Abstract
An enantioselective synthesis of the core framework of neurotrophic Illicium majucin-type sesquiterpenes is described here. This strategy is based on an organocatalyzed asymmetric Robinson annulation and provides an efficient approach for a diversity-oriented synthesis of Illicium natural products that holds remarkable therapeutic potential for neurodegenerative diseases.
Keywords: natural products, neurodegenerative diseases, neurotrophic small molecule, organocatalysis, total synthesis
Introduction
Neurotrophins are a family of endogenous proteins that are vital for neuron function, survival, and regeneration [1–3]. As such, they have prompted intense studies toward the treatment of various neurodegenerative diseases including Alzheimer’s disease [4] and Parkinson’s disease [5]. Despite their unambiguous importance, approaches to neurotrophin-based drug development have encountered problems associated with their limited oral availability, insufficient delivery to the central neural system and considerable manufacturing cost [6–7]. These limitations have stimulated the search for small molecules that can enhance or mimic neurotrophin activity as potential drug leads [8–12].
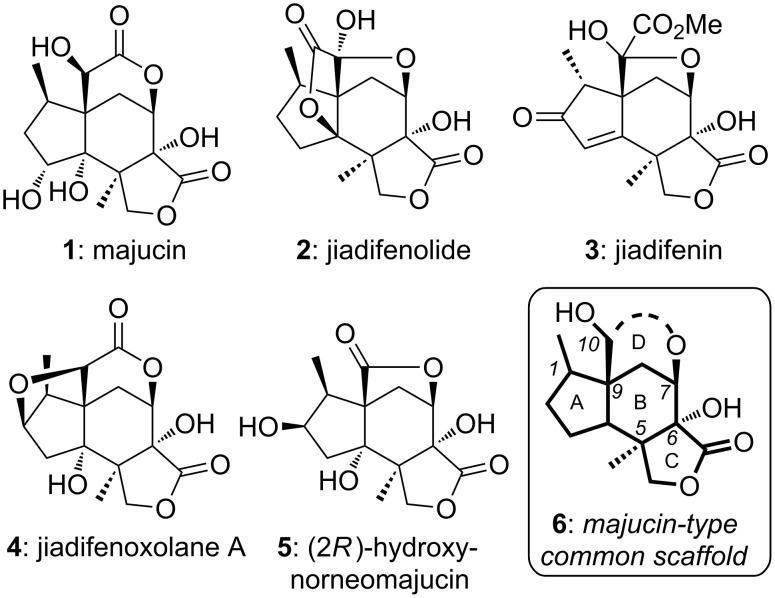
Majucin-type Illicium sesquiterpenes (Figure 1) [13], such as majucin (1) [14–15], jiadifenolide (2) [16], jiadifenin (3) [17], jiadifenoxolane A (4) [16] and (2R)-hydroxynorneomajucin (5) [18], share a caged tetracyclic scaffold (6). These compounds (2–5) have shown a great potential in enhancing neurite outgrowth in primary cultured rat cortical neurons at low nanomolar to low micromolar concentrations. Thus, to develop an efficient synthetic approach toward the complex core skeleton of these natural products is of paramount importance. Consequently, this family of neurotrophic sesquiterpenes has been the focus of extensive synthetic studies in which asymmetric and efficient construction of the tetracyclic core presents the principal challenge [19–23].
Figure 1.
Representative majucin-type Illicium sesquiterpenes.
We have recently reported a unified synthetic strategy of 2, 3 and designed analogues using scaffold 7 as the key intermediate (Figure 2) [24–26]. A potential drawback of this strategy is the late-stage modification of the A ring motif of 7 that requires additional steps for the synthesis of the target molecules. In an effort to overcome this issue, we describe here a second-generation strategy of framework 9 in which the C-1 center has been methylated early in the synthesis. As such, it represents an efficient route toward a diversity-oriented synthesis of several Illicium sesquiterpenes. The enantioselective entry to these molecules is based on an organocatalyzed asymmetric Robinson annulation that allows access to the enantiomerically enriched bicyclic motif 8 from achiral diketone 11 (Figure 2).
