Abstract
Mechanical ventilation is one of the most commonly applied interventions in intensive care units. Despite its life-saving role, it can be a risky procedure for the patient if not applied appropriately. To decrease risks, new ventilator modes continue to be developed in an attempt to improve patient outcomes. Advances in ventilator modes include closed-loop systems that facilitate ventilator manipulation of variables based on measured respiratory parameters. Adaptive support ventilation (ASV) is a positive pressure mode of mechanical ventilation that is closed-loop controlled, and automatically adjust based on the patient's requirements. In order to deliver safe and appropriate patient care, clinicians need to achieve a thorough understanding of this mode, including its effects on underlying respiratory mechanics. This article will discuss ASV while emphasizing appropriate ventilator settings, their advantages and disadvantages, their particular effects on oxygenation and ventilation, and the monitoring priorities for clinicians.
Keywords: Adaptive support ventilation, closed-loop ventilation, mechanical ventilation
Introduction
Mechanical ventilation support is a procedure often used in intensive care units. Despite being a technology that can potentially save lives, it is not devoid of risk, and if not used properly, it may even exacerbate lung damage or worsen the clinical outcomes.
The main objective of most ventilation support systems is to maintain both adequate oxygenation and ventilation, it reduces the work of breathing (WOB) and improves the comfort of the patient until the condition that forced the need for this technique has been reversed or alleviated. In an effort to meet these objectives, a variety of ventilatory modes have been developed that can potentially reduce complications, shorten the duration of mechanical ventilation and thus improve clinical outcomes.
The adaptive support ventilation (ASV) is one of the newer modes. It is considered a closed-loop controlled ventilatory mode, which is designed to ensure optimization of the patient's WOB. This article seeks to review the current literature in order to offer the reader updated operating principles, practical applications, and evidence in different clinical scenarios.
Closed-Loop Systems
There is a variety of ways to manipulate the control variables during mechanical ventilation. The two basic categories are the open-loop control and closed-loop control. The closed-loop control involves a positive or negative feedback of the information on the respiratory mechanics of the patient, based on measurements made almost continuously, which can be modified or adapted in a more physiological and individualized ventilatory support manner.[1,2] The two basic methods for closed-loop include: Control between breaths (inter-breath) which refers to the setting of control between each breath, but keeping it constant throughout the breath cycle; and intra-breath control, which does it within the same breath.[3]
In the closed-loop system, the output of gas is measured by providing a feedback signal that can be compared with the input value. The classical system of negative feedback control differentiates between input and output of gases, thus generating an error signal used to adjust the output so that it matches the input. The feedback control forces the gas output to become stable in the presence of environmental changes (such as leakage of the circuit, changes in lung mechanics, and respiratory muscle strain). This also automatically applies lung protection strategies, reducing the risk of errors committed by the operators [Figure 1].
Figure 1.
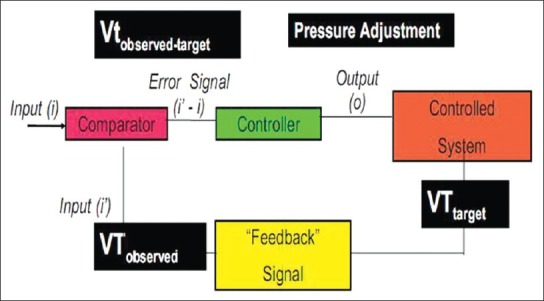
Closed-loop feedback system. The operator preset a target tidal volume (VT), through a feedback signal, the system measures the tidal volume of the patient (VT observed). The target VT and VT observed are compared (added or subtracted) and then an error signal is sent to the controller, which regulates the received signal and makes adjustments as needed to send an output signal, resulting in a desired breathing pattern, which can be eventually measured
Among the closed-loop systems available are Proportional Assist Ventilation (PAV), Neurally Adjusted Ventilatory Assistance (NAVA), Knowledge-Based Systems (KBS), and ASV. The first three (PAV, NAVA, and KBS) are basically advanced versions of Positive Pressure Support Ventilation (PSV) and therefore are considered to be “ventilatory modes”.
