Abstract
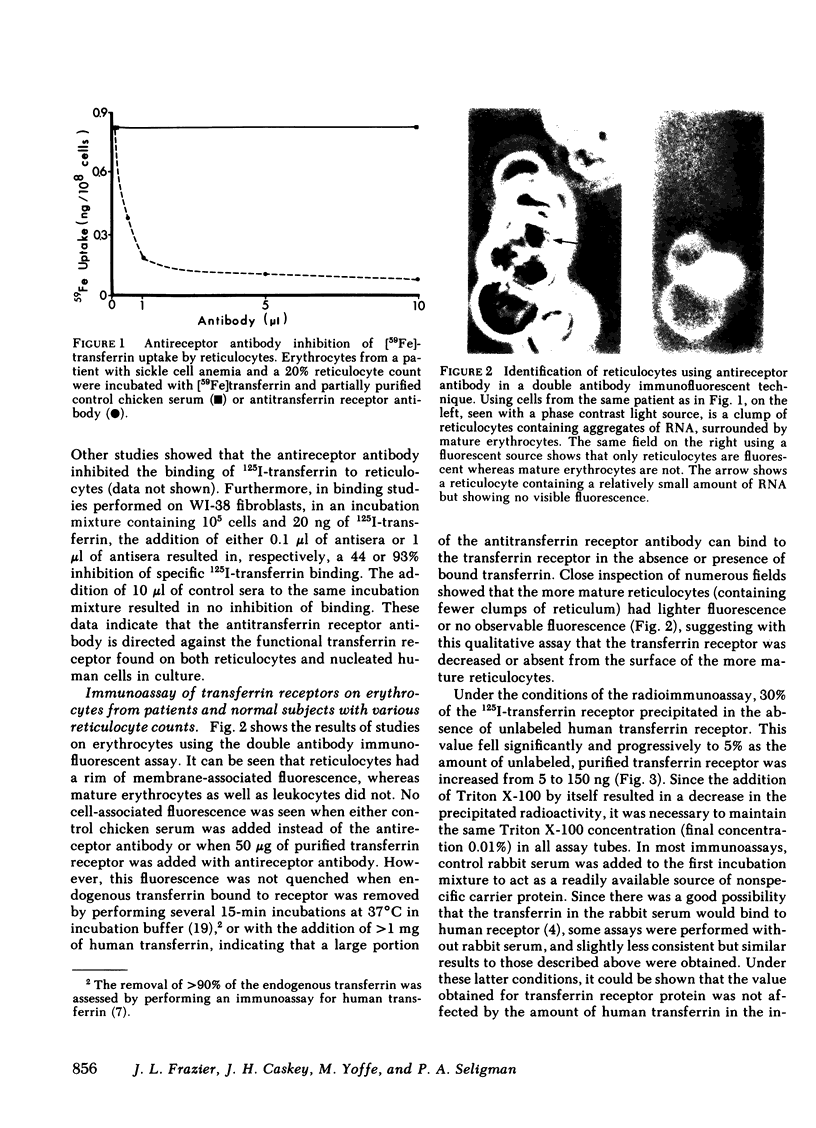
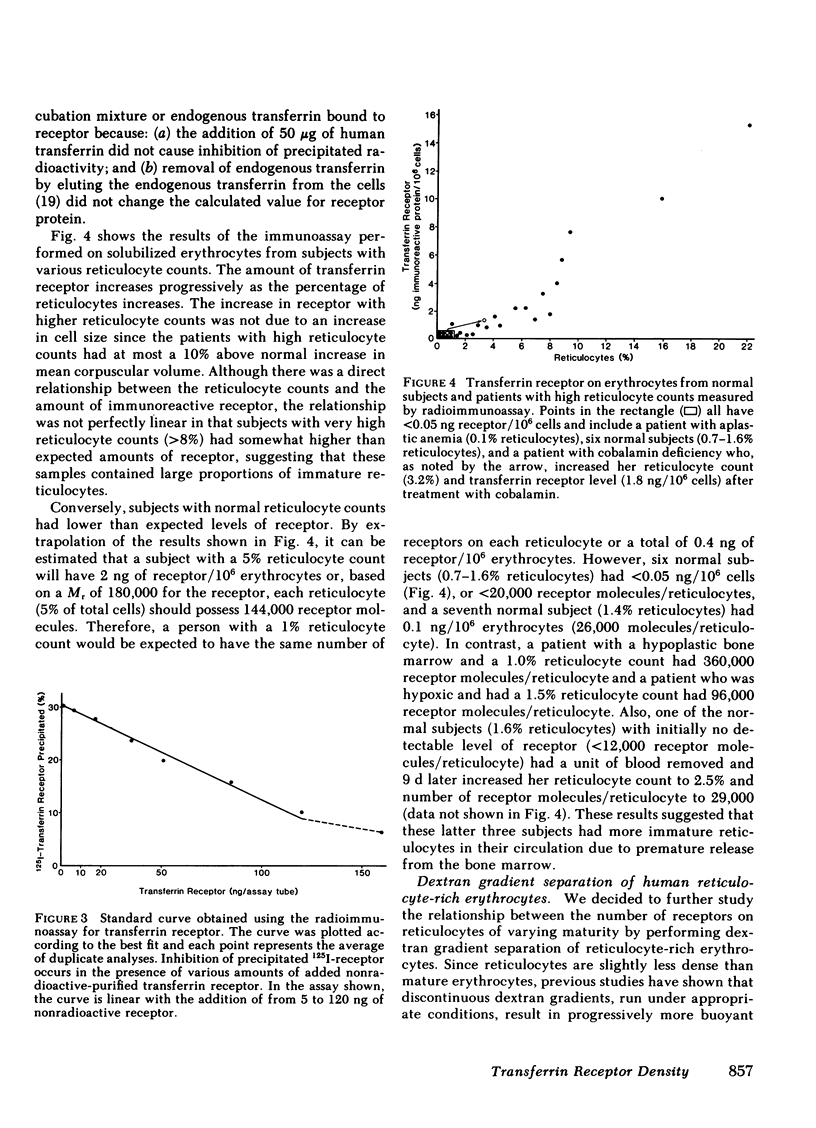
The transferrin receptor, present on reticulocytes and nucleated cells in tissue culture, has been measured with both immunoassay techniques and transferrin binding studies. The total cellular immunoreactive receptor is rapidly lost from erythrocytes during the process of reticulocyte maturation (from as many as 400,000 molecules to <20,000 molecules/reticulocyte). This event parallels the loss of cell surface transferrin binding sites and RNA content, and correlates with previous studies that have measured the decline in hemoglobin synthesis.
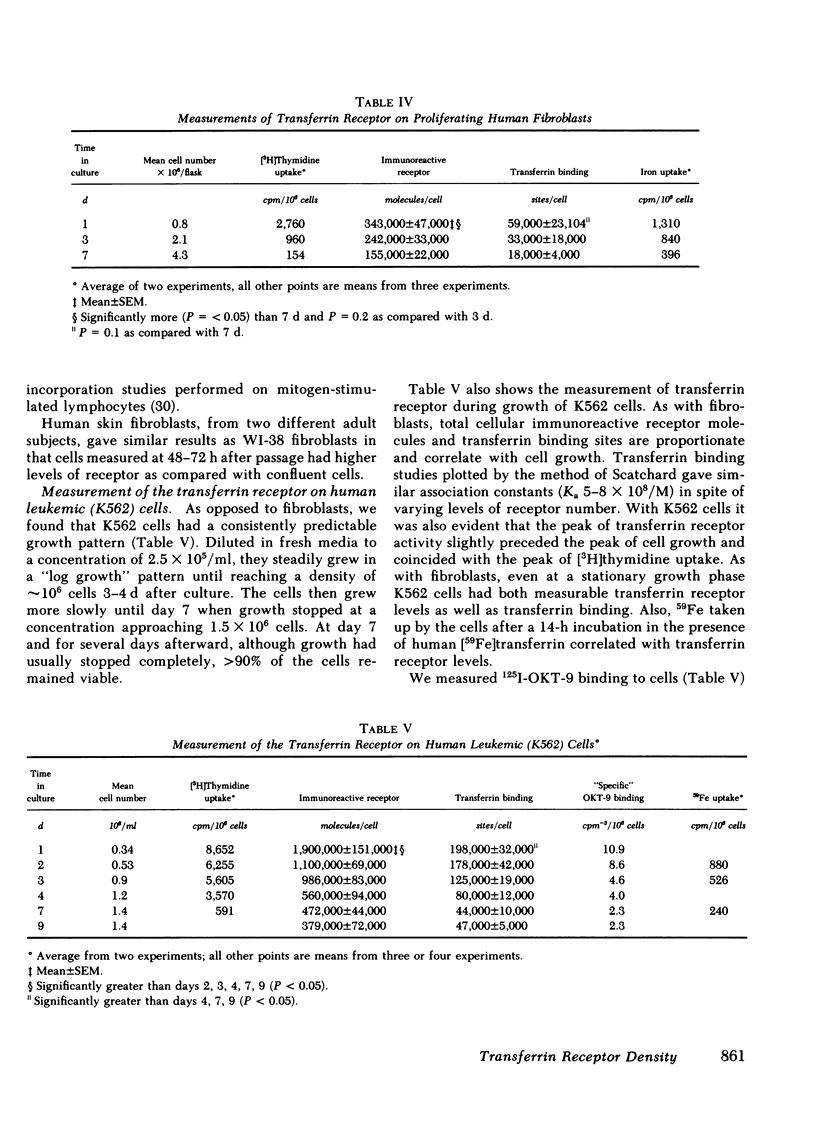
Nonhemoglobin-producing normal human fibroblasts, which appear to have a much lower iron requirement than reticulocytes, contain similar numbers of immunoreactive receptors per cell (400,000 receptor molecules), when in an active state of proliferation. Although receptor density on fibroblasts is directly related to cell proliferation, our studies demonstrate that nonproliferating fibroblasts still retain significant numbers of immunoreactive receptors (150,000 molecules/cell) and transferrin binding sites. Since additional studies indicate that proliferating cells have increased iron uptake, a simple hypothesis would predict that the parallel increase in transferrin binding sites and total cellular immunoreactive receptor associated with proliferation is related to an increased cellular iron requirement. However, the number of immunoreactive receptor molecules and transferrin binding sites is not changed when cells are grown in iron-deficient media, or in media with added transferrin-iron. This result and the lack of marked differences in receptor number on both hemoglobin-producing and nonhemoglobin-producing cells indicate that other factors besides receptor density play major roles in the regulation of cellular iron uptake, retention, and loss.