Abstract
Background
Intrauterine exposure to alcohol may affect cardiovascular development, increasing risk of cardiovascular malformations. Intrauterine exposure to light maternal alcohol intake has been reported to affect human umbilical arterial contractility, and adult sheep exposed in utero have had altered cerebrovascular reactivity. In human adults, alcohol intake affects arterial stiffness.
Objectives
We investigated whether intrauterine exposure to alcohol was associated with childhood pulse wave velocity (PWV), a measure of arterial stiffness.
Methods
On postnatal day 4, mothers of 147 twin pairs born in Tasmania from 1991 to 1993 reported alcohol intake during each trimester of pregnancy. At 9 years, child PWV was assessed over carotid-femoral and femoral-dorsalis pedis arterial segments by applanation tonometry.
Results
Carotid-femoral PWV was 0.2 m/s (95% CI 0.06, 0.4) higher (indicating stiffer vessels) in children whose mothers drank alcohol in the 2nd trimester rather than abstained, after adjusting for potential confounding factors. A similar effect was not seen for femoral-dorsalis pedis PWV. Findings were independent of child blood pressure which correlated strongly with PWV. Alcohol intake varied little between trimesters, so it was not possible to assess the effect of timing of exposure.
Conclusions
Carotid-femoral PWV in adults is predictive of cardiovascular morbidity and mortality. The degree of continuity between childhood and adulthood PWV is unknown, but as we found an association between prenatal alcohol exposure and carotid-femoral PWV at 9 years, a permanent change in vessel wall structure or function is possible. These findings need to be confirmed in other and larger cohorts, and mechanistic animal studies are needed.
Key Words: Fetus, Ethanol, Pulse wave velocity, Blood pressure
Introduction
High levels of alcohol exposure during gestation can cause fetal abnormalities or disorders described as fetal alcohol syndrome (with some affected children having cardiac malformations), fetal alcohol spectrum disorders, fetal alcohol effects, alcohol-related neurodevelopmental disorder or alcohol-related birth defects [1]. There is increasing concern that exposure to even low levels of alcohol may harm the developing fetus [2,3].
There is limited evidence that fetal exposure to alcohol can affect cardiovascular development. In a Finnish study, maternal alcohol consumption during the 1st trimester of pregnancy was more common among the mothers of 50 infants with an atrial septal defect (the commonest cardiac malformation) than among randomly selected birth cohort members [adjusted OR 1.9 (95% CI 1.1, 3.4)] [4].
However, no published human studies have examined the relationship between maternal alcohol consumption and cardiovascular function in offspring without malformations. A recent study found that umbilical arteries from babies exposed to low doses of alcohol in utero had reduced contractile responses compared with control arteries, independently of endothelial release of the vasodilators nitric oxide or prostacyclin [5].
In adults, alcohol intake has been shown to affect pulse wave velocity (PWV), though effects have been variable [6,7,8,9,10]. Carotid-femoral PWV is a non-invasive measure and is the ‘gold standard’ for arterial stiffness [11], with higher values representing greater vessel wall stiffness. Arterial distensibility is an important mechanical property of the arterial tree and of particular relevance for cardiac perfusion. Increased PWV is a predictor of cardiovascular morbidity and mortality in adult populations [11,12,13]. Schack-Nielsen et al. [14] found that PWV over the aortic-femoral segment in 10-year-old children was positively associated with duration of breast-feeding (faster with longer duration), suggesting that exposures during early development could have a long-term influence on PWV.
We measured blood pressure and PWV in 294 children from 147 twin pairs in Tasmania, Australia, at a mean age of 9 years. Investigation of the relationship between maternal alcohol intake in pregnancy and these measures was not an a priori hypothesis, but in light of the evidence above we decided to use our data to examine this relationship. If aortic stiffness at this age were found to relate to an exposure in utero, this would suggest a persisting influence that may affect the individual across their life span. PWV relates to blood pressure [11], so we also investigated the possibility that any association between maternal alcohol intake and PWV may be mediated via changes in blood pressure.
Methods
Subjects
Children were recruited into the Tasmanian Infant Health Study (TIHS) soon after birth, originally to investigate sudden infant death syndrome. [15] Both TIHS and this current study were approved by the University of Tasmania Human Research Ethics Committee.
