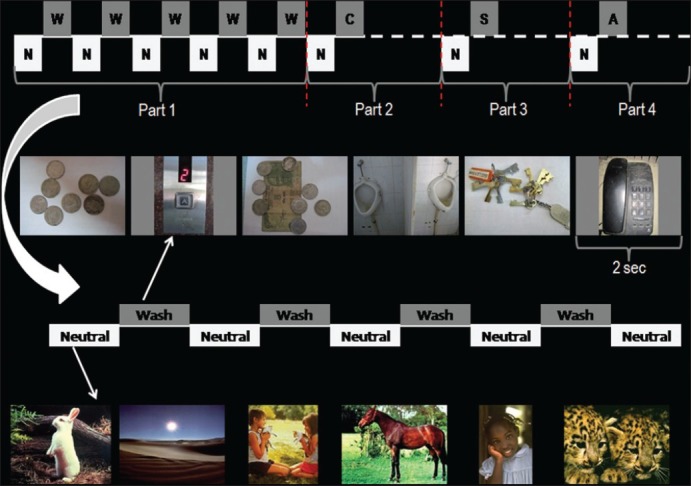
Schematic depiction of the symptom provocation paradigm. N=neutral block; W=washing/contamination relevant block; C=checking relevant block; S=symmetry relevant block; A=general aversive block. The schematic is for representational purposes only. All parts were of equal duration. Each block consisted of six pictures displayed for 2 s each
Subjects underwent a 5 min training procedure prior to the scan where they were familiarized with the instructions and the task. The pictures used for training were different from what were shown in the scanner. The following instructions were presented to the subjects. They have been modified from the MOCCS instructions to suit local needs. “Imagine that you have to touch what's shown in the following pictures and you are unable to wash your hands after that” (washing); imagine that you are not sure whether you switched off or locked the following objects and it is impossible for you to go back and check” (checking); imagine that somebody has messed up your things and you are unable to rearrange them”(symmetry/order); “imagine that you must touch or stand by the following objects” (aversive); “imagine that you are completely relaxed while looking at the following scenes” (neutral). Subjects received the instructions in full during the training phase. During the MRI, the gist of the instruction was presented in brief before the beginning of each block.
Image acquisition
Functional MRI (fMRI) scans were obtained with a Seimens Skyra scanner using a 32 channel coil. Three scans were rejected before the task began. The scan parameters are as follows: TR=2000 ms; TE=30 ms; flip angle=78; slice thickness=3 mm; slice order: Descending; slice number=37; gap=25%; matrix=64*64*64 mm 3, FOV=192*192, voxel=3.0 mm isotropic). A total of 240 scans were obtained during the paradigm, one for each picture. Hence, each part of the paradigm consisted of 30 emotionally relevant scans and 30 neutral scans. This paper describes results only for the contamination/washing relevant part.
Data analysis
Deoxyhemoglobinis paramagnetic, while it becomes diamagnetic when oxygenated. Hence, the magnetic signal of blood changes depending the relative concentration of oxy and Deoxyhemoglobin. This signal difference is detected during functional MRI as the blood oxygenation level-dependent (BOLD) contrast. The scan data were assessed using SPM8. SPM combines the General Linear Model and Gaussian field theory to draw statistical inferences from BOLD response data regarding deviations from the null hypothesis in three-dimensional brain space. Images were realigned, spatially normalized, and smoothed followed by first-level design specification and estimation where brain activation differences (Contamination/Washing - Neutral) were generated for each subject. Group level random effect analysis using a paired samples t-test was performed to examine for differences in BOLD response between patients and healthy controls. Inclusion masks comprising bilateral anterior prefrontal cortex (aPFC), orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), insula, parietal cortex, precuneus, thalamus, and basal ganglia were used for all analysis based on the a priori regions of interest. These masks were created using an automated software.[33] The coordinates of significant areas of activation were transformed from MNI space into the stereotactic space of Talairach and Tournoux,[34,35] using nonlinear transform.[36] With the help of automated software, brain regions were localized from the Talairach and Tournoux coordinates.[37] Activations surviving uncorrected P<0.005 were reported.
RESULTS
Demographic details
The patients were 29.8±7.2 years of age, four female, five male. Age of onset of illness was 22.9±7.0 years. They had studied for 13.9±1.6 years. The controls were 27.8±5.4, years of age, four female, five male, and had studied for 14.9±3.0 years. Subject-specific matching with subsequent paired-samples t-test did not show any significant difference (P>0.05). The sex ratio was similar in both groups (i.e., 5:4).