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. Structure and function of transferrin. Prog Hematol. 1975;9:25–56. [PubMed] [Google Scholar]
- Andersson L. C., Jokinen M., Gahmberg C. G. Induction of erythroid differentiation in the human leukaemia cell line K562. Nature. 1979 Mar 22;278(5702):364–365. doi: 10.1038/278364a0. [DOI] [PubMed] [Google Scholar]
- Baker E., Vicary F. R., Huehns E. R. Iron release from isolated hepatocytes. Br J Haematol. 1981 Apr;47(4):493–504. doi: 10.1111/j.1365-2141.1981.tb02678.x. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. H., Rankin M. C. Transferrin binding and iron uptake by mouse lymph node cells during transformation in response to concanavalin A. Immunology. 1981 Jun;43(2):393–398. [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman P. R., Beutler E., Finch C. A. Effects of iron deficiency exclusive of anaemia. Br J Haematol. 1978 Oct;40(2):179–184. doi: 10.1111/j.1365-2141.1978.tb03655.x. [DOI] [PubMed] [Google Scholar]
- Enns C. A., Shindelman J. E., Tonik S. E., Sussman H. H. Radioimmunochemical measurement of the transferrin receptor in human trophoblast and reticulocyte membranes with a specific anti-receptor antibody. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4222–4225. doi: 10.1073/pnas.78.7.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Hsi B. L., Stevens P. J. Transferrin and transferrin receptors in carcinoma of the breast. Lancet. 