Mothers of all live-born children from multiple pregnancies in Tasmania during the years 1988–1995 were approached for participation in TIHS. At an in-hospital interview soon after birth, information about obstetric history, socio-demographic factors and the twin pregnancy were collected from mothers who agreed to join the study. Maternal height, weight at the last measurement before delivery and obstetric data, including gestation length and twin infants’ birth weights, were copied from hospital records. Birth weight standard deviation scores (SDS, for gestation and sex) were calculated using British normative data [16]. Women were asked to report their average daily alcohol intake separately for each trimester.
Twin pairs recruited into TIHS during 1991, 1992 or 1993 were eligible for this study if they were still living in Tasmania and both agreed to participate.
Measurements at Follow-Up
Children whose parents had provided written informed consent and who had verbally agreed to the study were asked to fast overnight and then underwent measurements of height and weight, blood pressure and PWV in 2000, 2001 or 2002. Measurements were made at a comfortable indoor temperature by research assistants who had no access to the data which was collected soon after birth.
Blood pressure was measured 3 times using a Critikon Dinamap Pro 100 Monitor (Critikon, Tampa, Fla., USA). Cuff size was selected in accordance with the mid-upper arm girth measurement, and the child was seated with the left arm resting comfortably on a pillow and the elbow approximately level with the heart. The average of 3 blood pressure measurements was used in analyses of systolic and diastolic blood pressures.
PWV was measured using the SphygmoCor Pulse Wave Velocity (Vx) system (AtCor Medical Pty. Ltd., West Ryde, NSW, Australia) [11], with children lying supine. ECG leads were applied and then pulse pressure waves were detected using applanation tonometers held over 2 superficial artery sites. Integral software measured the time between the tip of the ECG R wave and a specified point on the pulse wave, averaged for all acceptable waveforms over a period of 10 s for each site. The default algorithm measured the time between the tip of the R wave and 10% up from the foot of the flow wave. The difference between the times taken for the pulse wave to reach each of the 2 sites represented the time the pulse wave took to travel between the 2 sites. Thus, if T1 was the time between the tip of the R wave and pulse wave arrival at the proximal site, and T2 the time between the tip of the R wave and pulse wave arrival at the distal site, then T2 – T1 was the time taken by the pulse wave to travel between the proximal and distal sites. The distance between the 2 sites was measured with a non-stretchable tape measure in a straight line, permitting calculation of PWV, the speed at which the pulse wave travelled between the 2 sites.
We measured PWV over 2 arterial segments, between the carotid artery in the neck and the femoral artery in the groin (a measure of aortic stiffness), and over the peripheral arterial segment between the femoral and dorsalis pedis arteries. We planned to obtain each PWV measure 3 times.
Height (with bare feet) was measured using a Leicester Height Measure. Weight in light clothing was measured using Heine Portable Professional Adult Scales that were calibrated daily using known weights. Child BMI was calculated as weight in kg divided by height in metres [2].
At the time of measurement, we asked mothers to tell us how old their twins were when they stopped breast-feeding.
Statistical Analyses
Characteristics of mothers who drank alcohol in pregnancy versus those who did not were summarised.
We used a mixed-effects regression approach (with random intercept for each twin pair) to estimate the effect of maternal alcohol consumption on child outcomes of interest, allowing for the lack of independence within twin pairs and adjusting for potentially confounding factors [17]. Regression analyses were performed using Stata Software Release 9 (2003; StataCorp, College Station, Tex., USA).
Variables were included in final regression models on the basis of direct estimation of the degree of confounding produced by each variable considered, assessed in terms of the effect estimate rather than on the basis of significance-testing algorithms [18].
Results
Altogether, 463 children from twin pregnancies were recruited soon after birth into TIHS in 1991, 1992 and 1993. Eleven of these had co-twins who did not survive or were too sick for recruitment as neonates. Of the 226 recruited pairs, 23 had left Tasmania, resulting in 203 eligible twin pairs.
Altogether, 14 pairs could not be traced and 42 pairs did not wish to participate, so 147 of 203 pairs (72%) agreed to participate in this study, comprising 149 boys and 145 girls. Mean age at the time of measurement was 9.0 years.
Mothers of non-participants were younger than participants [mean 28.3 years (SD 5.4) vs. 30.4 (4.6), respectively] and were less likely to have had tertiary education (7 vs. 16%).
Maternal Alcohol Intake
The number of women drinking alcohol during each trimester is shown in figure 1. There was a high degree of correlation between alcohol consumption in the 3 trimesters (r = 0.87, p < 0.0001 for the 1st and 2nd trimesters and r = 0.95, p < 0.0001 for the 2nd and 3rd trimesters). Two women reported drinking an average of 2–3 drinks per day in the 1st trimester, but all other women drank up to 1 drink per day (1–7 drinks per week). No woman reported drinking more than 1–7 drinks per week in the 2nd or 3rd trimesters. Therefore, there was very little scope to examine whether timing of alcohol exposure was important or to examine the possibility of a dose response.