Behavioral data
The “anxiety” and “no anxiety” responses provided by subjects to the contamination related pictures during the symptom provocation paradigm were tabulated. Each subject was shown 30 contamination related pictures. Among OCD patients (nine in all), a total of 202 “anxiety” responses were obtained while 68 responses were “no anxiety”. In healthy controls, 165 “anxiety” and 105 “no anxiety” responses were obtained. A Chi-square analysis revealed the difference between the two groups to be statistically significant (P=0.001).
Image analysis
Patients were found to have deficient activation in the following areas in comparison with healthy controls: Bilateral aPFC, bilateral DLPFC, bilateral OFC, bilateral ACC, bilateral insular cortex, bilateral parietal cortex, bilateral precuneus, and bilateral caudate [Table 1 and Figure 2].
Table 1.
Brain regions showing deficient activation in patients as compared to controls
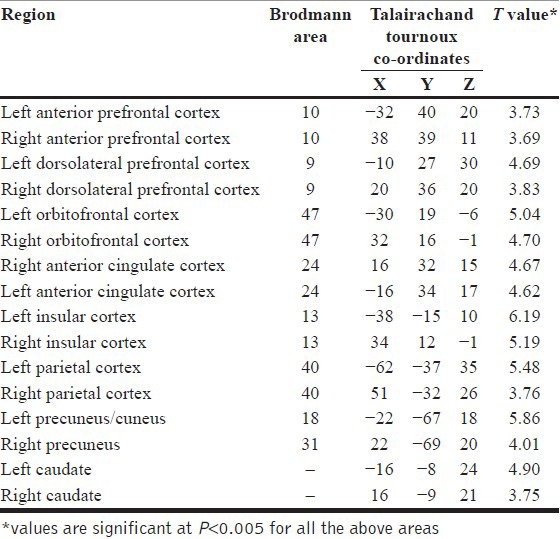
Figure 2.
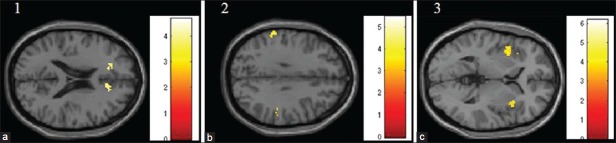
Deficient activation in brain areas in patients in comparison to controls. Brain images showing deficient activations in (a) bilateral anterior cingulate cortex; (b) bilateral parietal cortex; and (c) bilateral insula in patients in comparison to healthy controls. Images have been obtained using masks for the respective brain regions (uncorrected P<0.005). The colored bars are representative of t scores mentioned in Table 1
DISCUSSION
To the best of our knowledge, this is the first research report to evaluate medication-naïve or long-term medication-free OCD patients without any other Axis I co-morbidity using a multidimensional symptom provocation paradigm. They were assessed using standard tools and the dimensional nature of their pathology quantified using the D-YBOCS. The symptom provocation paradigm was modified from a standardized stimuli set and it was adapted to suit local needs better in a scientifically informed manner by taking into account the opinion of three experienced clinicians and 23 pilot subjects independent of the main study sample. These methodological strengths add to uniqueness of this study in examining OCD patients without the potential confounds of medications and co-morbidity; however, the inherent difficulties in recruiting such patients (i.e., medication-naïve/free and co-morbidity free) has resulted in relatively smaller sample. Though this might be construed as a potential limitation, it has to be noted that this is among the largest study sample that has reported fMRI observations in medication-naïve/free adult OCD subjects.
Our results demonstrate deactivation in several of the areas implicated in the pathophysiology of OCD that is broadly not in keeping with the published literature till date. The symptom provocation literature focusing on different dimensions in OCD is quite scanty. The only functional imaging study in adults using a dimensional approach, where washing/contamination dimension was looked at,[19] found that patients showed increased activation in OFC, ACC, DLPFC, and caudate among other brain regions in comparison to healthy controls. In this study, patients showed decreased activation in ventrolateral prefrontal cortex, lingual gyrus, and middle occipital gyrus. The only other fMRI study investigating the provocation of contamination/washing symptoms has been done on children.[23] This study reports decreased activation in right insula, putamen, thalamus, DLPFC, and left OFC in patients when compared to controls during viewing of contamination relevant stimuli, which is in line with our findings. Another study that evaluated adult washers with perfusion weighted imaging also reported hyperactivation in the frontostriatal areas, but this study did not include a control group.[24] Other studies in the recent past have reported increased activation in various OCD relevant brain areas but have not evaluated the washing/contamination dimension.[14,15,18,38] Hence, most studies have looked at a composite group of patients without consideration of dimensions involved. In cases where dimensional approach has been used, medication and co-morbidity effects confound findings. In this study, a homogenous, albeit small, group of co-morbidity and drug naïve/free patients have been studied. These reasons might explain why our findings appear to be not in line with most of the symptom provocation literature.
