Abstract
Previous behavioral work suggests that processing information in relation to the self enhances subsequent item recognition. Neuroimaging evidence further suggests that regions along the cortical midline, particularly those of the medial prefrontal cortex, underlie this benefit. There has been little work to date, however, on the effects of self-referential encoding on source memory accuracy or whether the medial prefrontal cortex might contribute to source memory for self-referenced materials. In the current study, we used fMRI to measure neural activity while participants studied and subsequently retrieved pictures of common objects superimposed on one of two background scenes (sources) under either self-reference or self-external encoding instructions. Both item recognition and source recognition were better for objects encoded self-referentially than self-externally. Neural activity predictive of source accuracy was observed in the medial prefrontal cortex (BA 10) at the time of study for self-referentially but not self-externally encoded objects. The results of this experiment suggest that processing information in relation to the self leads to a mnemonic benefit for source level features, and that activity in the medial prefrontal cortex contributes to this source memory benefit. This evidence expands the purported role that the medial prefrontal cortex plays in self-referencing.
Keywords: Medial prefrontal cortex, Self-reference, Source memory
Introduction
Most every individual has a sense of self they have accrued over a lifetime of experiences with the world. This schema for the self both guides aspects of social behavior (Amodio & Frith, 2006) and underlies many cognitive processes from theory of mind (Frith & Frith, 2003) to perspective taking (Vogeley & Fink, 2003) to the processing of reward (Knutson, Fong, Bennett, Adams, & Hommer, 2003). Within the laboratory, a variety of tasks have been used to induce self-relevant processing to investigate its effects on neural activity. Perhaps the most common task involves the evaluation of adjective traits for self-relevance. In this task, participants judge, for instance, whether they are “charming” or “kind.” While the adjective trait judgment directly evokes self-relevant processing, other experimental tasks have encouraged self-referential processing in a less direct manner. These other tasks induce self-relevant processes in participants through soliciting opinions, personal preferences, or subjective aesthetic evaluations. In one such study, participants rated their agreement with opinion statements such as “I think George Bush is a good president” (Zysset, Huber, Ferstl, & von Cramon, 2002) while in another study, participants selected which of two objects they preferred (S. C. Johnson et al., 2005). In yet another task, participants judged whether they found objects aesthetically pleasing (Jacobsen, Schubotz, Hofel, & Cramon, 2006). These tasks, as well as those based on adjective judgments, are self-referential to the extent that they depend on evaluation of stimuli based on a subjective, internal metric.
Given the central role the self-schema plays in numerous cognitive processes, it should come as little surprise that this sense of self serves as a useful lens through which we distill and retain new information. Work within the domain of memory has shown that processing information in relation to the self boosts subsequent item recognition relative to processing information via non-self-referential tasks (Rogers, Kuiper, & Kirker, 1977; Symons & Johnson, 1997). This memory effect—the self-reference effect in memory—is theorized to result from enhanced elaboration and organization the self schema provides to newly learned materials (Klein & Kihlstrom, 1986; Rogers, et al., 1977). While the self-reference effect has been well-established for item recognition, it is unclear whether episodic memory for contextual details of the episode, such as list membership, spatial location, or temporal order, might also benefit from self-referencing. Memory for these kinds of contextual associations can be measured objectively via a source memory task, in which participants indicate which experimentally manipulated context or detail (i.e. the source) was associated with an item during its initial exposure (M. K. Johnson, Hashtroudi, & Lindsay, 1993). Given the robust self-reference effect for item recognition (Symons & Johnson, 1997), it seems plausible that processing information in relation to the self might also improve source memory accuracy.
While little work has explicitly linked self-referencing to improved subsequent source memory, two lines of evidence suggests that such a relationship might exist. First, evidence suggests that source memory is improved for materials that are encoded in an emotional or social manner, such as deciding if food is spoiled/unsafe to eat or remembering whether information came from a trustworthy or a dishonest individual (May, Rahhal, Berry, & Leighton, 2005; Rahhal, May, & Hasher, 2002). In both of these tasks, it is plausible that participants engaged in self-relevant processing to meet task demands, which resulted in improved source accuracy. Second, in contrast to these studies that have measured source memory success objectively (i.e., by testing for a specific contextual detail), there is evidence that self-referencing yields higher subjective reports of recollection, as measured by the “remember-know” procedure (Conway & Dewhurst, 1995). Due to the subjective nature of recollection, however, it is difficult to know what contextual details were disproportionately supported by self-referencing in the Conway & Dewhurst study (1995). Overall, these lines of evidence converge on the notion that self-referential processing may strengthen memory for contextual details.
Neuroimaging work has found that cortical midline areas located within the prefrontal and parietal cortices show enhanced activity during the on-line performance of self-referential tasks compared to non-self relevant tasks (Craik et al., 1999; D’Argembeau et al., 2005; Fossati et al., 2003; Gutchess, Kensinger, & Schacter, 2007; Heatherton et al., 2006; Kelley et al., 2002; Kjaer, Nowak, & Lou, 2002; Lou et al., 2004; Northoff & Bermpohl, 2004; Northoff et al., 2006; Raposo, Vicens, Clithero, Dobbins, & Huettel, 2010). Further, accruing evidence suggests that these regions, specifically the medial prefrontal cortex (PFC), contribute to item recognition for self-referenced information. Two studies that have examined subsequent memory effects at encoding, (Gutchess, Kensinger, & Schacter, 2010; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004) and one study that has examined successful retrieval (Fossati et al., 2004) have reported medial prefrontal involvement. Specifically, Macrae et al. (2004) reported greater subsequent memory activity (subsequently recognized > subsequently forgotten) within the medial PFC for self-referenced adjectives compared to adjectives encoded self-externally. Similarly, Gutchess (2010) reported greater activity for subsequently recognized self-referenced adjectives compared to subsequently recognized other-referenced adjectives in a similar medial PFC region. Further, Fossati (2004) reported that activity in the medial PFC supported successful item recognition (hits > misses) of adjectives processed in reference to the self. Despite evidence of medial parietal involvement in online self-referential processing none of these memory studies reported memory effects within medial parietal cortex suggesting a somewhat limited role of this region for self-referential memory accuracy (but see Sajonz, et al. 2010). Taken together, these studies suggest a consistent role of the medial prefrontal cortex in the encoding and subsequent retrieval of material processed in reference to the self.
Previous neuroimaging reports investigating the effects of self-referencing on memory have almost exclusively focused on item recognition. As a consequence, little is known about the role that self-processing regions might play in source memory. One study, however, does offer evidence suggesting a role for the medial PFC in support of source memory accuracy, at least at the time of test. Benoit et al. (2010) examined test phase activity for a subset of trials where participants retrieved both item and source information and reported that ventromedial prefrontal cortex (BA 10) was more active during retrieval of these self-referenced trials relative to items encoded in a self-external task. While the Benoit study provides evidence that medial prefrontal cortex supports source retrieval at the time of test, it is unknown whether study phase activity in medial prefrontal cortex might also support subsequent source memory.
The current study was designed to assess the potential benefit to source memory accuracy for items encoded under self-reference and to determine the role of the medial PFC at the time of study and test on accurate source memory judgments. During study, participants made either self-referential (pleasant?) (Gusnard, Akbudak, Shulman, & Raichle, 2001; Raposo, et al., 2010), or self-external (similar color?) decisions about pictures of concrete objects presented against one of two background scenes (sources). During test, studied and unstudied objects were presented and participants judged which items they had seen previously and made two source decisions about which encoding context (task) and with which background (scene) the item was initially presented. Probing memory for multiple source features allows us to examine if self-referential encoding produces memory representations that are richer, containing more contextual details, and further, if medial PFC contribute to source accuracy for multiple self-referenced associations. Importantly, a response option was included at test that allowed participants to indicate that they did not know which source was associated with the recognized object, as has been implemented in prior studies (Duarte, Henson, & Graham, 2008; Duarte, Henson, Knight, Emery, & Graham, 2009; Gottlieb, Uncapher, & Rugg, 2010; Morcom, Li, & Rugg, 2007; Smith, Dolan, & Rugg, 2004). This procedure minimizes the possible dilution by guesses on estimates of source memory accuracy and associated neural activity (Rugg & Morcom, 2005). We hypothesized:
Source and item memory accuracy would be greater for objects encoded in the self-referential condition relative to the self-external condition.
Activation in regions including the hippocampus, lateral prefrontal and lateral posterior parietal cortex are likely to support source memory accuracy in a domain-general fashion, for a variety of contextual details and stimuli (Badre & Wagner, 2007; Dobbins & Wagner, 2005; Eichenbaum, Yonelinas, & Ranganath, 2007; Uncapher & Wagner, 2009; Vilberg & Rugg, 2008). Thus, source memory effects should be observed in these domain-general regions both for items encoded self-referentially and self-externally.
Critically, a subset of the regions engaged in self-referential processing of objects would also support successful memory of source information for those stimuli at study and at test. Specifically, we predict that areas within the medial PFC implicated in self-referential processing in previous studies (see above) may exhibit source memory effects for items self-referentially encoded to a greater extent than those encoded self-externally. Likewise, extrastriate regions sensitive to the color processing tapped by the self-external task (Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1990) may also show source memory effects particularly for items encoded under these instructions. It is reasonable to predict that these task-sensitive regions will contribute to recollection of source details at both study and test. That is, according to the cortical reinstatement hypothesis, regions active at the time of study are reactivated or reinstated at the time of test in support of successful episodic retrieval (Rugg, Johnson, Park, & Uncapher, 2008). Indeed recent neuroimaging evidence supports the notion that such a study-test overlap in functional activity occurs for episodes that are successfully recognized (J. D. Johnson, McDuff, Rugg, & Norman, 2009; J. D. Johnson & Rugg, 2007).