Table 1.
Maternal characteristics according to alcohol consumption in the 2nd trimester of pregnancy
| No | Drank | |
|---|---|---|
| alcohol | alcohol | |
| Mothers, n | 102 | 45 |
| Mean (SD) maternal age, years | 30 (5) | 31 (4) |
| Mean (SD) maternal height, cm | 163 (7) | 162 (6) |
| Mean (SD) last maternal weight in pregnancy, kg | 80 (18) | 72 (26) |
| Mother's first children, % | 38 | 27 |
| Did not smoke during pregnancy, % | 78 | 76 |
| Mothers with tertiary education, % | 21 | 24 |
| Fathers with tertiary education, % | 13 | 14 |
Four women delivered at 28 weeks, so fewer pregnancies are included in the 3rd trimester data, and most delivered before term. Recall of alcohol intake in the immediate postnatal period is possibly more reliable for mid-to-late gestation than for early gestation. We therefore present data relating to alcohol consumption in the 2nd trimester; the findings for the other trimesters are summarised in the text.
Characteristics of women who drank alcohol during the 2nd trimester versus those of women who abstained are shown in table 1. Mothers in the alcohol group were lighter than those in the no alcohol group when last weighed in pregnancy, and there was weak evidence that they had a longer gestation (table 2, p = 0.06).
Table 2.
The relationship between maternal alcohol consumption in the 2nd trimester of pregnancy and child anthropometric measurements at birth and at 9 years. Differences, 95% CI and p values are derived from mixed-effects regression models(n = 294)
| Mother abstained | Mother drank alcohol | Unadjusted difference | 95% CI | p | Adjusted difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|
| Measures at birth | ||||||||
| Mean (SD) gestation, weeks | 36.0 (3.0) | 36.9 (2.2) | 0.9 | −0.04, 1.9 | 0.06 | 1.0a | 0.02, 2.0 | 0.06 |
| Mean (SD) birth weight, kg | 2.43 (0.6) | 2.55 (0.5) | 0.12 | −0.08, 0.31 | 0.2 | −0.05b | −0.1, 0.05 | 0.3 |
| Mean (SD) birth length, cm | 45.7 (3.7) | 46.5 (3.0) | 0.8 | −0.4, 1.9 | 0.2 | −0.1b | −0.8, 0.6 | 0.7 |
| Mean (SD) birth weight SDS | −0.5 (0.8) | −0.6 (0.8) | 0.1 | −0.4, 0.1 | 0.2 | 0.1a | −0.4, 0.1 | 0.2 |
| Measures at 9 years of age | ||||||||
| Mean (SD) weight, kg | 31.2 (0.6) | 32.3 (0.7) | 1.07 | −1.4, 3.6 | 0.4 | −0.12c | −2.3, 2.1 | 0.9 |
| Mean (SD) height, cm | 133.4 (7.9) | 136.2 (7.7) | 2.7 | 0.2, 5.3 | 0.04 | 1.0d | −0.9, 2.9 | 0.3 |
| Mean (SD) BMI | 17.3 (2.9) | 17.3 (2.5) | −0.04 | −0.9, 0.8 | 0.9 | −0.2c | −1.1, 0.6 | 0.6 |
SDS = Birth weight standard deviation score, for gestation and sex.
Adjusted for maternal smoking.
Adjusted for gestational length in weeks.
Adjusted for age and sex of the child.
Adjusted for child age and maternal weight close to delivery.
Age at follow-up was 9.2 years for children whose mothers drank alcohol versus 8.9 years of age for the others. Altogether, 49% of abstainers’ children were boys versus 56% from mothers who drank alcohol.
Child PWV and Blood Pressure
Blood pressure was measured in all of the children, but PWV data were missing for 5 of 294; in one case the ECG leads were incorrectly attached and in another case there was a software problem. The remaining 3 children declined PWV measurement.
Mean (SD) systolic and diastolic blood pressures were 97 mm Hg (12) and 53 mm Hg (7), respectively. Apart from 12 children (5 in the control and 7 in the exposed group), all children had at least 2 PWV measurements. For each child, we calculated the mean of the first 2 measurements, unless there was only one, in which case that value was used in analyses. Mean (SD) PWV values were 6.0 (0.7) m/s for the carotid-femoral segment and 7.4 (1.2) m/s for the femoral-dorsalis pedis segment.