Figure 2.
Comparison of core skeleton synthetic strategies.
Results and Discussion
During the past 20 years, organocatalysis has emerged as an important field in asymmetric stereoselective synthesis due to its advantages, which include high enantioselectivity, environmental friendliness and ease of handling [27–50]. Organocatalyzed asymmetric Robinson annulation has long been proven to be one of the most powerful strategies to construct bicyclic systems with a chiral quaternary center [51–58]. Among them, the Hajos–Wiechert and Wieland–Miescher ketones represent two of the most famous examples [59–65]. With this background information in mind, we devised an enantioselective synthesis of 8 starting from commercially available dione 12, and the synthesis of 8 was previously published [25–26]. Tsuji–Trost allylation [66–68] of 12 produced compound 11, which was readily converted to 13 by an acid-catalyzed Michael addition with methyl vinyl ketone (MVK) (two steps, 63% overall yield) [69–71]. The organocatalyzed cyclization of 13 was achieved by optimizing the previously reported Tu/Zhang conditions [71] using D-prolinamide as the organocatalyst (Scheme 1). Performing this reaction at 80 °C gave rise to bicyclic motif 8 in about 70% ee (70 % yield after 12 h), while decreasing the temperature to 25 °C increased the enantioselectivity to over 99% (70% yield after 60 days). To compromise between high enantioselectivity and short reaction time, we decided to pursue this conversion at 40 °C where we obtained an enantiomeric excess of 90% (70% yield after 14 days).
Scheme 1.
Organocatalyzed asymmetric Robinson annulation.
The enantiomerically enriched Hajos–Wiechert-like diketone 8 (ee > 90%) was then subjected to a selective protection of the C-6 enone motif to yield dithioketal 14 (86% yield) [72–74]. Wittig olefination of the C-1 ketone with methoxymethylenetriphenylphosphine [75] yielded the corresponding enol methyl ether, which was hydrolyzed to the aldehyde under acidic conditions and reduced with NaBH4 to form alcohol 15 with desired diastereoselectivity at the C-1 center (dr = 9:1) in 81% yield (over three steps) [76]. The stereochemistry of 15 was unambiguously confirmed by single-crystal X-ray analysis of the related tosylate derivative 16 [77]. Deoxygenation of the C-15 primary alcohol was performed by: (a) mesylation of the alcohol with MsCl; and (b) reductive deoxygenation with LiEt3BH (super hydride). The thioketal protecting group was then removed under oxidative conditions with [bis(trifluoroacetoxy)iodo]benzene (PIFA) to yield ketone 10 in good yield (66% over three steps, Scheme 2) [78]. This approach allowed us to produce a sufficient amount of enone 10 (>10 grams) for further functionalization.
Scheme 2.
Early stage A-ring functionalization.
Conversion of 10 to 9 was accomplished based on our previously reported strategy (Scheme 3) [25]. Treatment of 10 with magnesium methyl carbonate (MMC) [79–81] yielded the C-5 carboxylic acid that, without further purification, was esterified under Meerwein’s conditions to afford β-ketoester 17. Treatment of 17 with TMSOTf/Et3N followed by enolate alkylation [82] under TBAF/MeI conditions afforded the desired C-5 quaternary center of 18 as a single isomer (35% over four steps). Global reduction of 18 with lithium aluminium hydride produced the corresponding C-6/C-14 diol motif. Selective TBS protection of the C-14 primary alcohol followed by an IBX oxidation of the C-6 secondary alcohol yielded ketone 19 in 80% combined yield over three steps. Triflation of the C-6 ketone with McMurry’s reagent (PhNTf2) [83–86] followed by a Pd(0)-catalyzed carbomethoxylation [87–90] produced the desired C-ring lactone 20 in 61% yield. Epoxidation of the C-6/C-7 enone with NaOH/H2O2 followed by oxidative cleavage of the C-11 terminal alkene under OsO4/NaIO4 conditions [91–92] afforded the corresponding C-11 aldehyde. Exposure of this intermediate to Jones oxidation triggered a highly efficient oxidation–epoxide opening [93–98] reaction cascade [99–100] to construct the critical D-ring of 9 (46% yield, over 3 steps). Notably, this scalable approach rendered us several hundred milligrams of compound 9, paving the way for a diversity-oriented synthesis. For example, a Mn(III) promoted C-2 allylic oxidation [24,101–102] would provide a C-2 oxygenated functionality. Similarly, C-10 α-substitution would provide a large diversity of neurotrophic analogues based on our recent findings [26].