Methods
Participants
Fifteen young adults (range 18 – 30) recruited from the Georgia Institute of Technology and community solicitation participated in the experimental procedures. One participant was excluded on the basis of poor memory performance and excessive head movement resulting in the inclusion of 14 younger adults (mean age: 23.1, [18–30], 9 females). All participants were right-handed native-English speakers. None reported a history of psychiatric or neurological disorders (e.g. stroke, epilepsy, multiple sclerosis, etc.), vascular disease, or psychoactive drug use. None of the participants were taking CNS-active medications. Participants had normal or corrected-to-normal vision. Informed written consent in accordance with the Institutional Review Board at the Georgia Institute of Technology was obtained and all participants were paid $10 per hour for their participation.
Stimuli
A total of 320 objects and two scene backgrounds served as stimuli. At encoding, 240 objects were presented superimposed on one of two background scenes, the remaining objects were presented as novel items at retrieval. Across participants, objects appearing as study items and test items were counterbalanced. The objects were color photographs of common objects (e.g., saxophone, dog, chair, etc.) taken from the Hemera Technologies ® Photo-Objects DVDs and the two background scenes were color photographs of one mountain and one beach landscape. Objects subtended a maximum vertical and horizontal visual angle of approximately 2.2 degrees. Scenes subtended a maximum viewing angle of approximately 8.8 degrees.
Procedure
Prior to scanning, participants were trained on a short version of the study and test tasks. Training included a minimum of 14 practice study trials and 12 practice test trials. Participants verbally reported task comprehension of the study and test phases to the experimenter and, if necessary, continued practice on additional trials. No participant needed additional practice trials, however. Once in the MRI suite, participants were given noise-dampening ear-plugs and headphones, a 4-button response box in their right hand, and, further, were instructed to minimize all movements, especially head movements.
The study phase of the experiment was conducted over four scanning runs. A total of 240 trials were presented (half in each encoding condition). For each run a total of 30 trials per condition were displayed. Each study trial lasted 3750 ms including presentation of the object-scene pair for 3500 ms followed by a 250 ms central fixation (See Figure 1). To reduce task-switching costs, trials were presented in short blocks, or “mini-blocks”, of 10 trials per encoding condition. Before each mini-block, an instruction prompt saying “get ready for the self/color task” was displayed for 6000 ms. There were two encoding conditions during the study phase. In the self-referential condition, subjects judged whether they found the object-scene pairing pleasant (yes or no). The self-referential task instructions emphasized consideration of both the object and the scene while making the pleasantness judgment. Participants were encouraged to base their judgments on the perceptual features and discouraged from focusing on semantic features or the function of the object in relation to the scene (e.g. “I would like to play golf in the mountains”). In the self-external condition, participants were instructed to pick the dominant color of the object and then judge whether the background contained a similar color (yes or no). Importantly, in both the self-referential and self-external conditions, task instructions emphasized attention to both the object and the scene and to perceptual features of the pair. Further, because both decisions were designed to be subjective, task instructions made it clear there were no “correct” answers. All yes/no responses at the time of study were made with the index and middle fingers, respectively, of the right hand. Trials with no responses or too many responses as well as trials with response times less than 200 ms were excluded from analysis.
Fig. 1.
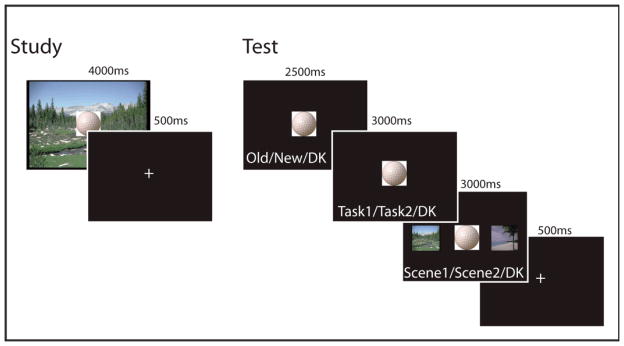
Trial schematic for the study and test phases respectively.
As in some previous neuroimaging self-referential investigations of both concrete words (Raposo, et al., 2010) and images (Gusnard, et al., 2001), we chose to use a pleasantness task to serve as the self-relevant condition. Similarly, previous experimental designs have employed self-relevant tasks that require subjective responses based on personal preference (Johnson, et al., 2005), aesthetics (Jacobsen et al., 2006), or personal opinions (Zysset, et al., 2002). Given that all of these studies have identified similar regional neural activity associated with self-referential processing, our use of the pleasantness task is quite suitable, we argue, for use as the self-referential encoding condition.
Following the study session, memory retrieval was tested over four scanning runs. All 240 objects seen at study were presented along with an additional 80 novel objects. For each run, 60 old items (half from each encoding condition, in a pseudorandom order such that no more than 4 trials of any one type were presented sequentially) and 20 novels items were presented. Each trial consisted of three recognition decisions—an item recognition decision followed by two source judgments (See Figure 1). Retrieval trials lasted 9750 ms and proceeded as follows: First, objects were displayed for 3250 ms with the prompt “Old | New | Don’t Know” written below the object in white font. This text served as the cue to make the item recognition judgment. The prompt subsequently changed to display “Self-Referential | Self-External | Don’t Know” for 3250 ms which served as the cue to make the task source judgment. Finally, the prompt changed to display “Mountain | Beach | Don’t Know” for 3250 ms. Coinciding with this prompt change, an image of the mountain scene and the beach scene appeared to the left and right of the object, respectively, serving as the cue to make the scene source decision. The “don’t know source” option was offered to reduce potential contamination by guessing on the source decision, as has been implemented in several similar studies (Duarte, et al., 2008; Duarte, et al., 2009; Gottlieb, et al., 2010; Morcom, et al., 2007; Smith, et al., 2004).
Within each retrieval run, equal numbers of studied objects from each of the four study scans were presented half of which were from each encoding conditions (self-referential, self-external). Trial types (studied, unstudied, self-referential, self-external) were presented in a pseudorandom order. Piloting showed equivalent source accuracy results regardless of which source decision occurred first (task or scene), as a consequence, we chose to present the task source prior to scene source decision for all subjects. Only those trials containing responses for all three retrieval judgments were included in the functional analysis. Trials with greater or fewer than three responses as well as trials with response times less than 200 ms were excluded. On average, about 1% of trials were excluded for these reasons at study and at test.
fMRI acquisition
All scans were acquired with a Siemens Trio 3T full body scanner (Siemens, Erlangen, Germany) using a 12-channel parallel imaging head coil. First, T1-weighted magnetization-prepared rapid gradient echo scans (MP-RAGE; TE= 4.52 ms, 256 × 256 FOV) were acquired over 160, 1-mm thick, sagittal slices to obtain high-resolution structural images. Functional data were acquired using a gradient echo pulse sequence (t2*-weighted, TR = 2000 ms, TE = 30ms, flip angle = 90°, 3-mm in-plane resolution), collected in 37 slices (interslice gap 17%) aligned to the anterior-posterior commissural line covering the entire cerebrum. A total of 134 volumes were collected during each study run and 313 volumes during each test run.
fMRI Analysis
Data were analyzed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology) implemented in MATLAB R2008a (The Mathworks Inc., Natick, MA). The first five volumes of each session were discarded to allow for equilibration effects. The resultant echo planar image (EPI) volumes were corrected for differences in slice acquisition time using the middle slice of each volume as the reference, and spatially realigned to the first acquired volume. Each participant’s structural scan was coregistered to the mean EPI image produced from the realignment and subsequently segmented and normalized to the Montreal Neurological Institute T1 average brain template. These normalization parameters were applied to all EPI volumes and the normalized EPIs were resliced to 3mm × 3mm × 3mm resolution and then spatially smoothed using an 8-mm full-width at half-maximum Gaussian kernel.
Analyses of the functional data from the study and test phases, which were modeled separately, were carried out in two steps. In the first step, neural activity was modeled as a series of one second epochs for study and three second epochs for test coinciding with onsets of the various event types and convolved with a canonical hemodynamic response function. The test phase included three response prompts but activity was modeled to the onset of the first prompt only, as participants were aware of all three response prompts and were likely planning for subsequent responses making it difficult to accurately model activity for each prompts separately. The time courses were down-sampled to the middle slice to form the covariates for the General Linear Model (GLM). For each participant and session, 6 covariates representing residual movement-related artifacts, determined by the spatial realignment step, were included in the first level model to capture residual (linear) movement artifacts. For both the study phase and the test phase GLMs, trials with too many or too few responses as well as trials with reaction times under 200 ms were not modeled. Voxel-wise parameter estimates for all covariates were obtained by Restricted Maximum-Likelihood (ReML) estimation, using a temporal high-pass filter (cut-off 128 seconds) to remove low-frequency drifts. As each session was modeled separately, the high pass filtering was conducted for each separate session. Intrinsic autocorrelations within each session were corrected by applying a first-order autoregressive [AR(1)] model. The data were also scaled to a grand mean of 100 over all voxels and scans (Friston, Ashburner, Kiebel, Nichols, & Penny, 2007).