We also calculated mean of all available PWV data for each child. Mean (SD) values were identical to those above.
Potentially Confounding Variables
We examined a number of variables for potential confounding effects on the association between maternal alcohol intake and child PWV. These included maternal weight, whether the mother and/or father had a tertiary education, whether the family owned the house in which they lived, whether these were the mother's first children, how much the mother smoked in pregnancy, gestation length in weeks, sex of the child, whether 1st or 2nd born twin, and age at the time of these measurements.
In mixed-effects regression models, PWV over the carotid-femoral segment was strongly positively related to systolic blood pressure (0.01 m/s per mm Hg, 95% CI 0.007, 0.021), diastolic blood pressure (0.03 m/s per mm Hg, 95% CI 0.02, 0.04) and child age (0.3 m/s per year, 95% CI 0.2, 0.4), but there was little evidence of association with any other potentially confounding variable listed above or with child height, weight or BMI.
For PWV over the femoral-dorsalis pedis segment, there were also strong positive associations with both systolic and diastolic blood pressure (0.02 m/s per mm Hg, 95% CI 0.004, 0.030, and 0.05 m/s per mm Hg, 0.03, 0.07, respectively), but with no other variable.
Systolic blood pressure was negatively related to gestation length (by −0.7 mm Hg per week; 95% CI −1.2, −0.1), but not diastolic. Both systolic and diastolic blood pressure increased with age, by 2 mm Hg per year (95% CI 0.5, 4.3) and 1 mm Hg per year (95% CI −0.08, 2.2), respectively. There was little evidence that blood pressure was related to any other variable listed above, but as expected, both systolic pressure and diastolic pressure were positively related to height, weight and BMI (data not shown) [19].
There has been interest in the relationship between birth weight, diastolic and systolic blood pressure, and PWV [20]. We found a weak negative association between birth weight and systolic pressure (−1.5 mm Hg per kg, 95% CI −3.9, 1.0) but little evidence for diastolic pressure or for PWV over either the carotid-femoral or femoral-dorsalis pedis segments [0.05 m/s per kg (−0.1, 0.2) and −0.03 m/s per kg (−0.3, 0.2), respectively]. Likewise, there was little evidence of association between these outcomes and other measures of birth size (birth length or birth weight SDS).
Maternal Alcohol Intake and Child Anthropometry
There was no evidence of association between maternal alcohol consumption and child weight, or BMI (table 2). Children from the group exposed in utero to alcohol were 2.7 cm taller (p = 0.04) than those from the no alcohol group. However, at the time of measurement, children from the exposed group were older than the unexposed group, and this difference in height was attenuated to 1 cm (p = 0.3) after adjustment for child age and maternal weight close to delivery.
Maternal Alcohol Intake and Child Blood Pressure
Using unadjusted mixed-effects regression models, there was little evidence that either systolic or diastolic blood pressure was related to maternal alcohol intake (table 3). After adjustment for potentially confounding factors (as detailed in table 3, or after further adjustment for birth weight, gestation length, birth size, child height, weight or BMI, twin number or any of the potentially confounding variables), our findings were not materially changed.
Table 3.
The relationship between maternal alcohol consumption in the 2nd trimester of pregnancy and child blood pressure and PWV at 9 years. Regression coefficients (CI) are from mixed-effects regression models and represent the ‘effect’ of a mother drinking alcohol vs. abstaining (n = 294 for blood pressure and n = 289 for PWV)
| Mother abstained | Mother drank alcohol | Unadjusted difference | 95% CI | p | Adjusted difference | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) systolic blood pressure, mm Hg | 97.3 (11.9) | 97.8 (9.6) | 0.8 | −2.6, 4.3 | 0.6 | −0.3a | −3.7, 3.2 | 0.9 |
| Mean (SD) diastolic blood pressure, mm Hg | 53.3 (6.7) | 53.2 (6.2) | 0.1 | −1.9, 2.2 | 0.9 | −0.4b | −2.5, 1.7 | 0.7 |
| Mean (SD) carotid-femoral PWV, m/s | 5.8 (0.7) | 6.1 (0.7) | 0.3 | 0.1, 0.5 | 0.001 | 0.2c | 0.03, 0.4 | 0.02 |
| Mean (SD) femoral-dorsalis pedis PWV, m/s | 7.5 (1.3) | 7.3 (1.1) | −0.2 | −0.5, 0.1 | 0.3 | −0.2c | −0.6, 0.1 | 0.2 |
Adjusted for maternal smoking in the second trimester and home ownership.