Scheme 3.
Synthesis of core scaffold 9.
Conclusion
We describe here an efficient and enantioselective approach to tetracyclic lactone 9 representing a key motif toward the synthesis of various neurotrophic [103–110] Illicium sesquiterpenes. Key to the strategy was a highly enantioselective Robinson annulation reaction that proceeded under organocatalytic conditions to form the Hajos–Wiechert-like enone 8. The overall strategy highlights the importance of organocatalytic approaches in the modern synthesis of bioactive natural products [111–116].
Supporting Information
Experimental procedures for the syntheses of all new compounds.
Acknowledgments
We gratefully acknowledge the National Institutes of Health (NIH) for financial support of this work through Grant Number CA 133002. We thank the National Science Foundation for instrumentation grants CHE9709183 and CHE0741968. We also thank Dr. Anthony Mrse (UCSD NMR Facility), Dr. Yongxuan Su (UCSD MS Facility) and Dr. Arnold L. Rheingold and Dr. Curtis E. Moore (UCSD X-Ray Facility).
This article is part of the Thematic Series "Transition-metal and organocatalysis in natural product synthesis".
References
- 1.Sofroniew M V, Howe C L, Mobley W C. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 2.Chao M V. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 3.Huang E J, Reichardt L F. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Querfurth H W, LaFerla F M. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 5.Shulman J M, De Jager P L, Feany M B. Annu Rev Pathol: Mech Dis. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 6.Skaper S D. CNS Neurol Disord: Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 7.Skaper S D. Curr Pharm Des. 2011;17:2704–2718. doi: 10.2174/138161211797415995. [DOI] [PubMed] [Google Scholar]
- 8.Me Y, Longo F M. Prog Brain Res. 2000;128:333–347. doi: 10.1016/S0079-6123(00)28030-8. [DOI] [PubMed] [Google Scholar]
- 9.Massa S M, Xie Y M, Longo F M. J Mol Neurosci. 2003;20:323–326. doi: 10.1385/JMN:20:3:323. [DOI] [PubMed] [Google Scholar]
- 10.Longo F M, Yang T, Knowles J K, Xie Y M, Moore L A, Massa S M. Curr Alzheimer Res. 2007;4:503–506. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 11.Joyner P M, Cichewicz R H. Nat Prod Rep. 2011;28:26–47. doi: 10.1039/c0np00017e. [DOI] [PubMed] [Google Scholar]
- 12.Williams P, Sorribas A, Howes M-J R. Nat Prod Rep. 2011;28:48–77. doi: 10.1039/c0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urabe D, Inoue M. Tetrahedron. 2009;65:6271–6289. doi: 10.1016/j.tet.2009.06.010. [DOI] [Google Scholar]
- 14.Yang C-S, Kouno I, Kawano N, Sato S. Tetrahedron Lett. 1988;29:1165–1168. doi: 10.1016/S0040-4039(00)86678-2. [DOI] [Google Scholar]
- 15.Kouno I, Baba N, Hashimoto M, Kawano N, Takahashi M, Kaneto H, Yang C-S, Sato S. Chem Pharm Bull. 1989;37:2448–2451. doi: 10.1248/cpb.37.2448. [DOI] [PubMed] [Google Scholar]