Contrasts of the parameter estimates for each participant were submitted to the second stage of analysis (treating participants as a random-effect). Incorrect responses to unstudied items (“false alarms”) were not considered further because of insufficient numbers for all participants (< 10 trials). Separate ANOVA models were created for study and test periods that allowed us to examine common memory effects (across self-referential and self-external conditions) as well as memory-by-condition interactions. The 2×3 model for the study period included factors of Condition (self-referential, self-external) and Memory (2SC - items subsequently associated with the correct source for both the task and scene source decisions; 1SC - items subsequently associated with the correct source for either the task or the scene but not both; and 0SC – items subsequently associated with no source recognition). Due to insufficient trial numbers for each source alone (< 10), the 1SC contrast included both trials where only the task source or the scene source was correctly recognized. Furthermore, there were insufficient numbers of item-only hits (without source) to analyze separately from item misses and so 0SC trials contained all trials where the source and/or the item was forgotten. This included item-only hit trials as well as item misses (i.e., forgotten). A similar procedure was implemented in Gottlieb, Uncapher, and Rugg (2010). As noted by Gottlieb et al., this procedure only allows us to make inferences about source memory effects but not item level effects, since we collapse over item hits and item misses. For the test period ANOVA model, an additional memory condition (correctly rejected new items) was included. The addition of correct rejections in the test period ANOVA allowed us to confirm basic “old-new” effects (data not shown) as a validation of our data. Fourteen covariates modeling the mean across conditions for each participant were also added to each model, to remove between-subject variance of no interest. Statistical Parametric Maps (SPMs) were created of the T-statistics for the various ANOVA effects of interest, using a single pooled error estimate for all contrasts, whose nonsphericity was estimated using ReML as described in Friston et al. (Friston et al., 2002).
In both the study and test phases, the primary contrast of interest was between the 2SC and 0SC trials (2SC > 0SC), allowing us to examine source memory accuracy effects. Because of our interest in this uni-directional contrast, we report the results from one-tailed t-contrasts, thresholded at p < 0.001, uncorrected with a minimum cluster size of 5. Inclusive masking was carried out at a threshold of p < 0.01. Inclusive masks were applied to determine the overlap between regions associated with task-specific processing (regardless of memory judgment) and task-dependent memory effects. Exclusive masking was carried out using a liberal uncorrected threshold of p < 0.05 for the mask1. Exclusive masking was applied to identify regions showing source memory effects common to both self-referential and self-external conditions, masking out the interactions between conditions. Both masked and unmasked contrasts were evaluated under a one-tailed uncorrected threshold of p < 0.001 and a minimum cluster size of 5 contiguous voxels. In addition to the whole brain analysis described above, we carried out region of interest analyses (ROI) in areas we had a priori interest and that had clear anatomical delineation, including the hippocampus and parahippocampus, using Small-Volume Correction (SVC) for bilateral masks derived from the Automatic Anatomical Labeling system (Tzourio-Mazoyer et al., 2002). Voxels within these ROIs were considered significant if they survived a family-wise error (FWE) correction of p < 0.05. For all effects, peak voxels satisfying our thresholds are reported in MNI coordinates. Where noted neural activity for these peak maxima were plotted for the 2SC and 0SC conditions. Neural activity for these peak voxels reflects the parameter estimates for the convolved regressors and is presented in arbitrary units.
Results
Behavioral Results
For the study decisions, participants rated items pleasant 52% (SD = 8%) of the time in the self-referential task and as having color similarity with the scene 38% (SD = 9%) of the time in the self-external task. To parallel the imaging analysis, response times (RTs) as measured during encoding are reported for the trials subsequently associated with accurate retrieval of two sources, one source (collapsed over scene and task), or zero sources (2SC, 1SC, 0SC, respectively) in Table 1. A 2 × 3 repeated measures analysis of variance (ANOVA) for the RT data collected at the time of study with the factors of Condition (self-referential, self-external) and Source (0SC, 1SC, 0SC) resulted in a main effect of Condition, F(1, 13) = 27.03, p < 0.001, an effect of Source, F(2, 26) = 4.08, p < 0.05, and a Condition by Source interaction, F(2, 26) = 8.92, p < 0.05. Pairwise comparisons showed that the Condition effect was driven by faster response times for the self-external relative to the self-referential condition for the 0SC and 1SC trials, t’s > 2.15, p < 0.05, but equivalent between conditions for the 2SC trials, t(13) = 1.71, p = 0.11. Accounting for the interaction, RTs did not reliably differ in the self-referential condition across the trial types, t’s < 1.26, p’s > 0.23, whereas within the self-external condition, the 0SC trials were associated with faster responses relative to both the 1SC and the 2SC trials, t’s > 4.91, p’s < 0.01. 1SC and 2SC trials did not differ, t(13) = 0.1, p = 0.92.
Table 1.
Proportion of two source correct (2SC), one source correct (1SC) scene, one source correct task, item only hits (zero source correct), and item misses to studied items, split by encoding condition (A). Proportion of correct rejections (CR) and false alarms (FA) to unstudied items (B). Response times at study (C) and at test (D) for the 2SC, 1SC, and the 0SC trials, split by encoding condition.
| A. Studied Items | 2SC | 1SC Scene | 1SC Task | Item only hit | Item Miss (forgotten) |
|---|---|---|---|---|---|
|
|
|||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Self-referential | 0.53 (0.16) | 0.14 (0.07) | 0.08 (0.03) | 0.16 (0.08) | 0.09 (0.06) |
| Self-external | 0.35 (0.14) | 0.05 (0.04) | 0.29 (0.09) | 0.10 (0.05) | 0.21 (.13) |
|
| |||||
| B. Unstudied Items | Correct Rejections | False Alarms | |||
|
|
|||||
| Mean (SD) | Mean (SD) | ||||
| 0.93 (0.06) | 0.07 (0.06) | ||||
| C. Study RTs | |||||
| 2SC | 1SC | 0SC | |||
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Self-referential | 1796 (344) | 1808 (358) | 1892 (476) | ||
| Self-external | 1733 (327) | 1736 (356) | 1511 (351) | ||
|
| |||||
| D. Test RTs | |||||
| 2SC | 1SC | 0SC | |||
|
|
|||||
| Mean | Mean | Mean | |||
| Self-referential | 1119 (163) | 1161 (160) | 1348 (257) | ||
| Self-external | 1178 (164) | 1200 (145) | 1343 (185) | ||
Response times are listed in milliseconds. The 0SC trials in (C) and (D) contain Item only hits, Item misses, and Item “don’t know” trials. SD = standard deviation; RTs = response times.
The mean proportions of 2SC, 1SC scene, 1SC task, item only hits, and item misses (forgotten) to studied items for each encoding task along with the proportions of correctly rejected (CR) and falsely recognized new items (false alarms) are presented in Table 1. A corrected measure of item recognition (Pr) was calculated by subtracting the probability of false alarms (FA) from the probability of hits (Snodgrass & Corwin, 1988). Corrected item recognition, regardless of source accuracy, was 0.88 (SD = 0.08) for the self-referential condition and 0.71 (SD = 0.13) for the self-external condition. Pairwise comparisons showed that item recognition was significantly better for the items encoded under the self-referential relative to the self-external task, t(13) = 6.87, p < 0.05.
A 2 × 5 ANOVA with Condition (self-referential, self-external) and Memory (2SC, 1SC scene, 1SC task, item only hit, item miss) as factors revealed a main effect of Memory, F(4, 52) = 29.4, p < 0.001, a Condition by Memory interaction, F(4, 52) = 41.1, p < 0.001, but no effect of Condition, F(1, 13) < 1. Pair-wise comparisons showed that the main effect of Memory was driven by a greater proportion of both source correct (2SC) trials relative to the other memory categories for both encoding conditions, t’s > 5.9, p’s < 0.001. The interaction resulted from greater proportions of 2SC trials, 1SC scene, and item only hits and reduced proportions of 1SC task and item misses for the self-referential condition relative to the self-external condition, t’s > 4.3, p’s < 0.01. It is important to note that the greater proportion of 2SC trials for the self-reference condition suggests that source accuracy for both sources (task and scene) was improved when participants engaged in the self-reference task.
Response times measured at test for the old/new recognition decision are reported in Table 1. To parallel the imaging analysis, RTs collected at test are reported for the 2SC, 1SC (collapsed over source and task) and 0SC trial types. A 2 × 3 ANOVA with the factors of Condition (self-referential, self-external) and Source (2SC, 1SC, 0SC) showed a main effect of Source, F(2, 26) = 26.6, p < 0.001, but no other effects, F’s < 1. Across both the self-referential and self-external conditions, the Source effect reflected longer RTs for the 0SC trials relative to the 2SC and the 1SC trials, t’s > 3.0, p’s < 0.01. 2SC and 1SC trials did not reliably differ within either condition, t’s < 0.4.
Following the procedure of Uncapher, Otten, and Rugg (2006), we next calculated the conditional probability of retrieving one source as influenced by the other source. Specifically, we calculated the probability of retrieving the task source when the scene was also retrieved [pBoth/(pSceneOnly + pBoth)], as well as the probability of retrieving the task source when the scene was not retrieved [pTaskOnly/(pItemOnly + pTaskOnly)]. An analogous procedure was carried out for the scene source. These conditional probability estimates are reported in Table 2 and were entered into a 2 × 2 × 2 ANOVA with factors of Condition (self-referential, self-external), Source (scene, task) and Accuracy of the other source (correct, incorrect). Results revealed a main effect of Source, F(1, 13) = 17.9, p = 0.001, Accuracy, F(1, 13) = 126.3, p < 0.001, and a three-way interaction between these factors, F(1, 13) = 33.6, p < 0.001. In both conditions, the Accuracy effect arose because the probabilities of getting two sources correct (e.g., Task given scene) was always higher than getting only one source correct (e.g., Task without scene; t’s > 4.15 p’s < 0.001). Further, both “scene” probabilities (e.g. Scene given task, Scene without task) were higher for the self-reference condition relative to the self-external condition, t’s > 4.09, p’s < 0.001, whereas both “task” probabilities were higher for the self-external condition, t’s > 4.16, p’s < 0.001. For both encoding conditions, these results suggest that source memory accuracy is greater when both sources were retrieved, suggesting that these sources were not independent. Importantly, these data further suggest that, to a greater extent than self-external encoding, self-referential encoding increases the likelihood that both sources will be accurately retrieved, relative to one source only, while self-external encoding increased the probability of retrieving the task source in particular.