1980 Aug 23;2(8191):390–392. doi: 10.1016/s0140-6736(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Galbraith R. M., Werner P., Arnaud P., Galbraith G. M. Transferrin binding to peripheral blood lymphocytes activated by phytohemagglutinin involves a specific receptor. Ligand interaction. J Clin Invest. 1980 Nov;66(5):1135–1143. doi: 10.1172/JCI109943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W., Burns G. F. Monoclonal antibody OKT-9 recognizes the receptor for transferrin on human acute lymphocytic leukemia cells. J Immunol. 1981 Sep;127(3):1256–1258. [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. Transferrin and iron uptake by human cells in culture. Exp Cell Res. 1974 Jul;87(1):207–212. doi: 10.1016/0014-4827(74)90543-6. [DOI] [PubMed] [Google Scholar]
- Hoffbrand A. V., Ganeshaguru K., Hooton J. W., Tattersall M. H. Effect of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br J Haematol. 1976 Aug;33(4):517–526. doi: 10.1111/j.1365-2141.1976.tb03570.x. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., KATZ J. H. The plasma-to-cell cycle of transferrin. J Clin Invest. 1963 Mar;42:314–326. doi: 10.1172/JCI104718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd W., Poodry C. A., Strominger J. L. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J Exp Med. 1980 Nov 1;152(5):1430–1435. doi: 10.1084/jem.152.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Small B., Chang H. Maturation of rabbit reticulocytes: degradation of specific reticulocyte proteins. Dev Biol. 1975 Nov;47(1):59–67. doi: 10.1016/0012-1606(75)90263-8. [DOI] [PubMed] [Google Scholar]
- Loh T. T., Higuchi D. A., van Bockxmeer F. M., Smith C. H., Brown E. B. Transferrin receptors on the human placental microvillous membrane. J Clin Invest. 1980 May;65(5):1182–1191. doi: 10.1172/JCI109773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio B. B., Lozzio C. B. Absence of erythrocytic components in the original K562 cell line. Int J Cancer. 1979 Oct 15;24(4):513–513. doi: 10.1002/ijc.2910240421. [DOI] [PubMed] [Google Scholar]