On the other hand, ASV combines various ventilatory modes: PSV, if the patient's respiratory rate (RR) is higher than the target: Pressure controlled ventilation, if there is no spontaneous breathing; and synchronized intermittent mandatory ventilation (SIMV), when patient's RR is lower than target. This has led many authors to call it the “no mode” or “integrated mode” or “three in one way.”[4]
History
ASV evolved as a form of mandatory minute ventilation (MMV) implemented with adaptive pressure control, and described by Hewlett in 1977.[5]
MMV is a mode that allows the operator to preset a target minute ventilation, the ventilator then supplies either volume or pressure-controlled mandatory breaths if the patient's spontaneous breaths generate a lower minute ventilation. Despite the efforts of manufacturers to popularize it, the acceptance of this mode was not widespread, possibly due to a combination of limitations and clinician's lack of understanding of it. MMV limitations include: Development of fast and ineffective breathing, development of auto-positive end-expiratory pressure (auto-PEEP) delivering dangerously high-tidal volumes (VTs) and increased dead space. Clinician's lack of understanding results in inappropriate programming.[6]
ASV technology invention is credited to Dr. Fleur T. Tehrani (clinical engineering professor at the University of California, USA) who used a modified version of the equation described by Otis et al. in 1950.[7,8]
ASV first clinical application was described in 1994 by Laubscher et al.[9,10] It became commercially available in Europe in 1998, but it was not until 2007 that it was marketed in the United States. It is considered to be the first commercially available ventilator system that uses an “optimal” targeting schema[11]. In this context, “Optimal” means minimizing the mechanical WOB: The machine selects a VT and frequency that the patient's brain would presumably select if the patient were not connected to a ventilator. This pattern is assumed to encourage the patient to generate spontaneous breaths. This mode provides specific minute ventilation and a breathing pattern optimized to the point of the smallest total energy expenditure, and it is based on patient's requirements. Thus, the ASV replaces other ventilatory modes designed solely for a specific phase of mechanical ventilation. It can be used for total or partial ventilatory support during the initiation, maintenance or weaning from mechanical ventilation.[11]
ASV Concept
ASV is a new ventilatory mode, which uses a closed-loop controlled mode between breaths. The ventilator allows the clinician to set a maximum plateau pressure and desired minute ventilation based on the patient's ideal weight. It automatically selects the target ventilatory pattern based on user inputs, as well as taking into account the respiratory mechanics data from the ventilator monitoring system (resistance, compliance, auto-PEEP). Thus, this mode can be safely used during initiation, maintenance, or weaning phases of the mechanical ventilation. ASV's goal is to ensure an effective alveolar ventilation level, minimizing the WOB, land leading the patient to an optimal ventilatory pattern in order to reduce complications such as volutrauma or barotrauma and air trapping.[6]
Operating Principles
The clinician, at the bedside, sets a target percent of minute ventilation (% MV) to be given to the patient, based on the ideal body weight (IBW). Additionally, the operator must assign PEEP, FiO2 and maximum pressure alarm (Pmax). Thus, minute ventilation (Ve) is calculated as the ratio between the ventilation resulting from IBW and the minute ventilation (% VM) set by the user. The ideal weight is calculated according to the Radford nomogram, which takes into account the patient's height.
Then, ASV automatically calculates the dead space based on the ideal weight (dead space [Vd] =2.2 ml/kg).
Ve[l/min] = Min Vol [%] × IBW/1000 (to IBW > 15 kg) or
Ve[l/min] = Min Vol [%] × IBW/500 (to IBW < 15 kg)
ASV selects the respiratory pattern in terms of RR, VT, inspiratory:Expiratory time (I:E ratio) for mandatory breathing and reaches the respiratory pattern selected. Thus, it is volume and pressure limited. Basically, ASV uses the Otis et al. and Mead et al. equation developed in 1950, that states that for a given level of alveolar ventilation, there is a particular RR which achieves a lower WOB. Therefore is more energy efficient to minimize the cumulative effects of elastic and resistive load imposed on the respiratory system.[12]
Otis Equation
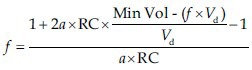
Where f is the RR, RC = airway resistance * respiratory compliance = time constant, Min Vol = minute ventilation, Vd= dead space, a = (2π2)/60 = 0.33 (constant for sinusoidal flow).
This is best exemplified in Figure 2 where Point A clearly shows that in order to maintain alveolar ventilation with very low RR, it is needed to use large VTs, which implies a high level of WOB. On the other hand, Point B, shows that a high amount of muscular effort is required to maintain adequate alveolar ventilation at high RRs (and low volume) in an attempt to overcome the resistance to flow. However, there is an optimal RR, which is the least costly in terms of WOB (Point C).