Adjusted for gestational length in weeks.
Adjusted for child age, maternal weight at the end of pregnancy and whether mother or father had tertiary education.
Maternal Alcohol Intake and Child PWV
In table 3, we show that exposure to alcohol in the 2nd trimester of pregnancy was strongly positively related to PWV over the carotid-femoral segment: it was 0.3 m/s greater in exposed than in non-exposed children. The effect size was slightly attenuated after adjustment for child age, maternal weight and whether each parent had tertiary education (table 3), but was not further altered by adjustment for gestation length, any measure of size at birth, growth or current body size or BMI, sex of the child, or for any other of the potentially confounding factors listed above or in table 1. After adjustment, the mean difference in carotid-femoral PWV between children exposed to alcohol and non-exposed children was approximately one third of a standard deviation.
Distribution of PWV values was close to normal, with the exception of one high outlier in the no alcohol group, identified using residual plots. Exclusion of this case strengthened the association between maternal alcohol intake and carotid-femoral PWV, and did not materially influence other associations.
The associations between maternal alcohol intake and PWV shown in table 3 were not attenuated by further adjustment for systolic blood pressure: the carotid-femoral PWV difference was 0.2 m/s (95% CI 0.04, 0.41) and femoral dorsalis pedis PWV was −0.2 m/s (−0.6, 0.1). Likewise, there was little difference in our findings after adjustment for diastolic pressure [0.2 m/s (0.08, 0.4) for carotid-femoral PWV and −0.3 m/s (−0.6, 0.04) for femoral-dorsalis pedis PWV].
Furthermore, the findings described above were unchanged if we used the mean of all available PWV data in the models, rather than the mean of the first 2 measures, or if we adjusted for which of the 2 research assistants (who worked together) actually made the measurement.
We also analysed our data using recorded maternal alcohol intake in the 1st and 3rd trimesters. For 3rd trimester drinking, our findings were not materially changed from those shown in table 3. For alcohol intake in the 1st trimester, the relationship with PWV over the carotid-femoral segment was weaker. The unadjusted difference was 0.3 m/s (95% CI 0.08, 0.48; p = 0.007) and the adjusted difference was 0.2 m/s (95% CI −0.02, 0.37; p = 0.08).
Duration of Breast-Feeding and Child PWV
Breast-feeding duration values were skewed and values ranged from zero (no breast-feeding) to 42 weeks. We found no evidence that breast-feeding duration differed between women who drank alcohol versus those who abstained [median (IQR) 4 months (0, 10) and 3 months (0, 9), respectively, p for difference 0.8, by rank-sum test].
Children were categorised into 4 approximately equal groups: not breast-fed (group 1, n = 82), breast-fed for 1–3 weeks (group 2, n = 74), breast-fed for 4–10 weeks (group 3, n = 72) or breast-fed for 11+ weeks (group 4, n = 66). There was little evidence that the duration of breast-feeding was related to carotid-femoral PWV (p = 0.2 by ANOVA). However, femoral-dorsalis pedis PWV was positively related to breast-feeding duration (p = 0.02 by ANOVA). Mean values were 7.1 m/s (SD 1.2) in group 1 children, 7.4 m/s (1.3) in group 2, 7.5 m/s (1.2) in group 3 and 7.7 m/s (1.1) in group 4.
When we additionally included duration of breast-feeding in the regression models shown in table 3, as either a continuous variable or categorised as above, the association between maternal alcohol consumption and both measures of PWV was unchanged.
Discussion
We found that maternal alcohol consumption during pregnancy was associated with faster transmission of the pulse pressure wave between the carotid and femoral arteries at 9 years of age, indicating increased stiffness of the aorta. This association was independent of child systolic and diastolic blood pressure, neither of which was influenced by maternal alcohol intake; therefore, not mediated via any influence on blood pressure. We also found very weak evidence of decreasing stiffness in the group exposed to alcohol over the peripheral arterial segment (between the femoral artery in the groin and the dorsalis pedis artery).