- 16.Kubo M, Okada C, Huang J-M, Harada K, Hioki H, Fukuyama Y. Org Lett. 2009;11:5190–5193. doi: 10.1021/ol9021029. [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama R, Huang J M, Yang C S, Fukuyama Y. J Nat Prod. 2002;65:527–531. doi: 10.1021/np010571k. [DOI] [PubMed] [Google Scholar]
- 18.Kubo M, Kobayashi K, Huang J-M, Harada K, Fukuyama Y. Tetrahedron Lett. 2012;53:1231–1235. doi: 10.1016/j.tetlet.2011.12.107. [DOI] [Google Scholar]
- 19.Cho Y S, Carcache D A, Tian Y, Li Y M, Danishefsky S J. J Am Chem Soc. 2004;126:14358–14359. doi: 10.1021/ja045939p. [DOI] [PubMed] [Google Scholar]
- 20.Carcache D A, Cho Y S, Hua Z, Tian Y, Li Y-M, Danishefsky S J. J Am Chem Soc. 2006;128:1016–1022. doi: 10.1021/ja056980a. [DOI] [PubMed] [Google Scholar]
- 21.Harada K, Imai A, Uto K, Carter R G, Kubo M, Hioki H, Fukuyama Y. Org Lett. 2011;13:988–991. doi: 10.1021/ol103024z. [DOI] [PubMed] [Google Scholar]
- 22.Mehta G, Shinde H M, Kumaran R S. Tetrahedron Lett. 2012;53:4320–4323. doi: 10.1016/j.tetlet.2012.06.001. [DOI] [Google Scholar]
- 23.Yang Y, Fu X, Chen J, Zhai H. Angew Chem, Int Ed. 2012;51:9825–9828. doi: 10.1002/anie.201203176. [DOI] [PubMed] [Google Scholar]
- 24.Trzoss L, Xu J, Lacoske M H, Mobley W C, Theodorakis E A. Org Lett. 2011;13:4554–4557. doi: 10.1021/ol201742j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J, Trzoss L, Chang W K, Theodorakis E A. Angew Chem, Int Ed. 2011;50:3672–3676. doi: 10.1002/anie.201100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trzoss L, Xu J, Lacoske M H, Mobley W C, Theodorakis E A. Chem–Eur J. 2013;20:6398–6408. doi: 10.1002/chem.201300198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalko P I, Moisan L. Angew Chem, Int Ed. 2004;43:5138–5175. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]
- 28.List B. Acc Chem Res. 2004;37:548–557. doi: 10.1021/ar0300571. [DOI] [PubMed] [Google Scholar]
- 29.Lelais G, MacMillan D W C. Aldrichimica Acta. 2006;39:79–87. [Google Scholar]
- 30.Taylor M S, Jacobsen E N. Angew Chem, Int Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]
- 31.Marion N, Díez-González S, Nolan I P. Angew Chem, Int Ed. 2007;46:2988–3000. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]
- 32.Gaunt M J, Johansson C C C, McNally A, Vo N T. Drug Discovery Today. 2007;12:8–27. doi: 10.1016/j.drudis.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Bertelsen S, Jorgensen K A. Chem Soc Rev. 2009;38:2178–2189. doi: 10.1039/b903816g. [DOI] [PubMed] [Google Scholar]
- 34.List B, Lerner R A, Barbas C F., III J Am Chem Soc. 2000;122:2395–2396. doi: 10.1021/ja994280y. [DOI] [Google Scholar]
- 35.Jen W S, Wiener J J M, MacMillan D W C. J Am Chem Soc. 2000;122:9874–9875. doi: 10.1021/ja005517p. [DOI] [Google Scholar]