Figure 2.
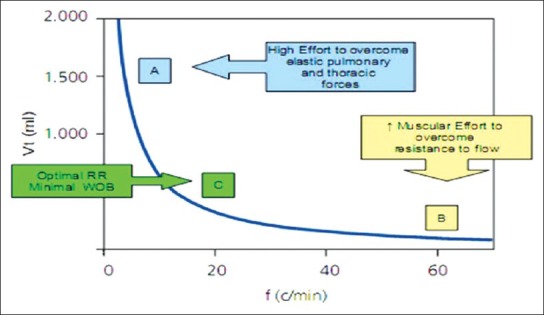
Tidal volume against respiration rate
Then the ASV mode uses a logarithm that calculates a target RR and objective VT based on Otis equation described above. ASV may select a pattern of high- and low-VT RRs in a patient with restrictive lung disease, and respiratory pattern with low frequency and high-VT in case of obstructive pathology.[6]
The expiratory time constant is calculated based on lung air resistance and respiratory compliance (RC = airway resistance × respiratory compliance = time constant) and is determined by the analysis of the flow-volume curve, in which ASV can adjust I:E relation and the target rate in order to keep the target volume within a margin of safety.[13] Moreover, this constant describes that a change in pressure (in time) equals a volume change, thus indicating the percentage of volume moved by the alveoli at a given time. ASV respiratory mechanics is calculated using the mathematical approach of setting least squares adjustment, which is based on the equation of motion in a relaxed atmosphere.
Airway pressure (PVA) = F × Rsr+ VT/PEEP Dsr+ Total
Where airway pressure (PVA) equals the product of resistive loads (flow (F) × respiratory system resistance (Rsr)), elastic loads (VT/Dsr), and total PEEP. These measurements are performed 200 times per second, for an average respiratory cycle of each variable.
These principles are outlined in Figure 3. The operator sets % VM, Pmax, PEEP and FiO2. The system by calculations described above and by a dual closed-loop system (RR goal and target VT) it calculates the RR and volume in which there is the lowest WOB (thus more efficient ventilation) within safety margins, adjusting inspiratory pressure and I:E ratio to achieve the desired goals.
Figure 3.
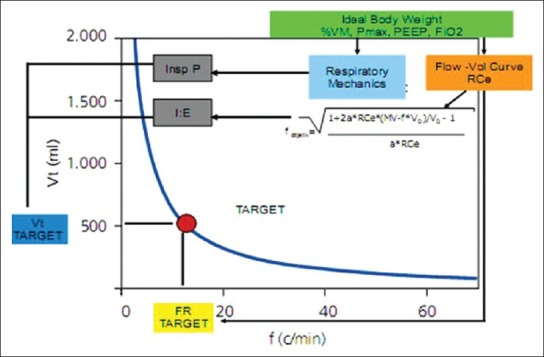
Adaptive support ventilation operating principles
ASV mode is based lung protective strategies, which aims to reach the RR and VT target, inside a security boundary area, where the highest energy efficiency is obtained and complications such as apnea, volutrauma, barotrauma and/or dead space ventilation are avoided [Figure 4].
Figure 4.