Table 2.
The probabilities of retrieving one source feature accurately, conditionalized on whether the other source feature was retrieved, as a function of study condition.
| Conditionalized response | Self-referential Mean (SD) | Self-external Mean (SD) |
|---|---|---|
| Task correct given Scene correct | 0.78 (0.11) | 0.87 (0.10) |
| Task correct given Scene incorrect | 0.34 (0.11) | 0.74 (0.11) |
| Scene correct given Task correct | 0.86 (0.08) | 0.53 (0.14) |
| Scene correct given Task incorrect | 0.47 (0.14) | 0.32 (0.17) |
fMRI results
We present the following functional results: First, we examined the main effects of encoding task at study regardless of memory outcome to verify that the self-referential and self-external tasks elicited activity in the a priori predicted regions discussed in the Introduction. Second, we examined regions associated with source memory accuracy shared by both the self-referential and self-external conditions, for the study and test phases separately, with common activity defined using exclusive masking (see methods). Third, we examined regions showing source memory effects unique to self-referential and self-external conditions, for study and test phases separately.
Encoding task effects
In the first analysis, we established evidence of study task effects for the self-referential and self-external tasks. For this analysis, we collapsed across all studied items, regardless of subsequent memory outcome, for each separate task. Regions showing greater activity for the self-referential task relative to the self-external task are reported in Table 3A and shown in Figure 2. These regions included cortical midline regions in medial PFC (BA 10/11) and the precuneus (BA 30). This analysis confirmed that the self-referential task engaged regions previously associated with self-referencing. Regions showing greater activity for self-external processing are reported in Table 3B and shown in Figure 2 and included lateral posterior parietal cortices (inferior parietal lobule and angular gyrus), bilateral inferior and middle prefrontal cortex and middle occipital regions.
Table 3.
Regions showing the task main effect at the time of study for the self-reference condition (A) and the self-external condition (B).
| Contrast | Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|---|
| A. Self-reference > Self-external | ||||||
| Superior Frontal Gyrus | Left | −12 53 28 | 32 | 5.64 | 293 | |
| Medial Frontal Gyrus | Left | −3 41 −17 | 11 | 5.02 | 66 | |
| Angular Gyrus | Left | −48 −73 28 | 39 | 4.31 | 52 | |
| Right | 57 −64 31 | 39 | 4.28 | 35 | ||
| Superior Frontal Gyrus | Right | 6 11 64 | 6 | 4.24 | 21 | |
| Inferior Temporal Gyrus | Left | −54 −7 −26 | 20 | 4.03 | 15 | |
| Superior Frontal Gyrus | Left | −9 11 67 | 6 | 3.98 | 16 | |
| Middle Temporal Gyrus | Right | 48 −10 −17 | 20 | 3.8 | 6 | |
| Anterior Cingulate | Left | −3 23 19 | 24 | 3.63 | 6 | |
| Medial Frontal Gyrus | Right | 12 56 7 | 10 | 3.63 | 6 | |
| Precuneus | Left | −6 −55 13 | 30 | 3.52 | 5 | |
| Inferior Frontal Gyrus | Left | −48 20 16 | 45 | 3.49 | 6 | |
| B. Self-external > Self-reference | ||||||
| Inferior Parietal Lobule | Left | −24 −67 43 | 7 | 7.26 | 552 | |
| Angular Gyrus | Right | 36 −58 52 | 7 | 6.14 | 700 | |
| Orbitofrontal cortex | Right | 15 59 −11 | 10 | 5.69 | 17 | |
| Middle Frontal Gyrus | Right | 42 32 34 | 45 | 5.35 | 205 | |
| Middle Frontal Gyrus | Right | 27 2 52 | 6 | 4.88 | 70 | |
| Precentral Gyrus | Left | −48 2 31 | 6 | 4.57 | 34 | |
| Superior Frontal Gyrus | Left | −21 2 52 | 6 | 4.44 | 15 | |
| Middle Frontal Gyrus | Left | −48 29 37 | 45 | 4.19 | 30 | |
| Left | −48 44 16 | 45 | 4.1 | 18 | ||
| Middle Temporal Gyrus | Right | 66 −28 −11 | 21 | 3.86 | 14 | |
| Lingual Gyrus | Right | 18 −76 −5 | 18 | 3.83 | 33 | |
| Middle Occipital Gyrus | Left | −27 −79 22 | 19 | 3.78 | 45 | |
| Right | 36 −79 16 | 19 | 3.73 | 22 | ||
| Superior Frontal Gyrus | Right | 6 32 43 | 8 | 3.45 | 6 | |
| Inferior Frontal Gyrus | Left | −39 50 −2 | 47 | 3.37 | 6 | |
Regions are listed from highest to lowest t-value. Regions listed without a cluster size are subsumed by the larger cluster listed directly above. BA: Brodmann’s Area.
Fig. 2.
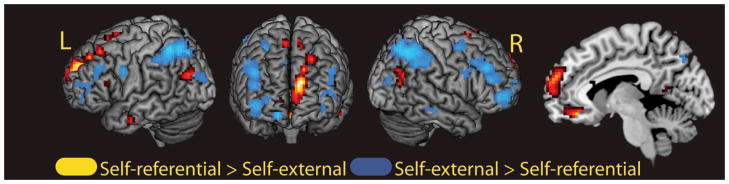
Main effect of task at study, collapsed across subsequent source memory judgment. Regions showing the effect of self-referential processing (hot colors; self-referential > self-external) and the effect of self-external processing (cool colors: self-external > self-referential) are depicted. All regions displayed on a standard surface-rendered brain in MNI space. The midline is shown through a sagittal slice at x = −8. Statistical threshold: p < 0.001, uncorrected, 5 contiguous voxels.
Task-invariant source memory effects
Study
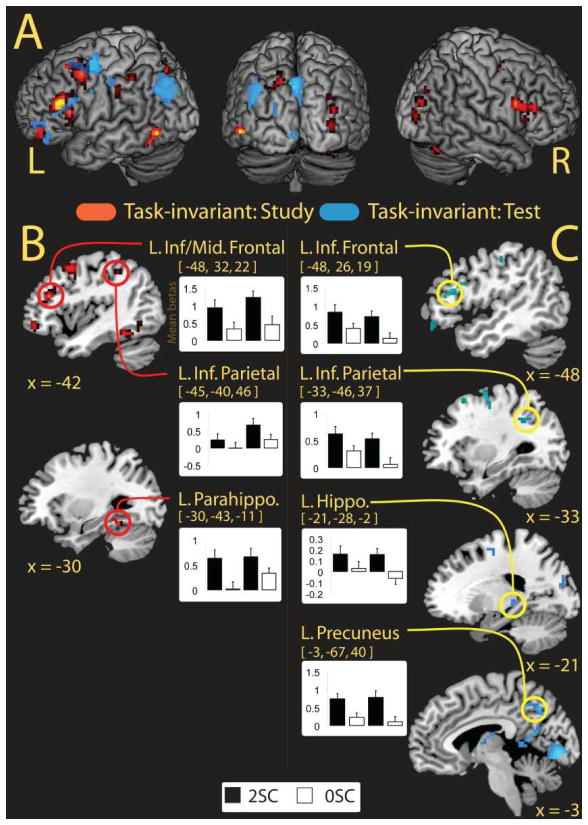
As a second analysis, we investigated regions showing task-invariant subsequent source memory effects. We examined these source effects at the time of study by exclusively masking the subsequent source memory effects (2SC > OSC, collapsed over task) with the source memory by task interactions. The analysis yielded multiple regions (Figure 3A), most notably the left inferior/middle frontal gyrus, bilateral fusiform gyri, and the left inferior parietal lobule (Table 4A). An ROI analysis (see methods) for the MTL yielded a significant effect in the left (−30, −43, −11, t = 4.8, p < 0.001) and the right posterior parahippocampi (24, −31, −17, t = 3.6, p < 0.05), and a significant trend in the left posterior hippocampus (−30, −37, −8, t = 3.08, p = 0.09). Mean parameter estimates (betas) extracted from the peak voxel coordinate for the left inferior/middle frontal cortex, the left inferior parietal lobule and the left parahippocampus are shown in Figure 3B.
Fig. 3.
Task-invariant activity at study and test. (A) Source memory regions (2SC > 0SC) exhibiting task-invariant activation common to both the self-reference and self-external conditions at the time of study (hot colors) and at the time of test (cool colors) rendered on a standard brain in MNI space. (B) Anatomic overlays and graphs depict mean parameter estimates (betas) from the peak voxels exhibiting task-invariant activation at thetime of study. (C) Overlays and graphs depict the parameter estimates of peak voxels at the time of test. In both (B) and (C) bars represent, from left to right, 2SC and 0SC self-reference trials followed by 2SC and 0SC self-external trials. Error barsdepict the standard error of the mean. Statistical threshold: p < 0.001, uncorrected, 5 contiguous voxels. 2SC = 2 source correct trials; 0SC = 0 source correct trials; L. inf/Mid. Frontal = Left Inferior/Middle Frontal; L. Inf. Frontal = Left Inferior Frontal; L. Inf. Parietal = Left Inferior Parietal; L. Parahippo. = Left Parahippocampus; L. Hippo. = Left Hippocampus; L. Precuneus = Left Precuneus. Note: The left parahippocampal region depicted in panel (B) was isolated from an ROI approach (see methods).