- 36.Ahrendt K A, Borths C J, MacMillan D W C. J Am Chem Soc. 2000;122:4243–4244. doi: 10.1021/ja000092s. [DOI] [Google Scholar]
- 37.Beeson T D, Mastracchio A, Hong J B, Ashton K, MacMillan D W C. Science. 2007;316:582–585. [PubMed] [Google Scholar]
- 38.Zhu S L, Wang Y, Ma D W. Adv Synth Catal. 2009;351:2563–2566. doi: 10.1002/adsc.200900449. [DOI] [Google Scholar]
- 39.Marques-Lopez E, Herrera R P, Christmann M. Nat Prod Rep. 2010;27:1138–1167. doi: 10.1039/b924964h. [DOI] [PubMed] [Google Scholar]
- 40.Grondal C, Jeanty M, Enders D. Nat Chem. 2010;2:167–178. doi: 10.1038/nchem.539. [DOI] [PubMed] [Google Scholar]
- 41.Zhu S L, Yu S Y, Wang Y, Ma D W. Angew Chem, Int Ed. 2010;49:4656–4660. doi: 10.1002/anie.201001644. [DOI] [PubMed] [Google Scholar]
- 42.Knowles R R, Carpenter J, Blakey S B, Kayano A, Mangion I K, Sinz C J, MacMillan D W C. Chem Sci. 2011;2:308–311. doi: 10.1039/c0sc00577k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X, Ma D. Chem–Asian J. 2011;6:2157–2164. doi: 10.1002/asia.201100219. [DOI] [PubMed] [Google Scholar]
- 44.Pham P V, Ashton K, MacMillan D W C. Chem Sci. 2011;2:1470–1473. doi: 10.1039/c1sc00176k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhu S, Ma D. Org Lett. 2011;13:1602–1605. doi: 10.1021/ol200004s. [DOI] [PubMed] [Google Scholar]
- 46.Jones S B, Simmons B, Mastracchio A, MacMillan D W C. Nature. 2011;475:183–188. doi: 10.1038/nature10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zi W, Xie W, Ma D. J Am Chem Soc. 2012;134:9126–9129. doi: 10.1021/ja303602f. [DOI] [PubMed] [Google Scholar]
- 48.Huo L, Ma A, Zhang Y, Ma D. Adv Synth Catal. 2012;354:991–994. doi: 10.1002/adsc.201100903. [DOI] [Google Scholar]
- 49.Simonovich S P, Van Humbeck J F, MacMillan D W C. Chem Sci. 2012;3:58–61. doi: 10.1039/c1sc00556a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Xing H, Xie W, Wan X, Lai Y, Ma D. Adv Synth Catal. 2013;355:68–72. doi: 10.1002/adsc.201200782. [DOI] [Google Scholar]
- 51.Ling T, Xiang A X, Theodorakis E A. Angew Chem, Int Ed. 1999;38:3089–3091. doi: 10.1002/(SICI)1521-3773(19991018)38:20<3089::AID-ANIE3089>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 52.Ling T, Poupon E, Rueden E J, Kim S H, Theodorakis E A. J Am Chem Soc. 2002;124:12261–12267. doi: 10.1021/ja027517q. [DOI] [PubMed] [Google Scholar]
- 53.Brady T P, Kim S H, Wen K, Theodorakis E A. Angew Chem, Int Ed. 2004;43:739–742. doi: 10.1002/anie.200352868. [DOI] [PubMed] [Google Scholar]
- 54.Ling T, Poupon E, Rueden E J, Theodorakis E A. Org Lett. 2002;4:819–822. doi: 10.1021/ol025501z. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Rivas F, Fischer D, González M A, Theodorakis E A. Org Lett. 2004;6:941–944. doi: 10.1021/ol036492c. [DOI] [PubMed] [Google Scholar]