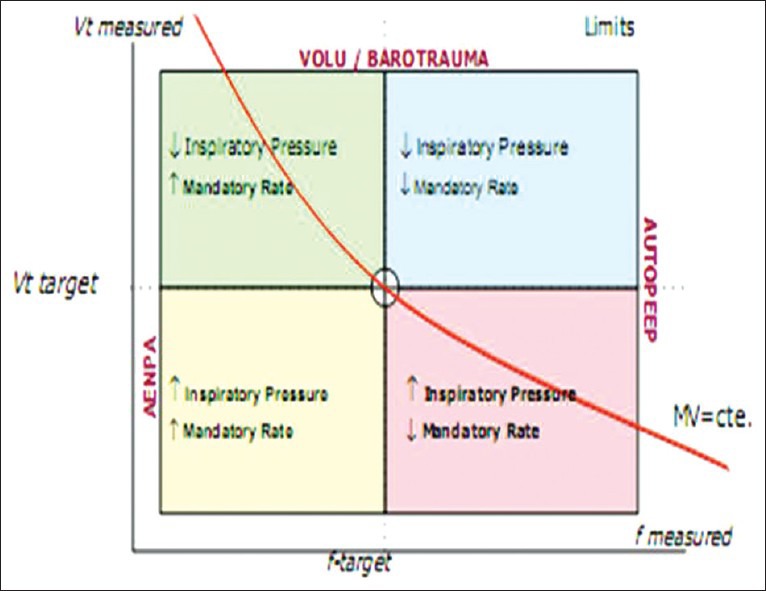
Security limits determined by adaptive support ventilation
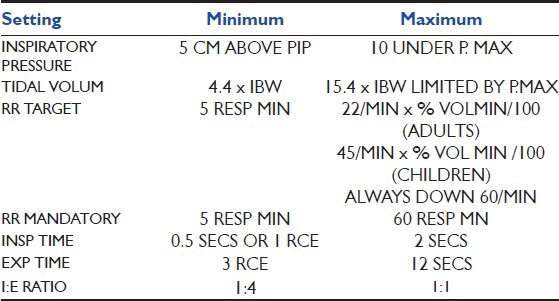
These safety limits are calculated based on the parameters of Table 1.
Table 1.
Minimum and Maximum parameters Protective Ventilation adaptive support ventilation
General Principles
The ASV is a ventilatory mode that adapts to patient respiratory effort. Depending on the spontaneous RR, ASV can work as PCV, if there is no spontaneous breathing; as pressure SIMV (P-SIMV), when patient RR is lower than target; or as PSV, if the RR is higher. Pressure level is then adapted to attain the target VT (within limits imposed by pressure alarms). Cycling off criteria is flow based in the case of assisted ventilation or time based for mandatory inspiration. To summarize it, any changes in respiratory mechanics and effort of the patient is accompanied by a dynamic pattern of breathing that gradually guides the patient to the new goal where there is a higher energy efficiency with minimal effort.
The safety rule, applied breath by breath, maintains ventilatory parameters within safe limits as shown in Figure 5. For this reason, if the patient is actively breathing, ASV automatically increases the number of pressure-controlled mandatory breaths necessary to maintain the target VM. Additionally, the safety limits prevent RRs and/or VT too high or too low, to minimize intrinsic PEEP, hyperventilation, increased dead space volume and barotrauma. In this way the operator can monitor trends and determine the patient condition and its interaction with this mode.
Figure 5.
Rules for safe use of adaptive support ventilation
Ventilator Settings
ASV ventilator settings are
Height of the patient (cm): Based on this it calculate the ideal body weight and dead space 2.2 ml/kg
Gender
-
% Min Vol: 25-350%
Normal 100%, asthma 90%, acute respiratory distress syndrome (ARDS) 120%, others 110%, Add 20% if T body >38.5°C (101.3°F) or add 5% for every 500 m (1640 feet) above sea level
Trigger: Flow trigger of 2 l/min
Expiratory trigger sensitivity: Start with 25% and 40% in Chronic obstructive pulmonary disease COPD
Tube resistance compensation: Set to 100%
High pressure alarm limit: 10 cm H2O be the limit of ↓ and ↑ least 25 cm H2O of PEEP/continuous positive airway pressure (CPAP)
PEEP
FiO2
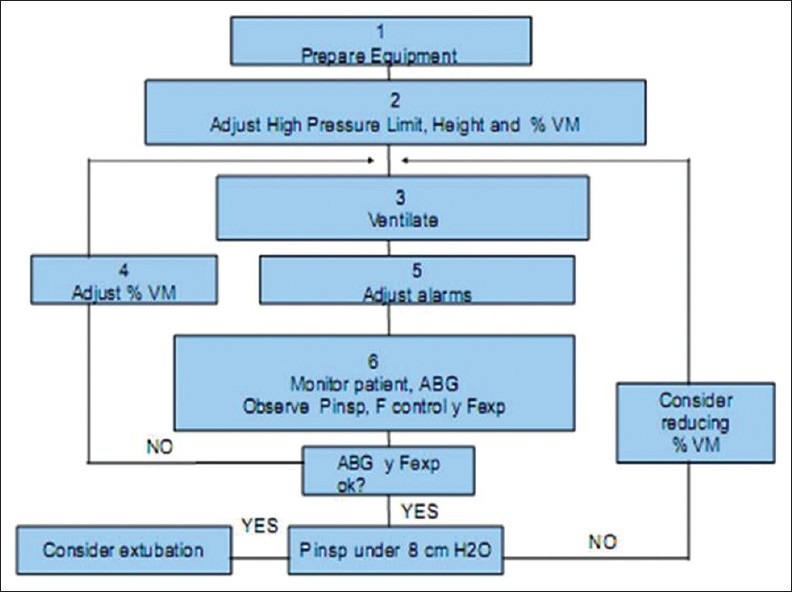
Once started, ASV provides a series of test breaths or test mode P-SIMV (with RRs between 10 and 15 min according to ideal body weight and assigned to inspiratory pressure above 15 cm H2O pressure basal), in which it measures the expiratory time constant for the respiratory system, and uses this along with the estimated dead space and normal minute ventilation in order to calculate an optimal breathing frequency in terms of mechanical work. During this breathing test, the ventilator measures compliance, Rce, VT, and RR based on selected inspiratory time (Ti), mandatory rate (f), and inspiratory pressure (Pinsp), according to the height (adult or pediatric age range) that the operator sets. In order to have a VT and RR target are determined within safety limits.[14]