We do not know why there might be an association between maternal alcohol intake and child PWV just over the aortic segment. However, the unit of compliance comprises a contribution from tissue matrix (e.g. collagen, elastin, proteoglycans) and tissue elements (vascular smooth muscle), and the contribution of smooth muscle versus matrix to vessel compliance differs considerably between conduit vessels such as the aorta versus muscular vessels like those in the femoral-dorsalis pedis segment. The thoracic aortic wall contains around 40% elastin, which is also a major component of the abdominal aorta and carotid and coronary arteries, increasing the elastic properties of these vessels [21]. Elastin is laid down mainly during development, which may make it vulnerable to intrauterine insult, and its longevity means that perturbations in pregnancy would likely persist throughout life [22]. Elastin content is roughly proportional to vessel radius [23], so there is less in the walls of peripheral vessels. Conversely, peripheral arteries can have a very thick layer of smooth muscle. Thus, the contractile state of smooth muscle (of which the vascular endothelium is a prominent regulator) has a relatively greater role in vessel biomechanics in these vessels than in conduit vessels. It is possible that alcohol exposure in utero affects vascular function via more than one mechanism: one adversely affecting development of the tissue matrix and resulting in a stiffer aortic segment, and another that acts via a mechanism involving vascular endothelium and/or smooth muscle to increase compliance in smaller vessels. If this were the case, it could explain the different findings for the aortic versus peripheral segments.
In studies of adults, the direction of the effect of alcohol on PWV is somewhat inconsistent. Excessive alcohol consumption was associated with increased arterial stiffness in people with normal blood pressure [9], though this study is difficult to interpret because compliance was measured between the brachial artery and ankle, a combination of central and peripheral vessels. Conversely, a study of young men and women showed that moderate alcohol intake was associated with lower PWV over the carotid-femoral segment [6]. Effects of acute and chronic alcohol intake have also been shown to differ, with the former reducing and the latter increasing vessel stiffness [24]. Alcohol acutely stimulates production of the vasodilator nitric oxide production [25,26], which would likely reduce stiffness. On the other hand, alcohol may enhance production of endothelin [27], which potently induces vasoconstriction, atherogenesis and inflammation, and alcohol-treated rats have an enhanced pressor response to endothelin-1 [27].
There is evidence from rat studies that exposures during gestation can affect aortic wall development. A brief period of growth inhibition about two thirds of the way through rat pregnancy, a period of fetal life when cellular growth in the developing aortic wall is rapid, led to a persistent reduction in the total content of elastin [28] and an increase in the collagen-to-elastin ratio [29]. Recent studies have shown that aortic wall thickness and elastin content were reduced in the offspring of rat dams fed a low-protein diet [30] or given extremely high doses of vitamin D [31]. Furthermore, a study of sheep demonstrated that intrauterine exposure to alcohol affected cerebral arteriolar dilator responses to vasoactive intestinal peptide in adulthood, indicating that fetal alcohol exposure can lead to persistent changes in vascular function [32]. However, to our knowledge, there are no published data on alcohol exposure in gestation and aortic development, apart from a study of zebrafish [33].
We, like Iveli et al. [5], found no evidence that low maternal intake of alcohol was associated with offspring birth weight. Further, we found little evidence of association between birth weight and blood pressure, or either measure of PWV.
There was a high correlation between reported alcohol intake in each of the 3 trimesters, and given that data were collected in the immediate postnatal period, we cannot be certain that women were able to recollect accurately their intake for the 3 trimesters separately. However, recall in the immediate postnatal period with respect to mid-to-late gestation is likely to be reasonably reliable and perturbations during this period have been shown to affect elastin formation in fetal rats and sheep.
Our data suggest that a low level of prenatal alcohol exposure may have a long-term (and possibly permanent) effect on vascular function. We found an approximately one third SD difference in PWV over the aortic segment between exposed and non-exposed children. If this were to persist into adulthood, it could be of clinical significance. In a Danish study of 40- to 70-year-olds [13], each 1 SD increment in aortic PWV increased the risk of a cardiovascular event (cardiovascular mortality, and fatal or nonfatal coronary heart disease) by 16%.
These findings should be regarded as preliminary and not definitive. Given the limited variation in maternal alcohol intake, we were unable to investigate whether there may be a threshold or dose-response relationship. Our findings require confirmation in other cohorts with detailed measures of factors like current exercise, dietary intake and alcohol intake, preferably with a greater degree of variation in maternal intake than we observed. Measures of heart rate and vessel diameter at the time of measurement, both of which affect PWV, would also be important.
Fig. 1.