Possible mechanism behind reduced brain activation
An attempt to understand deactivation of brain regions during provocation of anxiety leads one to other anxiety disorders. Intense anxiety, especially, in cases where the subject has not been pre-exposed to the anxiety provoking cues, has been reported to cause hypoactivation of the cingulate cortex.[39] It is hypothesized to reflect defective top-down emotional regulation leading to an intense anxiety state. Moreover, production of intense anxiety has been shown to decrease cerebral blood flow in several OCD-relevant areas like the orbitofrontal and insular cortex.[40,41] Since, the patients taken for the study had significant contamination related symptoms and the stimuli were novel for the patients while also being washing relevant, it might have elicited very strong anxiety reactions resulting in the hypoactivation of several brain regions. That patients reported significantly more pictures as anxiety provoking in comparison to healthy controls lends weight to this line of argument.
OCD as a disorder of neural circuitry
The current neurobiological understanding of OCD largely implicates the frontostriatal circuitry and dysfunction in its interconnections in the causation of the symptoms.[4,5,42] Persuasive arguments have been put forward concerning the role of the orbitofrontal cortex – chiefly its lateral division – in symptom generation.[43] However, recent studies have expanded our understanding of this debilitating disorder beyond the traditional frontostriatal model and have put forth a network level dysfunction involving several other brain areas such as the parietal cortex, cerebellum, DLPFC, insula, precuneus among others.[4,5,9] The larger network model proposes a dysfunctional interaction between the ventral circuit comprising the OFC (mostly posterior), ACC, ventral striatum, and limbic areas including the hippocampus and the dorsal circuit that comprises of the lateral and anterior OFC, DLPFC, dorsal striatum with probable interaction with the parietal cortex and cerebellum.[4] The ventral circuit is emotionally responsive while the dorsal responds to executive demands and has been hypothesized to underlie cognitive deficits seen in OCD. Our results also highlight the importance of these areas and appear to be in line with this larger conceptualization of this disorder.
To summarize, we have reported novel findings during symptom provocation in drug naïve/drug comorbidity free washers in comparison to healthy controls. We found group differences in brain areas previously known to be implicated in OCD, but these differences were in the opposite direction of published findings in adult OCD patients. This discrepancy could be due to confounding factors of heterogeneity, comorbidity, and psychotropic medication in the previous reports. Our results implicate frontal, striatal, parietal, and occipital regions and underscore their importance in the pathophysiology of this illness. These results need replication in a larger and more diverse sample of patients.
ACKNOWLEDGMENT
Indian Council of Medical Research Financial Grant Award to Dr. Sri Mahavir Agarwal for MD Thesis. Department of Biotechnology Research Grant to Professor Y.C. Janardhan Reddy.
Footnotes
Source of Support: This study is supported by the Department of Biotechnology (DBT), Government of India Grant awarded to YCJR. Indian Council of Medical Research (ICMR) gave a Financial Grant for MD Thesis to SMA. DJ and UB are supported by the DBT. VS is supported by the ICMR. SVK is supported by the Well come Trust/DBT India Alliance
Conflict of Interest: None.
REFERENCES
- 1.Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. doi: 10.1016/S0140-6736(02)09620-4. [DOI] [PubMed] [Google Scholar]
- 2.Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, et al. Pathophysiology of obsessive-compulsive disorder: A necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. 2004;72:195–221. doi: 10.1016/j.pneurobio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Fenske JN, Schwenk TL. Obsessive compulsive disorder: Diagnosis and management. Am Fam Physician. 2009;80:239–45. [PubMed] [Google Scholar]
- 4.Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Rev Neurother. 2009;9:255–69. doi: 10.1586/14737175.9.2.255. [DOI] [PubMed] [Google Scholar]
- 5.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mataix-Cols D. Deconstructing obsessive-compulsive disorder: A multidimensional perspective. Curr Opin Psychiatry. 2006;19:84–9. doi: 10.1097/01.yco.0000194809.98967.49. [DOI] [PubMed] [Google Scholar]
- 7.Mataix-Cols D, Lawrence NS, Wooderson S, Speckens A, Phillips ML. The maudsley obsessive-compulsive stimuli set: Validation of a standardized paradigm for symptom-specific provocation in obsessive-compulsive disorder. Psychiatry Res. 2009;168:238–41. doi: 10.1016/j.psychres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kim K, Kim CH, Cha KR, Park J, Han K, Kim YK, et al. Anxiety provocation and measurement using virtual reality in patients with obsessive-compulsive disorder. Cyberpsychol Behav. 2008;11:637–41. doi: 10.1089/cpb.2008.0003. [DOI] [PubMed] [Google Scholar]