This means that the pressure limit is automatically adjusted to achieve an average delivered VT equal to the target. The ventilator continuously monitors the mechanics of the respiratory system and adjusts its settings accordingly.
Follow-up
Once some stability is achieved, it is recommended to monitor ventilatory settings and perform arterial blood gases, which can guide on how to adjust parameters [Table 2].
Table 2.
Min Vol optimize% based on blood gas
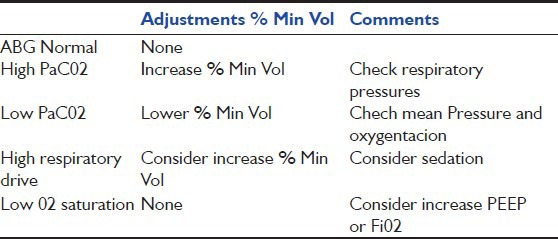
Decreases in % VM are performed according to clinical criteria and arterial blood gases results. % VM can be lowered 10% to 10% to 25% if there is an adequate patient effort and are above the requirements, until the withdrawal of ventilatory support when the settings are minimal. ASV parameters are considered to be minimal when all breaths are spontaneous (no mandatory breaths) and inspiratory pressure is ≤8 cm H2O above baseline. The patient monitoring practice can be done in two ways: By observing the level of support provided by the ventilator and observing patient effort in the monitor screen [Table 3].[6,15]
Table 3.
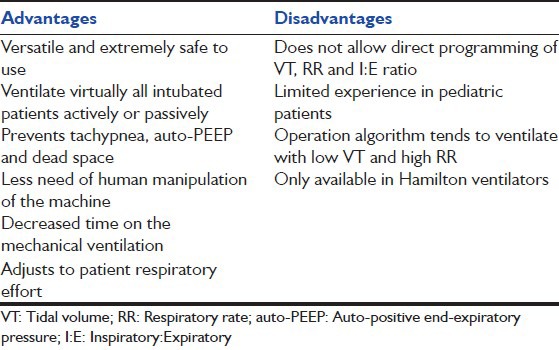
Advantages and disadvantages of adaptive support ventilation
Clinical Experience
Post-cardiac surgery
There are many reports about using ASV in patients after cardiac surgery. Sulzer et al.[16] conducted a prospective, randomized controlled trial in uncomplicated patients after cardiac surgery and found that patients on ASV mode required less time on mechanical ventilation than those on SIMV followed by pressure support (PS).
Petter et al.[17] conducted a similar study, comparing ventilatory extubation in ASV mode and SIMV + PS in postoperative cardiac surgery patients, both approaches showed equal outcomes. However, those patients on ASV had fewer ventilator manipulations and fewer alarms that could be interpreted as a medical resource saving benefit.
Cassina conducted a prospective observational cohort study, including 155 consecutive patients after cardiac surgery, confirming the safety benefit of ASV mode. 134 patients (86%) were successfully extubated within 6 h, with an average intubation of 3.6 (2.53-4.83) hours (quartiles), and no re-intubation due to respiratory failure.[18] Concluding that ASV allows rapid extubation in selected patients and can facilitate respiratory management of post-surgery patients.