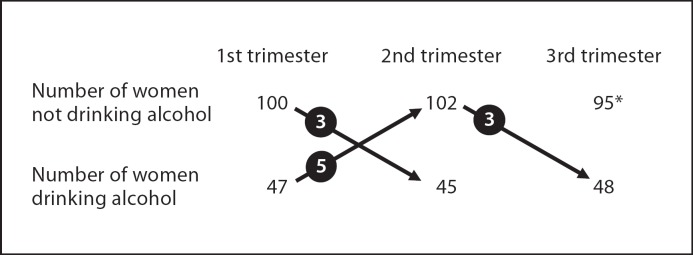
Number of women drinking alcohol in each trimester. Arrows denote women changing from one group to another and numbers in circles denote the number of women changing in that direction. * Four women delivered at 28 weeks of gestation.
Acknowledgements
This study was funded by the National Heart Foundation of Australia, and Ruth Morley is supported by a Senior Research Fellowship (350250) from the National Health and Medical Research Council of Australia (NHMRC).
The Tasmanian Infant Health Study was funded by the NHMRC, US National Institutes of Health, Tasmanian State Government, Australian Rotary Health Research Fund, Sudden Infant Death Research Foundation, National Sudden Infant Death Syndrome Council of Australia, Community Organisations’ Support Program of the Department of Human Services and Health, Zonta International, Wyeth Pharmaceuticals and Tasmanian Sanatoria After-Care Association.
We thank the research nurses (Teresa Skerratt and Sue Davoren) and research assistant (James Dilger), who undertook fieldwork, and we are especially grateful to the children who participated, and their families.
References
- 1.Manning MA, Eugene Hoyme H. Fetal alcohol spectrum disorders: A practical clinical approach to diagnosis. Neurosci Biobehav Rev. 2007;31:230–238. doi: 10.1016/j.neubiorev.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 2.Jones KL, Chambers CD, Hill LL, Hull AD, Riley EP. Alcohol use in pregnancy: inadequate recommendations for an increasing problem. BJOG. 2006;113:967–968. doi: 10.1111/j.1471-0528.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary CM, Heuzenroeder L, Elliott EJ, Bower C. A review of policies on alcohol use during pregnancy in Australia and other English-speaking countries, 2006. Med J Aust. 2007;186:466–471. doi: 10.5694/j.1326-5377.2007.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 4.Tikkanen J, Heinonen OP. Risk factors for atrial septal defect. Eur J Epidemiol. 1992;8:509–515. doi: 10.1007/BF00146368. [DOI] [PubMed] [Google Scholar]
- 5.Iveli MF, Morales S, Rebolledo A, Savietto V, Salemme S, Apezteguía M, Cecotti N, Drut R, Milesi V. Effects of light ethanol consumption during pregnancy: increased frequency of minor anomalies in the newborn and altered contractility of umbilical cord artery. Pediatr Res. 2007;61:456–461. doi: 10.1203/pdr.0b013e3180332c59. [DOI] [PubMed] [Google Scholar]
- 6.van den Elzen AP, Sierksma A, Oren A, Vos LE, Witteman JC, Grobbee DE, Hendriks HF, Uiterwaal CS, Bots ML. Alcohol intake and aortic stiffness in young men and women. J Hypertens. 2005;23:731–735. doi: 10.1097/01.hjh.0000163140.82212.16. [DOI] [PubMed] [Google Scholar]
- 7.Sierksma A, Muller M, van der Schouw YT, Grobbee DE, Hendriks HF, Bots ML. Alcohol consumption and arterial stiffness in men. J Hypertens. 2004;22:357–362. doi: 10.1097/00004872-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Sierksma A, Lebrun CE, van der Schouw YT, Grobbee DE, Lamberts SW, Hendriks HF, Bots ML. Alcohol consumption in relation to aortic stiffness and aortic wave reflections: a cross-sectional study in healthy postmenopausal women. Arterioscler Thromb Vasc Biol. 2004;24:342–348. doi: 10.1161/01.ATV.0000110784.52412.8f. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara T, Tomiyama H, Hashimoto H, Yamamoto Y, Yano E, Yamashina A. Excessive alcohol intake increases the risk of arterial stiffening in men with normal blood pressure. Hypertens Res. 2004;27:669–673. doi: 10.1291/hypres.27.669. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi N, Yoshida H, Kawashimo H, Suzuki K, Nakamura K, Tatara K. Alcohol consumption and risk for increased aortic pulse wave velocity in middle-aged Japanese men. Angiology. 2001;52:533–542. doi: 10.1177/000331970105200805. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-Invasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 13.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 14.Schack-Nielsen L, Molgaard C, Larsen D, Martyn C, Michaelsen KF, Arterial stiffness in 10-year-old children current and early determinants. Br J Nutr. 2005;94:1004–1011. doi: 10.1079/bjn20051518. [DOI] [PubMed] [Google Scholar]