Table 4.
Regions showing task-invariant source accuracy effects at study (A) and test (B).
| Contrast | Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|---|
| A. Task-invariant, study | ||||||
| Fusiform Gyrus | Left | −33 −46 −17 | 37 | 5.57 | 218 | |
| Inferior/Middle Frontal Gyrus | Left | −48 32 22 | 45 | 5.15 | 110 | |
| Fusiform Gyrus | Right | 24 −34 −20 | 30 | 4.86 | 28 | |
| Inferior Frontal Gyrus | Right | 54 11 25 | 44 | 4.47 | 59 | |
| Precentral Gyrus | Left | −45 8 52 | 6 | 4.43 | 103 | |
| Inferior Orbitofrontal Gyrus | Left | −39 50 −5 | 47 | 4.14 | 26 | |
| Thalamus | Left | −9 −10 7 | 4.04 | 9 | ||
| Precentral Gyrus | Right | 27 −1 49 | 6 | 3.96 | 12 | |
| Superior Parietal Lobule | Left | −15 −73 46 | 7 | 3.93 | 22 | |
| Cerebellum | Right | 30 −64 −29 | 3.89 | 19 | ||
| Middle Occipital Gyrus | Right | 36 −79 22 | 19 | 3.87 | 20 | |
| Left | −24 −61 37 | 7 | 3.84 | 18 | ||
| Right | 33 −85 7 | 19 | 3.78 | 21 | ||
| Inferior Parietal Lobule | Left | −45 −40 46 | 40 | 3.75 | 15 | |
| Superior Frontal Gyrus | Left | −3 23 52 | 8 | 3.72 | 26 | |
| Inferior Parietal Lobule | Left | −51 −25 37 | 3 | 3.62 | 12 | |
| Caudate | Right | 12 −1 16 | 3.61 | 9 | ||
| Precuneus | Right | 33 −76 31 | 19 | 3.54 | 6 | |
| B. Task-invariant, test | ||||||
| Lingual Gyrus | Left | −6 −85 −5 | 17 | 6.16 | 124 | |
| Precuneus | Left | −3 −67 40 | 7 | 5.44 | 150 | |
| Retrosplenial/Posterior Cingulate Cortex | Left | −9 −55 16 | 30 | 4.94 | 37 | |
| Precentral Gyrus | Left | −33 −4 58 | 6 | 4.91 | 83 | |
| Middle Occipital Gyrus | Left | −36 −73 40 | 7 | 4.47 | 87 | |
| Inferior Frontal Gyrus | Left | −48 26 19 | 45 | 4.34 | 47 | |
| Lingual Gyrus | Left | −12 −52 4 | 17 | 3.93 | 5 | |
| Inferior Parietal Lobule | Left | −33 −46 37 | 40 | 3.73 | 22 | |
| Inferior Orbitofrontal Gyrus | Left | −48 44 −17 | 45 | 3.72 | 14 | |
| Thalamus | Left | −3 −10 13 | 3.71 | 26 | ||
| Middle Occipital Gyrus | Left | −21 −82 16 | 18 | 3.68 | 7 | |
| Hippocampus | Left | −21 −28 −2 | 3.62 | 10 | ||
| Inferior Frontal Gyrus | Left | −39 47 −2 | 47 | 3.58 | 11 | |
| Precentral Gyrus | Left | −39 −4 31 | 6 | 3.57 | 6 | |
| Postcentral Gyrus | Left | −51 −19 46 | 4 | 3.46 | 13 | |
| Cingulate Gyrus | Left | 0 −37 31 | 31 | 3.37 | 6 | |
Task-invariant regions were defined by exclusively masking subsequent source regions (2SC>0SC) with regions showing the memory by condition interaction.
Regions are listed from highest to lowest t-value; Regions listed in bold are depicted in Figure 3; 2SC: 2 Source correct trials; 0SC: 0 Source correct trials; BA: Brodmann’s Area.
In a subsidiary analysis we tested for regions showing a graded pattern of activity for subsequent source accuracy (2SC > 1SC > 0SC). To perform this analysis, we inclusively masked (see methods) the 2SC > 0SC contrast with 2SC > 1SC and 1SC > 0SC contrasts. No region survived this analysis.
Test
An analogous procedure was used to assess task-invariant source memory effects at the time of test (Figure 3A). This analysis revealed activity in left-lateralized inferior frontal cortex, medial parietal (precuneus, retrosplenial/posterior cingulate cortex), inferior parietal lobule, and the posterior hippocampus (Table 4B). Extracted parameter estimates for a subset of these regions are shown in Figure 3C. ROI analysis additionally uncovered activation in the left posterior parahippocampus (−24, −43, −11, t = 3.6, p = 0.02).
As at study, we tested for regions showing a graded pattern of source memory accuracy at the time of test. Results showed that two regions, the left lingual gyrus (−21, −73, −8, t = 5.9, cluster size = 15) and the left calcarine cortex (−12, −52, 10, t = 5.6, cluster size = 6), showed increasing activation as more source information was retrieved (2 SC > 1 SC > 0 SC).
Task-selective source memory effects
Study
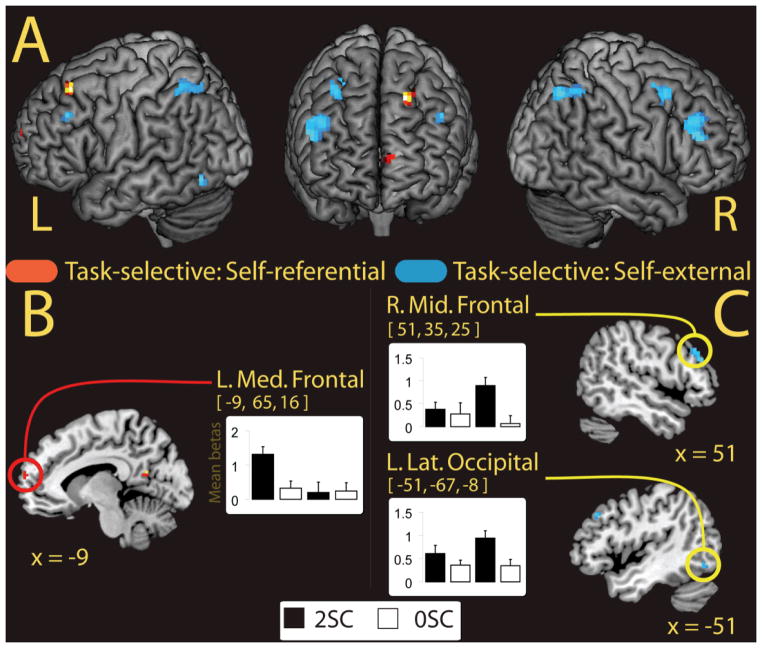
The third analysis examined task-selective source memory effects. We first examined these memory effects constrained by the general task effects. To accomplish this, we inclusively masked the subsequent source memory effect for the self-referential condition (2SC > 0SC) with the interaction where the magnitude of the source memory effect was greater for the self-referential task relative to the self-external task ([2SC > 0SC, self-reference] > [2SC > 0SC, self-external], See Park, Uncapher, & Rugg, 2008 for a similar procedure) and then inclusively masked the resultant spm with the self-referential task main effect (self-referential > self-external). The results of this analysis yielded effects in two regions, the left medial prefrontal cortex (BA 10; see Figure 4B) and the left superior frontal gyrus (see Table 5A). Using a similar procedure for the self-external condition, we found task-selective source memory effects in the right middle frontal gyrus, the right angular gyrus, and the lateral occipital cortex (Table 5B). Effects for the right middle frontal gyrus and left lateral occipital cortex are depicted in Figure 4C.
Fig. 4.
Task-selective activity at study. (A) Source memory regions (2SC > 0SC) showing task-selective activation for the self-referential condition (hot colors) and for the self-external condition (cool colors) at the time of study rendered on a standard brain in MNI space. (B) Anatomic overlays and graphs depict mean parameter estimates (betas) from the peak voxels exhibiting task-selective self-referential activation at the time of study constrained by the task main effect. (C) Overlays and graphsdepict the parameter estimates of peak voxels for the self-external condition at the time of study constrained by the task main effect. In both (B) and (C) bars represent, from left to right, 2SC and 0SC self-reference trials followed by 2SC and 0SC self-external trials. Error bars depict the standard error of the mean. Statistical threshold: p < 0.001, uncorrected, 5 contiguous voxels. 2SC = 2 source correct trials; 0SC = 0 source correct trials. L. Med. Frontal = Left Medial Frontal; R. Mid. Frontal =Right Middle Frontal; L. Lat. Occipital = Left Lateral Occipital.
Table 5.