Recently, Dongelman et al.[19] conducted a randomized controlled study in patients with post-coronary bypass, comparing extubation time in patients with ASV against PCV/pressure support and demonstrated that ASV is safer and more useful even when the extubation time was similar in both modes.
ASV Weaning Mode
ASV can be used as a method of weaning in both acute and chronic patients. Linton et al.[20] conducted some weaning trials in chronic respiratory patients, showing that ASV is a cost-saving mode in terms of need for respiratory therapists and intensive care personnel. On admission, 27 patients were assigned to 90% ASV target VM being reduced by 10% weekly to a target VM percentage of 60% according to patient tolerance.[21] Twenty patients were successfully weaned within 2 weeks to 2 months in the first year after the establishment of the facility.[16,22]
Lung Protective Strategy
Belliato et al.[23] evaluated ASV mode in patients with normal lungs, patients with restrictive pulmonary diseases, obstructive diseases and in pulmonary physical model with a normal level and high minute ventilation. In postoperative patients with normal lungs, ASV selected a ventilatory strategy close to the physiological one. In patients with COPD, ASV selected a high expiratory time pattern and in patients with restrictive conditions a pattern with low VTs. In the model, the selection was similar. In the hyperventilation test, ASV assigned a balanced increase between VT and RR. The authors concluded that ASV can select an adequate ventilatory pattern for a wide range of lung conditions.
Arnal et al. conducted a prospective observational cohort study, similar to the former, in order to determine the breathing pattern generated by ASV in various pulmonary conditions. Included 243 patients categorized as: Normal lung, acute lung injury (ALI), COPD, and restrictive lung disease. Daily information was collected on ventilation parameters, respiratory patterns and arterial blood gases.[15] They concluded that in passively ventilated patients ASV is able to change the delivered pattern (VT and respiratory frequency FR) based on the underlying condition, offering higher VT and lower RR in COPD patients than in those with ALI.
Tassaux et al.[24] conducted a prospective crossover study in 10 patients with acute respiratory failure of diverse etiology during an early weaning period. The results showed that for a given level of minute ventilation, ASV patients had a lower WOB based on the measurement of respiratory muscle activity (electromyography), and also achieved better patient-ventilator interaction when compared to in the SIMV mode connected patients.
Recently, Sulemanji et al.[25] compared ASV mode with ventilation strategy using a fixed VT 6 ml/kg. They concluded that in a lung model with different mechanics, ASV was superior in preventing potentially harmful effects of excessive plateau pressure (greater than 28 cm H2O) than with fixed VT 6 ml/kg, this is because ASV automatically adjusts PVA, which results in lower VTs.
ASV Versus Other Ventilatory Modes
Gruber et al.[26] compared ASV with pressure regulated volume controlled (PRVC) in patients undergoing cardiac surgery (without complications) and showed that ASV results in a shorter intubation time and with less clinical intervention (by the operator) compared with PRVC[27].
Iotti et al. conducted a prospective multi-centered crossover study in 6 European intensive care units, with 88 patients who were categorized as: 22 healthy lung, 36 with restrictive lung disease, and 30 with obstructive lung disease, and compared the short-term effects of ASV with conventional ventilation modes (volume or pressure) and ventilated patients with acute respiratory failure, thus demonstrating the benefits of ASV over this modes.[1]
In-Pediatric Patients
It is limited to case reports. Brown and Duthie describe a case of a pediatric patient (11 years) with status asthmatics, demonstrating that ASV results in a decrease peak pressure without causing auto-PEEP.[28] Georgiev reported her experience in neonatal intensive care unit with ASV.[29] She reported less days of stay in mechanical ventilation, less sedation and reduction in hospital stay. Pediatric studies are not yet published: A protocol called ASV in ARDS in children over 12 years started in 2010 with completion date in 2011; another protocol of investigation concluded by the University of Montreal entitled a Safety and Feasibility Study of a Computerized Mechanical Ventilation Protocol: Intellivent (CloserPed) using ASV to assess the safety of this mode in pediatric patients, has not been published yet.
Other benefits
Jung et al.[30] conducted a study with healthy pigs and compared the effects of ASV versus controlled ventilation in vivo and in vitro in terms of the effects these modes could have on diaphragm muscle. They concluded that ASV protects the diaphragm against the deleterious effects of prolonged mechanical ventilation and also helps maintain an adequate contraction of the diaphragm as demonstrated by measurements of transdiaphragmatic pressure and phrenic nerve conduction.