- 56.Brady T P, Kim S H, Wen K, Kim C, Theodorakis E A. Chem–Eur J. 2005;11:7175–7190. doi: 10.1002/chem.200500513. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen T X, Dakanali M, Trzoss L, Theodorakis E A. Org Lett. 2011;13:3308–3311. doi: 10.1021/ol200966z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng F, Dai M-J, Angeles A R, Danishefsky S. J Chem Sci. 2012;3:3076–3080. doi: 10.1039/c2sc20868g. See for a recent update of Robinson annulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wieland P, Miescher K. Helv Chim Acta. 1950;33:2215–2228. doi: 10.1002/hlca.19500330730. [DOI] [Google Scholar]
- 60.Eder U, Sauer G, Weichert R. Angew Chem, Int Ed Engl. 1971;10:496–497. doi: 10.1002/anie.197104961. [DOI] [Google Scholar]
- 61.Hajos Z G, Parrish D R. J Org Chem. 1973;38:3239–3243. doi: 10.1021/jo00959a002. [DOI] [PubMed] [Google Scholar]
- 62.Hajos Z G, Parrish D R. J Org Chem. 1974;39:1615–1621. doi: 10.1021/jo00925a003. [DOI] [Google Scholar]
- 63.Bradshaw B, Bonjoch J. Synlett. 2012:337–356. doi: 10.1055/s-0031-1290107. [DOI] [Google Scholar]
- 64.Zhou P, Zhang L, Luo S, Cheng J-P. J Org Chem. 2012;77:2526–2530. doi: 10.1021/jo202433v. [DOI] [PubMed] [Google Scholar]
- 65.Winterfeldt E. Angew Chem, Int Ed. 2013;52:4723. doi: 10.1002/anie.201301415. [DOI] [Google Scholar]
- 66.Tsuji J, Takahashi H, Morikawa M. Tetrahedron Lett. 1965;6:4387–4388. doi: 10.1016/S0040-4039(00)71674-1. [DOI] [Google Scholar]
- 67.Trost B M, Fullerto T. J Am Chem Soc. 1973;95:292–294. doi: 10.1021/ja00782a080. [DOI] [Google Scholar]
- 68.Trost B M, Van Vranken D L. Chem Rev. 1996;96:395–422. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]
- 69.Ruprah P K, Cros J-P, Pease J E, Whittingham W G, Williams J M J. Eur J Org Chem. 2002:3145–3152. doi: 10.1002/1099-0690(200209)2002:18<3145::AID-EJOC3145>3.0.CO;2-3. [DOI] [Google Scholar]
- 70.Lacoste E, Vaique E, Berlande M, Pianet I, Vincent J-M, Landais Y. Eur J Org Chem. 2007:167–177. doi: 10.1002/ejoc.200600664. [DOI] [Google Scholar]
- 71.Zhang X-M, Wang M, Tu Y-Q, Fan C-A, Jiang Y-J, Zhang S-Y, Zhang F-M. Synlett. 2008:2831–2835. doi: 10.1055/s-0028-1083542. [DOI] [Google Scholar]
- 72.Coates R M, Shaw J E. Chem Commun. 1968:515–516. doi: 10.1039/c19680000515. [DOI] [Google Scholar]
- 73.Williams J R, Sarkisia G M. Synthesis. 1974:32–33. doi: 10.1055/s-1974-23227. [DOI] [Google Scholar]
- 74.Bosch M P, Camps F, Coll J, Guerrero A, Tatsuoka T, Meinwald J. J Org Chem. 1986;51:773–784. doi: 10.1021/jo00356a002. [DOI] [Google Scholar]
- 75.Pu X, Ma D. Angew Chem, Int Ed. 2004;43:4222–4225. doi: 10.1002/anie.200460128. [DOI] [PubMed] [Google Scholar]
- 76.Paquette L A, Wang T-Z, Philippo C M G, Wang S. J Am Chem Soc. 1994;116:3367–3374. doi: 10.1021/ja00087a023. [DOI] [Google Scholar]
- 77.CCDC 931875 contains the supplementary crystallographic data for compound 16. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/products/csd/request/.