Regions showing task-selective effects for the self-referential (A) and for the self-external condition (B) at the time of study constrained by the task main effect. Regions showing task-selective effects for the self-referential (C) and for the self-external condition (D) at the time of study unconstrained by the task main effect.
| Contrast | Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|---|
| A. Task-selective, Self-referential, Study constrained by Task Effect | ||||||
| Superior Frontal Gyrus | Left | −18 29 49 | 8 | 3.99 | 14 | |
| Medial Prefrontal | Left | −9 65 16 | 10 | 3.53 | 6 | |
| B. Task-selective, Self-external, Study constrained by Task Effect | ||||||
| Middle Frontal Gyrus | Right | 51 35 25 | 45 | 4.51 | 67 | |
| Angular Gyrus | Right | 30 −67 49 | 7 | 4.5 | 46 | |
| Lateral Occipital | Left | −51 −67 −8 | 37 | 4.4 | 9 | |
| Middle Frontal Gyrus | Right | 30 2 52 | 6 | 4.33 | 43 | |
| Left | −45 32 37 | 45 | 4.18 | 6 | ||
| Superior Parietal Lobule | Left | −24 −67 40 | 7 | 4.11 | 67 | |
| C. Task-selective, Self-Referential, Study unconstrained by Task Effect | ||||||
| Superior Frontal Gyrus | Left | −18 29 49 | 8 | 3.99 | 15 | |
| Precuneus | Left | −9 −58 19 | 23 | 3.87 | 7 | |
| Medial Prefrontal | Left | −9 65 16 | 10 | 3.53 | 6 | |
| D. Task-selective, Self-external, Study unconstrained by Task Effect | ||||||
| Middle Frontal Gyrus | Right | 51 35 25 | 45 | 4.51 | 67 | |
| Angular Gyrus | Right | 30 −67 49 | 7 | 4.5 | 46 | |
| Lateral Occipital | Left | −51 −67 −8 | 37 | 4.4 | 9 | |
| Middle Frontal Gyrus | Right | 30 2 52 | 6 | 4.33 | 43 | |
| Left | −45 32 37 | 45 | 4.18 | 6 | ||
| Superior Parietal Lobule | Left | −24 −67 40 | 7 | 4.11 | 67 | |
Task-selective effects were defined by inclusively masking source accuracy effects for each condition respectively (e.g., 2SC>0SC, self-referential) with the interaction where the accuracy effect was larger for one condition versus the other (e.g., ([2SC>0SC, self-referential > [2SC>0SC, self-external]).
In panels (A) and (B) task-selective effects were inclusively masked with the encoding task main effect (e.g., self-referential>self-external).
Regions are listed from highest to lowest t-value; Regions listed in bold are depicted in Figure 4; 2SC: 2 Source correct trials; 0SC: 0 Source correct trials; BA: Brodmann’s Area.
We also analyzed task-sensitive source accuracy effects that were not constrained by the task main effects. To this end, the same inclusive masking procedure was applied as above only without the additional inclusive mask of the task main effect. Results of this analysis for the self-referential source accuracy effects are reported in Table 5C and included all the same regions showing constrained effects with the inclusion of one additional region, the left precuneus. Unconstrained task-selective effects (Table 5D) for the self-external task were identical to those uncovered in the constrained analysis.
As a subsidiary analysis we again tested for graded memory effects within each condition respectively. No regions survived this analysis for either the self-referential or the self-external conditions.
The finding of a task-selective subsequent source memory region in the medial prefrontal cortex for the self-referential task, suggests a role for this region in the recollection of source features. Yet, because we collapsed across item only hits and item misses to form the 0SC category, it is possible that contributions of this region was driven in part by familiarity; as 2SC and item misses would differ in both recollection and familiarity. To account for this possibility, we examined a subset of participants (n = 6) who had sufficient numbers of item only hit trials to compare against the source accuracy trials (2SC, 1SC scene, and 1SC task trials). To perform this analysis we extracted the parameter estimates of our subset from the peak voxel in the medial PFC that showed task-selective self-referential effects (MNI coordinate = −9, 65, 16). The resulting parameter estimates are displayed in Figure 5. Results of this analysis showed greater activity for the 2SC trials relative to the item only hits, t(5) = 2.76, p < 0.05, confirming that the medial PFC supported recollection for self-referentially encoded source features. Furthermore, because our 1SC trials were collapsed across the scene and task sources in the analysis for all 14 participants, it may have been possible that medial PFC activity was more robust for one type of source relative to the other (e.g., greater activation for scene source judgments than task). A direct comparison between the 1SC trial types, separated into scene and task only trials, showed no significant difference between the 1SC Task and 1SC Scene trials, t(5) < 1, suggesting that this medial prefrontal region supported subsequent source memory accuracy for both source features uniquely. The 2SC trials did not differ from either the 1SC task, t(5) = 1.8, p = 0.13, or the 1SC scene trials, t(5) = 0.3, p = 0.79.
Fig. 5.
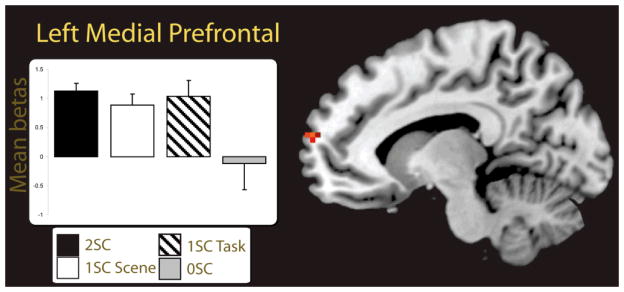
Parameter estimates are depicted from the peak voxel of activation in the left medial prefrontal region (MNI coordinates = −9, 65, 16) showing task-selective source memory effectsfor the self-reference condition (see Table 5). The parameter estimates were extracted from six participants who had sufficient 2SC, 1SC task, 1SC scene, and 0SC trials for stable beta estimation. Error bars depict the standard error of the mean. Statistical threshold: p < 0.001, uncorrected, 5 contiguous voxels. 2SC = 2 source correct trials; 1SC Task = 1 source correct task trials; 1SC Scene = 1 source correct scene trials; 0SC = 0 source correct trials.
Test
Task-selective source memory effects constrained by the encoding task main effect for the self-referential condition at the time of test were derived in the following manner: test phase self-referential source memory effects (2SC > 0SC) were inclusively masked with the interaction where the magnitude of the source memory effect was greater for the self-referential relative to the self-external condition ([2SC > 0SC, self-reference] > [2SC > 0SC, self-external]), and then inclusively masked with the self-referential main effect at the time of encoding (self-referential > self-external). This analysis yielded no suprathreshold voxels. The same procedure for the self-external task yielded task-selective regions in left inferior parietal lobule, the left middle occipital gyrus, and left inferior frontal gyrus (See Table 6B).
Table 6.
Regions showing task-selective effects for the self-referential (A) and for the self-external condition (B) at the time of test constrained by the encoding task main effect. Regions showing task-selective effects for the self-referential (C) and for the self-external condition (D) at the time of test unconstrained by the encoding task main effect.
| Contrast | Region | Hemisphere | MNI Coordinates | BA | T-value | Cluster Size |
|---|---|---|---|---|---|---|
| A. Task-selective, Self-referential, Test constrained by Task Effect | ||||||
| no surviving voxels | ||||||
| B. Task-selective, Self-external, Test constrained by Task Effect | ||||||
| Inferior Parietal Lobule | Left | −27 −70 46 | 7 | 7.38 | 370 | |
| Middle Occipital Gyrus | Left | −24 −64 34 | 19 | 6.74 | ||
| Middle Occipital Gyrus | Left | −27 −79 25 | 19 | 7.28 | 38 | |
| Globus Pallidum | Left | −21 2 52 | 5.81 | 14 | ||
| Inferior Frontal Gyrus | Left | −48 32 25 | 45 | 5.74 | 21 | |
| Middle Frontal Gyrus | Left | −51 23 37 | 44 | 4.69 | ||
| Precentral Gyrus | Left | −39 2 34 | 6 | 5.53 | 18 | |
| Lingual Gyrus | Right | 12 −64 49 | 7 | 4.01 | 49 | |
| Superior Parietal Lobule | Right | 21 −70 55 | 7 | 3.91 | ||
| Middle Occipital Gyrus | Right | 33 −61 40 | 7 | 3.51 | 19 | |
| C. Task-selective, Self-referential, Test unconstrained by Task Effect | ||||||
| Posterior Cingulate | Left | −6 −46 34 | 23 | 3.93 | 8 | |
| D. Task-selective, Self-external, Test unconstrained by Task Effect | ||||||
| Middle Occipital Gyrus | Left | −27 −79 28 | 19 | 7.75 | 1090 | |
| Fusiform Gyrus | Left | −24 −73 −8 | 18 | 6.5 | 573 | |
| Lateral Occipital | Left | −48 −55 −11 | 37 | 6.25 | ||
| Cuneus | Right | 21 −55 22 | 17 | 6.44 | 153 | |
| Inferior Frontal Gyrus | Left | −51 32 25 | 45 | 6.18 | 303 | |
| Superior Frontal Gyrus | Left | −21 2 52 | 6 | 5.81 | 148 | |
| Left | −6 17 49 | 32 | 5.06 | 60 | ||
| Middle Frontal Gyrus | Left | −45 47 −2 | 46 | 4.39 | 14 | |
| Precuneus | Left | −6 −49 49 | 7 | 4.25 | 10 | |
| Superior Parietal Lobule | Right | 21 −58 52 | 7 | 4.22 | 87 | |
| Middle Occipital Gyrus | Right | 36 −70 37 | 19 | 4.07 | 80 | |
| Middle Frontal Gyrus | Right | 51 29 34 | 45 | 3.7 | 9 | |
| Middle Occipital Gyrus | Left | −27 −94 4 | 18 | 3.43 | 5 | |
| Fusiform Gyrus | Right | 27 −40 −17 | 37 | 3.39 | 7 | |
Task-selective effects were defined by inclusively masking source accuracy effects for each condition respectively (e.g., 2SC>0SC, self-referential) with the interaction where the accuracy effect was larger for one condition versus the other (e.g., ([2SC>0SC, self-referential] > [2SC>0SC, self-external]).