Summary
ASV is an advanced mode with many advantages. First, it keeps normal ventilation and promotes the ventilatory pattern associated with the best energetic. Second, and taking into account spontaneous breathing, it is useful to prevent tachypnea, the development of auto-PEEP and excessive dead space ventilation. It can be safely used in cases of apnea or low respiratory drive, and adapting to respiratory effort of the patient (spontaneous or not) without exceeding the plateau pressure preset by the operator.
All this includes the advantage of a lung protective strategy and the decrease of the use of resources. It can be used in both acute and chronically ventilated patients, and as a strategy for initiation, maintenance or weaning. However, controlled studies are needed to clarify the role of ASV in clinical practice and its impact mortality in severely ill patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Iotti GA, Polito A, Belliato M, Pasero D, Beduneau G, Wysocki M, et al. Adaptive support ventilation versus conventional ventilation for total ventilatory support in acute respiratory failure. Intensive Care Med. 2010;36:1371–9. doi: 10.1007/s00134-010-1917-2. [DOI] [PubMed] [Google Scholar]
- 2.Campbell RS, Sinamban RP, Johannigman JA, Luchette FA, Frame SB, Davis K, Jr, et al. Clinical evaluation of a new closed loop ventilation mode: Adaptive supportive ventilation (ASV) Crit Care. 1999;3:P038. [Google Scholar]
- 3.Brunner JX. Principles and history of closed-loop controlled ventilation. Respir Care Clin N Am. 2001;7:341–62. doi: 10.1016/s1078-5337(05)70040-x. [DOI] [PubMed] [Google Scholar]
- 4.Wysocki M, Brunner JX. Closed-loop ventilation: An emerging standard of care? Crit Care Clin. 2007;23:223–40. doi: 10.1016/j.ccc.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Hewlett AM, Platt AS, Terry VG. Mandatory minute volume. A new concept in weaning from mechanical ventilation. Anaesthesia. 1977;32:163–9. doi: 10.1111/j.1365-2044.1977.tb11588.x. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin N Am. 2001;7:425–40. doi: 10.1016/s1078-5337(05)70049-6. [DOI] [PubMed] [Google Scholar]
- 7.Tehrani FT. The origin of adaptive support ventilation. Int J Artif Organs. 2005;28:1051–2. doi: 10.1177/039139880502801013. [DOI] [PubMed] [Google Scholar]
- 8.Tehrani FT, Roum JH. Flex: A new computerized system for mechanical ventilation. J Clin Monit Comput. 2008;22:121–30. doi: 10.1007/s10877-008-9113-4. [DOI] [PubMed] [Google Scholar]
- 9.Laubscher TP, Frutiger A, Fanconi S, Jutzi H, Brunner JX. Automatic selection of tidal volume, respiratory frequency and minute ventilation in intubated ICU patients as start up procedure for closed-loop controlled ventilation. Int J Clin Monit Comput. 1994;11:19–30. doi: 10.1007/BF01132840. [DOI] [PubMed] [Google Scholar]
- 10.Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng. 1994;41:51–9. doi: 10.1109/10.277271. [DOI] [PubMed] [Google Scholar]
- 11.Mireles-Cabodevila E, Diaz-Guzman E, Heresi GA, Chatburn RL. Alternative modes of mechanical ventilation: A review for the hospitalist. Cleve Clin J Med. 2009;76:417–30. doi: 10.3949/ccjm.76a.08043. [DOI] [PubMed] [Google Scholar]
- 12.Otis AB, Fenn WO, Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2:592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- 13.Veelo DP, Dongelmans DA, Binnekade JM, Paulus F, Schultz MJ. Adaptive support ventilation: A translational study evaluating the size of delivered tidal volumes. Int J Artif Organs. 2010;33:302–9. [PubMed] [Google Scholar]
- 14.Arnal JM, Wysocki M, Novotni D, Demory D. Hamilton Manual, 2009. Available from: http://www.hamilton-medical.com .