- 78.Angeles A R, Dorn D C, Kou C A, Moore M A S, Danishefsky S J. Angew Chem, Int Ed. 2007;46:1451–1454. doi: 10.1002/anie.200604308. [DOI] [PubMed] [Google Scholar]
- 79.Finkbeiner H L, Stiles M. J Am Chem Soc. 1963;85:616–622. doi: 10.1021/ja00888a031. [DOI] [Google Scholar]
- 80.Micheli R A, Hajos Z G, Cohen N, Parrish D R, Portland L A, Sciamanna W, Scott M A, Wehrli P A. J Org Chem. 1975;40:675–681. doi: 10.1021/jo00894a003. [DOI] [PubMed] [Google Scholar]
- 81.Frie J L, Jeffrey C S, Sorensen E J. Org Lett. 2009;11:5394–5397. doi: 10.1021/ol902168g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H M, Nieto-Oberhuber C, Shair M D. J Am Chem Soc. 2008;130:16864–16865. doi: 10.1021/ja8071918. [DOI] [PubMed] [Google Scholar]
- 83.Mcmurry J E, Scott W J. Tetrahedron Lett. 1983;24:979–982. doi: 10.1016/S0040-4039(00)81581-6. [DOI] [Google Scholar]
- 84.Scott W J, Mcmurry J E. Acc Chem Res. 1988;21:47–54. doi: 10.1021/ar00146a001. [DOI] [Google Scholar]
- 85.Nicolaou K C, Peng X-S, Sun Y-P, Polet D, Zou B, Lim C S, Chen D Y-K. J Am Chem Soc. 2009;131:10587–10597. doi: 10.1021/ja902939t. [DOI] [PubMed] [Google Scholar]
- 86.Ding H, Chen D Y K. Angew Chem, Int Ed. 2011;50:676–679. doi: 10.1002/anie.201006367. [DOI] [PubMed] [Google Scholar]
- 87.Cowell A, Stille J K. J Am Chem Soc. 1980;102:4193–4198. doi: 10.1021/ja00532a034. [DOI] [Google Scholar]
- 88.Cacchi S, Morera E, Ortar G. Tetrahedron Lett. 1985;26:1109–1112. doi: 10.1016/S0040-4039(00)98525-3. [DOI] [Google Scholar]
- 89.Magro A A N, Robb L M, Pogorzelec P J, Slawin A M Z, Eastham G R, Cole-Hamilton D. J Chem Sci. 2010;1:723–730. doi: 10.1039/c0sc00276c. [DOI] [Google Scholar]
- 90.Nicolaou K C, Ding H, Richard J-A, Chen D Y-K. J Am Chem Soc. 2010;132:3815–3818. doi: 10.1021/ja9093988. [DOI] [PubMed] [Google Scholar]
- 91.Zuo Z, Xie W, Ma D. J Am Chem Soc. 2010;132:13226–13228. doi: 10.1021/ja106739g. [DOI] [PubMed] [Google Scholar]
- 92.Richard J-A, Chen D Y-K. Eur J Org Chem. 2012:484–487. doi: 10.1002/ejoc.201101629. [DOI] [Google Scholar]
- 93.Nicolaou K C, Majumder U, Roche S P, Chen D Y-K. Angew Chem, Int Ed. 2007;46:4715–4718. doi: 10.1002/anie.200701947. [DOI] [PubMed] [Google Scholar]
- 94.Nicolaou K C, Dalby S M, Li S, Suzuki T, Chen D Y-K. Angew Chem, Int Ed. 2009;48:7616–7620. doi: 10.1002/anie.200904588. [DOI] [PubMed] [Google Scholar]
- 95.Nicolaou K C, Wu T R, Kang Q, Chen D Y-K. Angew Chem, Int Ed. 2009;48:3440–3443. doi: 10.1002/anie.200900438. [DOI] [PubMed] [Google Scholar]
- 96.Nicolaou K C, Kang Q, Wu T R, Lim C S, Chen D Y-K. J Am Chem Soc. 2010;132:7540–7548. doi: 10.1021/ja102623j. [DOI] [PubMed] [Google Scholar]
- 97.Peixoto P A, Richard J-A, Severin R, Chen D Y-K. Org Lett. 2011;13:5724–5727. doi: 10.1021/ol202053m. [DOI] [PubMed] [Google Scholar]