In panels (A) and (B) task-selective effects were inclusively masked with the encoding task main effect (eg., self-referential>self-external).
Regions are listed from highest to lowest t-value; Regions listed without a cluster size are subsumed by the larger cluster listed directly above; 2SC: 2 Source correct trials; 0SC: 0 Source correct trials; BA: Brodmann’s Area.
We next examined regions showing task-selective source memory effects at test unconstrained by the encoding task main effects. Starting first with the self-reference condition, we determined self-selective source memory effects by inclusively masking the self-reference source memory effects at test (2SC > 0SC, self-reference) with the interaction where the magnitude of the source memory effect was greater for the self-referential relative to the self-external condition ([2SC > 0SC, self-reference] > [2SC > 0SC, self-external]). This analysis yielded a single region in the left posterior cingulate (Table 6C). The analogous analysis for the self-external condition resulted in multiple regions reported in Table 6D including the left middle occipital gyrus, left fusiform, left lateral occipital, and several prefrontal and parietal sites.
An analysis of graded source memory effects (2 SC > 1 SC > 0 SC), analogous to those carried out at study, resulted in no significant voxels for the self-referential condition. In the self-external condition, graded effects were found in left inferior temporal cortex (−45, −61, −5, t = 5.3, cluster size = 25), left middle occipital gyrus (−33, −85, 16, t = 4.9, cluster size = 47), and left fusiform gyrus (−27, −52, −11, t = 4.4, cluster size = 7).
Discussion
The principal goal of this investigation was to examine behavioral and neural source memory effects for items studied under conditions of self-reference. This experiment yielded three major findings: first, we report behavioral evidence of a source accuracy benefit for items encoded self-referentially relative to self-externally, an extension of the mnemonic benefit attributable to self-referencing; second, we found source accuracy effects in the medial prefrontal cortex at the time of study for items processed in relation to the self. Critically, the medial prefrontal contributions were equivalent for the two source features, suggesting that memory for multiple source features benefits from self-referencing; third, consistent with prior reports of domain-general memory processes, activity in medial temporal (left hippocampus and parahippocampus), left inferior frontal, and left inferior parietal lobule supported source memory accuracy across both the self-reference and self-external conditions.
Behavioral Results
Consistent with prior investigations (Rogers, et al., 1977; Symons & Johnson, 1997), item recognition was better for stimuli encoded under conditions of self-reference relative to stimuli encoded under a non-self-referential task. In addition to the item recognition benefit, we report behavioral evidence of a source accuracy benefit for the self-referenced stimuli. This source accuracy benefit was evident in the greater proportion of trials where both source features were accurately retrieved in the self-reference relative to the self-external condition. This suggests that self-referencing facilitates the binding of multiple source features when materials are processed in a self-referential manner. This finding is consistent with the view that self-referencing serves to enhance memory through elaboration (Rogers, et al., 1977) and organization (Klein & Kihlstrom, 1986) of materials processed via one’s self schema. The finding that the self-external condition led to higher estimates of source accuracy for the task source, however, was somewhat unexpected. It may be the case, though, that for a subset of the self-referenced trials, participants were using color to make the pleasantness judgment (e.g. I like the color of the object against the scene background). Participants may have subsequently recollected their color-based decision during study when making source memory judgments about the task and mistakenly attributed this information to the color task. Future studies implementing debriefing questions for each retrieval trial will be necessary to determine the likelihood of this possibility.
While we provide evidence of an item and source memory benefit for self-referencing, one possible critique of the memory advantage observed for the self-reference condition might be explained, at least in part, by a depth of processing effect. Indeed, better item and source memory for the self-reference condition is consistent with this possibility. While this is a plausible critique in this study, it should be noted that this is a valid criticism of most every experiment examining self-referential processing. Wary of this confound, we took several steps to equate processing depth between the self-reference and self-external tasks in this study. First, for both tasks, participants were instructed to make judgments based on perceptual features rather than on other dimensions (e.g. function of the objects, semantic meaning). Second, both tasks focused attention on the relationship between the object and the background scene equating relational demands. Third, we constructed the self-external task to be subjective. Since the self-reference task in this study (and all other self studies for that matter) is subjective by definition, it was critical to match the self-external task along this dimension. Use of a color similarity task where there were no correct answers introduced subjectivity to the decision process for the self-external task. Finally, as discussed below, source memory effects for self-referenced items were identified in the medial PFC and not the left inferior frontal cortex, which has directly been implicated in depth of processing (Kapur et al., 1994). Further, study phase response times did not reliably differ for the two source correct trials across condition. If greater processing was occurring for the self-referenced items then it would be expected to be reflected in longer response times. This was not the case, arguing against the levels of processing criticism. In short, we attempted to equate the two encoding tasks to limit the validity of the processing depth critique and furthermore, our response time and imaging results are inconsistent with a depth of processing argument.
fMRI Results
Encoding task effects
Examination of task effects at the time of study, regardless of subsequent memory performance, revealed encoding activity consistent with expectations for the two tasks. Because our self-reference task used a pleasantness decision instead of an adjective judgment as several previous studies have used (Craik, et al., 1999; Gutchess, et al., 2007, 2010; Heatherton, et al., 2006; Kelley, et al., 2002; Macrae, et al., 2004; Moran, Macrae, Heatherton, Wyland, & Kelley, 2006), we examined task effects to establish correspondence between self-processing regions in our study with those of previous studies. As anticipated, online processing of the pleasantness task engaged a network of cortical midline regions similar to those that have typically been associated with self-relevant processing including ventral and dorsal medial prefrontal cortex as well as medial parietal cortex (Kelley, et al., 2002; Lou, et al., 2004; Northoff & Bermpohl, 2004; Northoff, et al., 2006). By comparison, activity associated with the self-external condition, in this case judging color similarity between objects and backgrounds, engaged numerous perceptual processing regions, including middle occipital sites bilaterally, consistent with attention to color features (Corbetta, et al., 1990).
Task-selective source memory effects
We found that activity in the medial prefrontal cortex supported subsequent source memory for self-referenced materials. Previous neuroimaging reports have found medial prefrontal activations in support of item recognition (Gutchess, et al., 2010; Macrae, et al., 2004). The current finding represents one of the first links, if not the first, between medial prefrontal activation and subsequent source memory accuracy for self-referential information. Critically, the task-selective source memory effects within the medial prefrontal cortex observed at the time of study overlapped with the task-main effect implying that the medial PFC involvement was not simply an artifact of better overall memory performance for the self-referential task. This evidence suggests that the cortical regions that are active during the on-line processing of material for self-relevance also contribute to establishing memories that are rich in contextual features.
The fact that medial PFC activity was tied to subsequent retrieval of source details suggests that this region was involved in the recollection of those contextual features (both task and scene) for self-referenced materials. However, because our 0SC trials contained both item only hits (no source) and item misses (i.e., forgotten), it is possible that that the contributions of the medial prefrontal cortex was partially familiarity-based (as 2SC and item misses likely differ for both recollection and familiarity). To rule out this possibility we examined activity in a subset of participants who had sufficient numbers of item only hit trials to segregate from the item misses. Results of this analysis showed that medial PFC activity was greater for items associated with correct source judgments than for item only hit trials (no sources correct), arguing against the familiarity account. Because we were unable to examine activity associated with item misses, however, future work should more closely inspect item misses to guard against the possibility that medial PFC activity supports retrieval via familiarity processes. The subsidiary analysis additionally allowed examination of the two source features separately and showed that activity in the medial prefrontal cortex supported subsequent source memory accuracy for both the task and scene sources in isolation. These results suggest that the medial prefrontal activity, at least for self-referenced materials, acts to facilitate memory formation of various episodic features. It should be noted that the source memory effects in the medial prefrontal cortex and superior frontal gyrus emerged within our modest sample size suggesting robust effects. It is possible that other medium sized effects were also apparent. Indeed, analysis of self-reference source memory effects at a reduced threshold (inclusive masking reduced to p < 0.05) revealed an additional region of activation in the left temporal gyrus (data not shown), a region that has been previously associated with self-reference memory effects (Gutchess, Kensinger, & Schacter, 2010). While we provide evidence that self-referencing underlies some types of source features, future work should elaborate on other types of contextual features that might also be supported by medial PFC involvement.
The medial prefrontal cortex has not typically been associated with recollection effects to the extent of other regions (e.g. hippocampus). Close inspection, however, of many neuroimaging investigations of subjective recollection using the remember/know procedure show that activity in medial prefrontal cortex consistently is associated with recollection (Daselaar, Fleck, & Cabeza, 2006; Henson, Rugg, Shallice, Josephs, & Dolan, 1999; J. D. Johnson & Rugg, 2007; Wheeler & Buckner, 2004; Yonelinas, Otten, Shaw, & Rugg, 2005). It is tempting to consider that medial prefrontal involvement in these previous studies might have supported memory for self-relevant details that were incidental to the task (e.g. “I remember wanting to play golf when I saw the golf ball”) that supported the endorsement of a “remember” judgment. More explicitly, it may be the case that activity of this region contributes to the recollection of subjective details that are part of the phenomenal experience of a recollection judgment, such as incidental thoughts or emotional responses elicited by studied materials. Future studies might address this possibility by gathering more precise information about the nature and type of information that lead to a subjective recollection endorsement to determine the extent to which recollection is based on retrieval of self-generated associations.