- 15.Dwyer T, Ponsonby AL, Newman NM, Gibbons LE. Prospective cohort study of prone sleeping position and sudden infant death syndrome. Lancet. 1991;337:1244–1247. doi: 10.1016/0140-6736(91)92917-q. [DOI] [PubMed] [Google Scholar]
- 16.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17:407–429. [PubMed] [Google Scholar]
- 17.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 18.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voors AW, Webber LS, Frerichs RR, Berenson GS. Body height and body mass as determinants of basal blood pressure in children – The Bogalusa Heart Study. Am J Epidemiol. 1977;106:101–108. doi: 10.1093/oxfordjournals.aje.a112439. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26:1049–1057. doi: 10.1097/HJH.0b013e3282f82c3e. [DOI] [PubMed] [Google Scholar]
- 21.Rucker RB, Tinker D. Structure and metabolism of arterial elastin. Int Rev Exp Pathol. 1977;17:1–47. [PubMed] [Google Scholar]
- 22.Martyn CN, Greenwald SE. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet. 1997;350:953–955. doi: 10.1016/s0140-6736(96)10508-0. [DOI] [PubMed] [Google Scholar]
- 23.Roach MR. The pattern of elastin in the aorta and large arteries of mammals. Ciba Found Symp. 1983;100:37–55. doi: 10.1002/9780470720813.ch4. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud A, Feely J. Divergent effect of acute and chronic alcohol on arterial stiffness. Am J Hypertens. 2002;15:240–243. doi: 10.1016/s0895-7061(01)02315-9. [DOI] [PubMed] [Google Scholar]
- 25.Deng XS, Deitrich RA. Ethanol metabolism and effects: nitric oxide and its interactions. Curr Clin Pharmacol. 2007;2:145–153. doi: 10.2174/157488407780598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinhenz DJ, Sutliff RL, Polikandriotis JA, Walp ER, Dikalov SI, Guidot DM, Hart CM. Chronic ethanol ingestion increases aortic endothelial nitric oxide synthase expression and nitric oxide production in the rat. Alcohol Clin Exp Res. 2008;32:148–154. doi: 10.1111/j.1530-0277.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirapelli CR, Legros E, Brochu I, Honore JC, Lanchote VL, Uyemura SA, de Oliveira AM, D’Orleans-Juste P. Chronic ethanol intake modulates vascular levels of endothelin-1 receptor and enhances the pressor response to endothelin-1 in anaesthetized rats. Br J Pharmacol. 2008;154:971–981. doi: 10.1038/bjp.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry CL, Looker T. An alteration in the chemical structure of the aortic wall induced by a finite period of growth inhibition. J Anat. 1973;114:83–94. [PMC free article] [PubMed] [Google Scholar]
- 29.Janzen K, Janzen J. Nutrition and fetal aortogenesis. J Atheroscler Thromb. 2006;13:265. doi: 10.5551/jat.13.265. [DOI] [PubMed] [Google Scholar]
- 30.Skilton MR, Gosby AK, Wu BJ, Ho LM, Stocker R, Caterson ID, Celermajer DS. Maternal undernutrition reduces aortic wall thickness and elastin content in offspring rats without altering endothelial function. Clin Sci (Lond) 2006;111:281–287. doi: 10.1042/CS20060036. [DOI] [PubMed] [Google Scholar]
- 31.Norman P, Moss I, Sian M, Gosling M, Powell J. Maternal and postnatal vitamin D ingestion influences rat aortic structure, function and elastin content. Cardiovasc Res. 2002;55:369–374. doi: 10.1016/s0008-6363(02)00444-3. [DOI] [PubMed] [Google Scholar]
- 32.Ngai AC, Mondares RL, Mayock DE, Gleason CA. Fetal alcohol exposure alters cerebrovascular reactivity to vasoactive intestinal peptide in adult sheep. Neonatology. 2008;93:45–51. doi: 10.1159/000105524. [DOI] [PubMed] [Google Scholar]
- 33.Qian L, Wang Y, Jiang Q, Zhong T, Song H. Ethanol disrupts the formation of hypochord and dorsal aorta during the development of embryonic zebrafish. Sci China C Life Sci. 2005;48:608–615. doi: 10.1360/062005-1. [DOI] [PubMed] [Google Scholar]