- 98.Peixoto P A, Severin R, Tseng C-C, Chen D Y-K. Angew Chem, Int Ed. 2011;50:3013–3016. doi: 10.1002/anie.201008000. [DOI] [PubMed] [Google Scholar]
- 99.Nicolaou K C, Edmonds D J, Bulger P G. Angew Chem, Int Ed. 2006;45:7134–7186. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 100.Wasilke J-C, Obrey S J, Baker R-T, Bazan G C. Chem Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]
- 101.Nicolaou K C, Toh Q-Y, Chen D Y-K. J Am Chem Soc. 2008;130:11292–11293. doi: 10.1021/ja804588r. [DOI] [PubMed] [Google Scholar]
- 102.Leung G Y C, Li H, Toh Q-Y, Ng A M-Y, Sum R J, Bandow J E, Chen D Y-K. Eur J Org Chem. 2011:183–196. doi: 10.1002/ejoc.201001281. [DOI] [Google Scholar]
- 103.Yuan C, Chang C-T, Axelrod A, Siegel D. J Am Chem Soc. 2010;132:5924–5925. doi: 10.1021/ja101956x. [DOI] [PubMed] [Google Scholar]
- 104.Fischer D F, Sarpong R. J Am Chem Soc. 2010;132:5926–5927. doi: 10.1021/ja101893b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jana C K, Hoecker J, Woods T M, Jessen H J, Neuburger M, Gademann K. Angew Chem, Int Ed. 2011;50:8407–8411. doi: 10.1002/anie.201101869. [DOI] [PubMed] [Google Scholar]
- 106.Scott L E, Telpoukhovskaia M, Rodriguez-Rodriguez C, Merkel M, Bowen M L, Page B D G, Green D E, Storr T, Thomas F, Allen D D, et al. Chem Sci. 2011;2:642–648. doi: 10.1039/c0sc00544d. [DOI] [Google Scholar]
- 107.Tun M K M, Wüstmann D-J, Herzon S B. Chem Sci. 2011;2:2251–2253. doi: 10.1039/c1sc00455g. [DOI] [Google Scholar]
- 108.Cheng X, Harzdorf N, Khaing Z, Kang D, Camelio A M, Shaw T, Schmidt C E, Siegel D. Org Biomol Chem. 2012;10:383–393. doi: 10.1039/c1ob06363d. [DOI] [PubMed] [Google Scholar]
- 109.Elamparuthi E, Fellay C, Neuburger M, Gademann K. Angew Chem, Int Ed. 2012;51:4071–4073. doi: 10.1002/anie.201200515. [DOI] [PubMed] [Google Scholar]
- 110.Newton J N, Fischer D F, Sarpong R. Angew Chem, Int Ed. 2013;52:1726–1730. doi: 10.1002/anie.201208571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Drouet K E, Theodorakis E A. J Am Chem Soc. 1999;121:456–457. doi: 10.1021/ja983429n. [DOI] [Google Scholar]
- 112.Tisdale E J, Slobodov I, Theodorakis E A. Proc Natl Acad Sci U S A. 2004;101:12030–12035. doi: 10.1073/pnas.0401932101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vong B G, Kim S H, Abraham S, Theodorakis E A. Angew Chem, Int Ed. 2004;43:3947–3951. doi: 10.1002/anie.200460203. [DOI] [PubMed] [Google Scholar]
- 114.Guizzunti G, Brady T P, Malhotra V, Theodorakis E A. J Am Chem Soc. 2006;128:4190–4191. doi: 10.1021/ja058259a. [DOI] [PubMed] [Google Scholar]
- 115.Xu J, Caro-Diaz E J E, Trzoss L, Theodorakis E A. J Am Chem Soc. 2012;134:5072–5075. doi: 10.1021/ja300807e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu J, Caro-Diaz E J E, Lacoske M H, Hung C-I, Jamora C, Theodorakis E A. Chem Sci. 2012;3:3378–3386. doi: 10.1039/c2sc21308g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures for the syntheses of all new compounds.