In contrast to the finding of medial PFC activity during study, we did not observe source memory effects for self-referenced items at the time of test in this region. This finding stands in contrast to Benoit et al. (2010) who reported study phase activity in ventromedial prefrontal cortex for self-referenced materials in a subset of trials where source information was retrieved. Unlike the analysis procedure we followed, however, Benoit et al. (2010) did not determine whether the ventromedial prefrontal regions overlapped with the regions engaged by the self-referential encoding task. Thus, it is possible that the ventromedial activity they report reflected better source memory performance for the self-relevant task and was not specific to source memory, per se. It may be the case, then, that medial PFC plays a generative role in establishing the memory representation between various features encoded self-referentially at study, but then, at the time of retrieval, other cortical regions are utilized to retrieve these representations. One region that did show greater source memory effects for self-referentially encoded items at test was located in the posterior cingulate, a region that has been implicated in self-referential processing (Sajonz et al., 2010). Further investigation of this region, however, revealed that it did not overlap with the encoding task main effect. It is possible, therefore, that the contribution of this region might not support self-referential source memory, per se, but might simply reflect the better memory accuracy for the self-referenced items relative to the self-externally encoded items.
One potential caveat to our medial PFC finding: it is possible that activity in this region was driven by a depth of processing effect given the better behavioral performance observed for the self-reference task. It should be noted however, that depth of processing effects have typically been associated with left inferior frontal activations (Kapur, et al., 1994), and not in medial prefrontal regions that served as the focus of our present study. Further evidence from self-reference studies that compare ‘self’ processing with ‘other’ processing, which are well-matched on depth of processing, routinely uncover medial prefrontal cortex involvement. Both of these points guard against the possibility that the medial PFC activity reported in this study was due to a depth of processing effect.
We also observed task-selective source memory effects for the self-external condition at the time of study in bilateral middle frontal gyri, the angular gyrus, and a region of the left lateral occipital cortex. While both encoding conditions focused attention to the scene, the self-external condition may have induced more scanning of the scene in order to make the color similarity judgment than did the self-reference condition. Consistent with this hypothesis, the middle frontal and parietal regions identified here, have previously been associated with exogenous allocation of attention (Corbetta, Patel, & Shulman, 2008; Corbetta & Shulman, 2002). Similarly, at the time of test, we observed self-external source memory effects in these frontoparietal regions as well as the lateral occipital cortex. Interestingly, the activity found in the left lateral occipital cortex during test (−48 −55 −11) overlapped spatially with the occipital region identified during study. Such overlap is consistent with the “cortical reinstatement” hypothesis which suggests that regions engaged at the time of study are reinstated at the time of test in the service of accurate episodic retrieval (J. D. Johnson & Rugg, 2007; Woodruff, Johnson, Uncapher, & Rugg, 2005). Overall the findings of task-selective source memory effects at study for both encoding tasks suggests that the regions involved in the online processing of stimuli (medial PFC for the self-reference condition and frontoparietal regions for the self-external condition) further contribute to episodic memory encoding for these stimuli (Moscovitch, 1992; Uncapher, et al., 2006).
Task-invariant source memory effects
Great attention has been paid to regions that contribute to source memory accuracy regardless of stimulus domain or task (Badre & Wagner, 2007; Dobbins & Wagner, 2005; Eichenbaum, et al., 2007; Uncapher & Wagner, 2009; Vilberg & Rugg, 2008). Consistent with these findings, we observed activity in left inferior/middle frontal cortex, medial and lateral posterior parietal cortex, and the posterior parahippocampus and hippocampus that supported source memory in a task-invariant manner at study and at test. Activity within the posterior parahippocampus is perhaps not surprising given that source memory accuracy depended on effective encoding and subsequent retrieval of a scene, and this region has been implicated in scene and/or spatial processing in numerous previous studies (Epstein, Graham, & Downing, 2003; Epstein, Harris, Stanley, & Kanwisher, 1999; Lee et al., 2005; Lee, Scahill, & Graham, 2008). It is further possible that this region contributed to the encoding and retrieval of contextual information consistent with the ‘Binding of item and context model’ (Eichenbaum, et al., 2007).
Our results parallel, to a degree, findings from a recent study examining hippocampal activation for multiple recollected details (Vilberg & Rugg, 2007). Vilberg and Rugg (2007) reported greater hippocampal activity for trials associated with the greatest amount of recollection (two recollected features) relative to items for which only one feature or no features were recollected. In neither our study nor in the study by Vilberg and Rugg was there evidence of a graded pattern of recollection in the hippocampus. Collectively, these findings suggest that activity in the hippocampus is associated with recollection but offer no support for the hypothesis that recollection effects within the MTL vary on a continuous scale (Wixted, 2007).
Finally, it is worth mentioning that a few regions demonstrated graded patterns of source recollection for both encoding conditions at test (2 SC > 1 SC > 0 SC). Specifically, activity sensitive to the amount of source features correctly recognized was found in the left lingual gyrus and the left calcarine cortex. Similarly, previous findings have shown graded recollection effects, measured subjectively, in extrastriate regions (Vilberg & Rugg, 2007). As discussed by Vilberg and Rugg, this pattern may reflect the fact that greater dependence on perceptual processing regions is necessary to support recollection of more features bound to recognized objects.
Conclusions
Overall, our data suggest that processing stimuli in reference to the self supports source memory accuracy and that encoding-related activity in the medial prefrontal cortex underlies this source memory benefit. Taken with previous findings, these data confirm and extend the link between self-referencing, memory accuracy, and the medial prefrontal cortex. This evidence supports the notion that processing information in relation to the self is a powerful mnemonic aide which facilitates memory for various episodic features.
Acknowledgments
The authors would like to thank Michael Dulas and Yashu Jiang for their invaluable assistance during the development of this project. This research was supported by a grant from the American Federation for Aging Research to A. Duarte and by NIH Grant T32 AG00175 to E.D. Leshikar.
Footnotes
Note that a liberal threshold for an exclusive mask is more conservative in excluding regions from the masked SPM. The procedure of exclusively masking main effects by their interaction is formally equivalent to the original definition of a “cognitive conjunction” (Price & Friston, 1997).
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50(3):1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Conway MA, Dewhurst SA. The Self and Recollective Experience. Applied Cognitive Psychology. 1995;9(1):1–19. [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248(4962):1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96(4):1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15(11):1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cereb Cortex. 2008;18(9):2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Knight RT, Emery T, Graham KS. Orbito-frontal Cortex is Necessary for Temporal Context Memory. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annu Rev Neurosci. 2007 doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37(5):865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23(1):115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22(4):1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Kiebel S, Nichols TE, Penny WD, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Elsevier; 2007. [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16(2):484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb LJ, Uncapher MR, Rugg MD. Dissociation of the neural correlates of visual and auditory contextual encoding. Neuropsychologia. 2010;48(1):137–144. doi: 10.1016/j.neuropsychologia.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2(2):117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48(1):211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen T, Schubotz RI, Hofel L, Cramon DY. Brain correlates of aesthetic judgment of beauty. Neuroimage. 2006;29(1):276–285. doi: 10.1016/j.neuroimage.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Johnson JD, McDuff SG, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 2009;63(5):697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the Reinstatement of Encoding-Related Cortical Activity. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Pschol Rev. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, Lee J, et al. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17(12):1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Tulving E, Wilson AA, Houle S, Brown GM. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci U S A. 1994;91(6):2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- Klein SB, Kihlstrom JF. Elaboration, organization, and the self-reference effect in memory. J Exp Psychol Gen. 1986;115(1):26–38. doi: 10.1037//0096-3445.115.1.26. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15(6):782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cereb Cortex. 2008;18(3):683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan JP, Nowak M, Kjaer TW, et al. Parietal cortex and representation of the mental Self. Proc Natl Acad Sci U S A. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- May CP, Rahhal T, Berry EM, Leighton EA. Aging, source memory, and emotion. Psychol Aging. 2005;20(4):571–578. doi: 10.1037/0882-7974.20.4.571. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci. 2006;18(9):1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age Effects on the Neural Correlates of Episodic Retrieval: Increased Cortical Recruitment with Matched Performance. Cereb Cortex. 2007 doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Park H, Uncapher MR, Rugg MD. Effects of study task on the neural correlates of source encoding. Learn Mem. 2008;15(6):417–425. doi: 10.1101/lm.878908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5(4 Pt 1):261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Rahhal TA, May CP, Hasher L. Truth and character: sources that older adults can remember. Psychol Sci. 2002;13(2):101–105. doi: 10.1111/1467-9280.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Vicens L, Clithero JA, Dobbins IG, Huettel SA. Contributions of frontopolar cortex to judgments about self, others and relations. Soc Cogn Affect Neurosci. 2010 doi: 10.1093/scan/nsq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Johnson JD, Park H, Uncapher MR. Encoding-retrieval overlap in human episodic memory: a functional neuroimaging perspective. Prog Brain Res. 2008;169:339–352. doi: 10.1016/S0079-6123(07)00021-0. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The relationship between brain activity, cognitive performance and aging: the case of memory. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging. Oxford: Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, Park SQ, Wittmann A, Stoy M, et al. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage. 2010;50(4):1606–1617. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Smith AP, Dolan RJ, Rugg MD. Event-related potential correlates of the retrieval of emotional and nonemotional context. J Cogn Neurosci. 2004;16(5):760–775. doi: 10.1162/089892904970816. [DOI] [PubMed] [Google Scholar]
- Snodgrass J, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. Journal of Experimental Psychology. 1988;116:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52(3):547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91(2):139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45(10):2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia. 2008;46(7):1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends Cogn Sci. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21(4):1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114(1):152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43(7):1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25(11):3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15(4):983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]