- Mott D. M., Howard B. V., Bennett P. H. Stoichiometric binding and regulation of insulin receptors on human diploid fibroblasts using physiologic insulin levels. J Biol Chem. 1979 Sep 25;254(18):8762–8767. [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Phillips J. L. Specific binding of zinc transferrin to human lymphocytes. Biochem Biophys Res Commun. 1976 Sep 20;72(2):634–639. doi: 10.1016/s0006-291x(76)80087-3. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Schulman H. M. The fractionation of rabbit reticulocytes in dextran density gradients. Biochim Biophys Acta. 1967 Oct 9;148(1):251–255. doi: 10.1016/0304-4165(67)90300-5. [DOI] [PubMed] [Google Scholar]
- Schulman H. M., Wilczynska A., Ponka P. Transferrin and iron uptake by human lymphoblastoid and K-562 cells. Biochem Biophys Res Commun. 1981 Jun;100(4):1523–1530. doi: 10.1016/0006-291x(81)90691-4. [DOI] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormey D. C., Mueller G. C. Biological effects of transferrin on human lymphocytes in vitro. Exp Cell Res. 1972 Sep;74(1):220–226. doi: 10.1016/0014-4827(72)90500-9. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Witt D. P., Woodworth R. C. Identification of the transferrin receptor of the rabbit reticulocyte. Biochemistry. 1978 Sep 19;17(19):3913–3917. doi: 10.1021/bi00612a004. [DOI] [PubMed] [Google Scholar]
- van Bockxmeer F. M., Morgan E. H. Transferrin receptors during rabbit reticulocyte maturation. Biochim Biophys Acta. 1979 Apr 18;584(1):76–83. doi: 10.1016/0304-4165(79)90237-x. [DOI] [PubMed] [Google Scholar]