- 9.Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocation of obsessive-compulsive symptoms: A quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 10.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 11.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 12.Adler CM, McDonough-Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM. fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res. 2000;34:317–24. doi: 10.1016/s0022-3956(00)00022-4. [DOI] [PubMed] [Google Scholar]
- 13.Rauch SL, Shin LM, Dougherty DD, Alpert NM, Fischman AJ, Jenike MA. Predictors of fluvoxamine response in contamination-related obsessive compulsive disorder: A PET symptom provocation study. Neuropsychopharmacology. 2002;27:782–91. doi: 10.1016/S0893-133X(02)00351-2. [DOI] [PubMed] [Google Scholar]
- 14.Shapira NA, Liu Y, He AG, Bradley MM, Lessig MC, James GA, et al. Brain activation by disgust-inducing pictures in obsessive-compulsive disorder. Biol Psychiatry. 2003;54:751–6. doi: 10.1016/s0006-3223(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–10. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int J Psychophysiol. 2005;57:69–77. doi: 10.1016/j.ijpsycho.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Sanematsu H, Nakao T, Yoshiura T, Nabeyama M, Togao O, Tomita M, et al. Predictors of treatment response to fluvoxamine in obsessive-compulsive disorder: An fMRI study. J Psychiatr Res. 2010;44:193–200. doi: 10.1016/j.jpsychires.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–38. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 19.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–76. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 20.Pallanti S. Transcultural observations of obsessive-compulsive disorder. Am J Psychiatry. 2008;165:169–70. doi: 10.1176/appi.ajp.2007.07111815. [DOI] [PubMed] [Google Scholar]
- 21.Zor R, Fineberg N, Hermesh H, Asigo G, Nelson S, Agha H, et al. Are there between-country differences in motor behavior of obsessive-compulsive disorder patients? CNS Spectr. 2010;15:445–55. doi: 10.1017/s1092852900000377. [DOI] [PubMed] [Google Scholar]
- 22.Fontenelle LF, Mendlowicz MV, Marques C, Versiani M. Trans-cultural aspects of obsessive-compulsive disorder: A description of a Brazilian sample and a systematic review of international clinical studies. J Psychiatr Res. 2004;38:403–11. doi: 10.1016/j.jpsychires.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert AR, Akkal D, Almeida JR, Mataix-Cols D, Kalas C, Devlin B, et al. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: A functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2009;48:936–44. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 24.Chen XL, Xie JX, Han HB, Cui YH, Zhang BQ. MR perfusion-weighted imaging and quantitative analysis of cerebral hemodynamics with symptom provocation in unmedicated patients with obsessive-compulsive disorder. Neurosci Lett. 2004;370:206–11. doi: 10.1016/j.neulet.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 26.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 27.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 30.Tolin DF, Frost RO, Steketee G. A brief interview for assessing compulsive hoarding: The hoarding rating scale-interview. Psychiatry Res. 2010;178:147–52. doi: 10.1016/j.psychres.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, et al. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): An instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. 2006;11:495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- 32.Lang PJ, Bradley MM, Cuthbert BN. New York: NIMH Center for the Study of Emotion and Attention; 1997. International Affective Picture System (IAPS): Technical manual and affective ratings. [Google Scholar]
- 33.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 34.Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, TM P. 3D statistical neuroanatomical models from 305 MRI volumes. IEEE Nucl Sci Symp Medical Imaging Conf Proc. 1993;108:1877–8. [Google Scholar]
- 35.Talairach P, JA T. Stuttgart: Thieme; 1988. A stereotactic co-planar atlas of human brain. [Google Scholar]
- 36.Brett M, Johnsrude IS, Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–9. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- 37.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. To discard or not to discard: The neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol Psychiatry. 2009;14:318–31. doi: 10.1038/sj.mp.4002129. [DOI] [PubMed] [Google Scholar]
- 39.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zohar J, Insel TR, Berman KF, Foa EB, Hill JL, Weinberger DR. Anxiety and cerebral blood flow during behavioral challenge. Dissociation of central from peripheral and subjective measures. Arch Gen Psychiatry. 1989;46:505–10. doi: 10.1001/archpsyc.1989.01810060025005. [DOI] [PubMed] [Google Scholar]
- 41.Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. Am J Psychiatry. 2001;158:1220–6. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 42.Huey ED, Zahn R, Krueger F, Moll J, Kapogiannis D, Wassermann EM, et al. A psychological and neuroanatomical model of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 2008;20:390–408. doi: 10.1176/appi.neuropsych.20.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rotge J-Y, Langbour N, Jaafari N, Guehl D, Bioulac B, Aouizerate B, et al. Anatomical alterations and symptom-related functional activity in obsessive-compulsive disorder are correlated in the lateral orbitofrontal cortex. Biol Psychiatry. 2010;67:e37–8. doi: 10.1016/j.biopsych.2009.10.007. [DOI] [PubMed] [Google Scholar]