- 15.Arnal JM, Wysocki M, Nafati C, Donati S, Granier I, Corno G, et al. Automatic selection of breathing pattern using adaptive support ventilation. Intensive Care Med. 2008;34:75–81. doi: 10.1007/s00134-007-0847-0. [DOI] [PubMed] [Google Scholar]
- 16.Sulzer CF, Chioléro R, Chassot PG, Mueller XM, Revelly JP. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: A randomized controlled study. Anesthesiology. 2001;95:1339–45. doi: 10.1097/00000542-200112000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Petter AH, Chioléro RL, Cassina T, Chassot PG, Müller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: The effect on duration of endotracheal intubation and patient management. Anesth Analg. 2003;97:1743–50. doi: 10.1213/01.ANE.0000086728.36285.BE. [DOI] [PubMed] [Google Scholar]
- 18.Cassina T, Chioléro R, Mauri R, Revelly JP. Clinical experience with adaptive support ventilation for fast-track cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17:571–5. doi: 10.1016/s1053-0770(03)00199-x. [DOI] [PubMed] [Google Scholar]
- 19.Dongelmans DA, Veelo DP, Paulus F, de Mol BA, Korevaar JC, Kudoga A, et al. Weaning automation with adaptive support ventilation: A randomized controlled trial in cardiothoracic surgery patients. Anesth Analg. 2009;108:565–71. doi: 10.1213/ane.0b013e318190c49f. [DOI] [PubMed] [Google Scholar]
- 20.Linton DM, Renov G, Lafair J, Vasiliev L, Friedman G. Adaptive Support Ventilation as the sole mode of ventilatory support in chronically ventilated patients. Crit Care Resusc. 2006;8:11–4. [PubMed] [Google Scholar]
- 21.Saddy F, Oliveira GP, Garcia CS, Nardelli LM, Rzezinski AF, Ornellas DS, et al. Assisted ventilation modes reduce the expression of lung inflammatory and fibrogenic mediators in a model of mild acute lung injury. Intensive Care Med. 2010;36:1417–26. doi: 10.1007/s00134-010-1808-6. [DOI] [PubMed] [Google Scholar]
- 22.Kirakli C, Ozdemir I, Ucar ZZ, Cimen P, Kepil S, Ozkan SA. Adaptive support ventilation for faster weaning in COPD: A randomised controlled trial. Eur Respir J. 2011;38:774–80. doi: 10.1183/09031936.00081510. [DOI] [PubMed] [Google Scholar]
- 23.Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs. 2004;27:709–16. doi: 10.1177/039139880402700809. [DOI] [PubMed] [Google Scholar]
- 24.Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient-ventilator interactions during partial ventilatory support: A preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med. 2002;30:801–7. doi: 10.1097/00003246-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Sulemanji D, Marchese A, Garbarini P, Wysocki M, Kacmarek RM. Adaptive support ventilation: An appropriate mechanical ventilation strategy for acute respiratory distress syndrome? Anesthesiology. 2009;111:863–70. doi: 10.1097/ALN.0b013e3181b55f8f. [DOI] [PubMed] [Google Scholar]
- 26.Gruber PC, Gomersall CD, Leung P, Joynt GM, Ng SK, Ho KM, et al. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology. 2008;109:81–7. doi: 10.1097/ALN.0b013e31817881fc. [DOI] [PubMed] [Google Scholar]
- 27.Chen CW, Wu CP, Dai YL, Perng WC, Chian CF, Su WL, et al. Effects of implementing adaptive support ventilation in a medical intensive care unit. Respir Care. 2011;56:976–83. doi: 10.4187/respcare.00966. [DOI] [PubMed] [Google Scholar]
- 28.Brown MK, Duthie SM. Adaptive support ventilation reduced peak pressures, improved blood gases and minimized air trapping in a child with status asthmaticus. Resp Care Open Forum Abstracts. 2000. Available from: http://www.rcjournal.com/abstracts/2000/?id=A00000262 .
- 29.Georgiev H. A breath of fresh air. ASV mode using Hamilton G5 Ventilator in NICU. www.therapytimes.com/content .
- 30.Jung B, Constantin JM, Rossel N, Le Goff C, Sebbane M, Coisel Y, et al. Adaptive support ventilation prevents ventilator-induced diaphragmatic dysfunction in piglet: An in vivo and in vitro study. Anesthesiology. 2010;112:1435–43. doi: 10.1097/ALN.0b013e3181d7b036. [DOI] [PubMed] [Google Scholar]


