Abstract
Purpose
Several adult studies have documented the importance of the peri-stroke areas to aphasia recovery. But, studies examining the differences in patterns of cortical participation in language comprehension in patients who have (LMCA-R) or have not recovered (LMCA-NR) from left middle cerebral artery infarction have not been performed up to date.
Methods
In this study, we compare cortical correlates of language comprehension using fMRI and semantic decision/tone decision task in 9 LMCA-R and 18 LMCA-NR patients matched at the time of stroke for age and handedness. We examine the cortical correlates of language performance by correlating intra- and extra-scanner measures of linguistic performance with fMRI activation and stroke volumes.
Results
Our analyses show that LMCA-R at least 1 year after stroke show a return to typical fMRI language activation patterns and that there is a compensatory reorganization of language function in LMCA-NR patients with shifts to the right hemispheric brain regions. Further, with increasing strength of the left-hemispheric fMRI signal shift there are associated improvements in performance as tested with standardized linguistic measures. A negative correlation between the size of the stroke and performance on some of the linguistic tests is also observed.
Conclusions
This right-hemispheric shift as a mechanism of post-stroke recovery in adults appears to be an ineffective mode of language function recovery with increasing right-hemispheric shift associated with lower language performance. Thus, normalization of the post-stroke language activation patterns is needed for better language performance while shifts of the activation patterns to the non-dominant (right) hemisphere and/or large stroke size are associated with decreased linguistic abilities after stroke.
Keywords: Functional MRI, aphasia, language, recovery, semantic decision, stroke
1. INTRODUCTION
Overall, approximately 30% of patients with stroke suffer from various types of aphasia with this deficit most common in stroke with left middle cerebral artery (LMCA) territory damage. Some of the affected patients recover to a certain degree in the months and years following the stroke. The recovery process is modulated by several factors including type, intensity and timing of the intervention, context in which the recovery occurs and/or the severity of the initial presentation. These factors have been investigated in numerous studies from various laboratories.(e.g., (Berthier and Pulvermuller, 2011; Kertesz and McCabe, 1977; Robey, 1998; Hart, 2010)) While the relative impact of these modulators on post-stroke aphasia recovery is mostly known, the degree of the contribution of brain areas unaffected by stroke to the recovery process is less clear. There is a lack of consensus in regards to the relative contributions of the dominant vs. non-dominant hemispheres to the recovery process.(e.g., (Anderson et al., 2011a)) The proposed mechanisms of post-stroke recovery include repair of damaged networks (sprouting), activation of compensatory areas (redundancy), or activation of previously functionally inactive pathways (unmasking).(Lee and van Donkelaar, 1995) The first two mechanisms may be expressed as a recovery of the peri-stroke areas or shift of functions within the language-dominant brain regions surrounding the stroke to compensate for damage to the dominant left-hemispheric language circuitry.(e.g., (Cao et al., 1999; Saur et al., 2006; Szaflarski et al., 2011a)) Compensation via the last mechanism may occur by shifting language functions to the brain regions in the other (non-dominant) hemisphere after damage to the dominant left-hemispheric language structures.(Abo et al., 2004; Tillema et al., 2008) However, this last mode of compensation may not be as efficient, at least in older subjects, as the intra-hemispheric shifts or recovery of the peri-stroke areas.(Raja Beharelle et al., 2010; Richter et al., 2008; Rosen et al., 2000; Saur et al., 2006; van Oers et al., 2010; Weiller et al., 1995) Based on the available data a hierarchical model of post-stroke aphasia recovery was recently developed. This model posits that post-stroke aphasia recovery and the relative contributions of the dominant and non-dominant hemispheres are dependent, in part, on the size of the lesion.(Hamilton et al., 2011; Heiss and Thiel, 2006)
There may be many reasons for the observed between-studies differences in the localization and lateralization of the aphasia recovery correlates. These may include timing of the assessment in regards to the incident stroke (acute vs. subacute vs. chronic) (Kertesz and McCabe, 1977; Saur et al., 2006), size of the lesion (Allendorfer et al., 2012a; Raja Beharelle et al., 2010), age of stroke onset (prenatal vs. postnatal; young vs. old) (Jacola et al., 2006; Kennard, 1942; Knoflach et al., 2012; Tillema et al., 2008), type of the language neuroimaging paradigm used (e.g., event related vs. block design)(Allendorfer et al., 2012a) and pre-stroke handedness.(Borod et al., 1985) The above and other factors including co-morbidities like hypertension, seizures/epilepsy or diabetes need to be taken into account when assessing post-stroke functional recovery.(Lazar et al., 2008; Nichols-Larsen et al., 2005)
Modern neuroimaging techniques (e.g., fMRI and/or DTI, PET/fMRI, SPECT, MEG) are frequently used for the assessment of the remaining language functions, of the longitudinal changes associated with post-stroke recovery, or for the assessment of changes in language function localization from before to after the intervention.(Allendorfer et al., 2012b; Breier et al., 2007; Eliassen et al., 2008; Jodzio et al., 2003; Richter et al., 2008; Saur et al., 2006; Szaflarski et al., 2011b; van Oers et al., 2010; Weiller et al., 1995) Post-stroke aphasia neuroimaging studies frequently focus on the localization of language functions after stroke in patients with chronic aphasia who continue to have substantially unrecovered deficits or on the correlates of language recovery (Anderson et al., 2011b; Elkana et al., 2011a; Elkana et al., 2011b; Fridriksson et al., 2012; Raja Beharelle et al., 2010), while studies specifically comparing patients with good vs. poor recovery of language functions (i.e., patients who were able to return to the pre-stroke language abilities vs. patients whose language functions continue to be partially or completely impaired) or studies evaluating post-stroke language localization in completely recovered subjects are scarce.(Abo et al., 2004) In contrast to the paucity of studies focusing on subjects who recovered from adult stroke, several studies addressing this issue in children who recovered from perinatal or early postnatal stroke have been conducted.(e.g., (Fair et al., 2010; Jacola et al., 2006; Raja Beharelle et al., 2010; Staudt et al., 2001; Tillema et al., 2008))
It is imperative for us to understand the differences that underlie good vs. poor post-stroke aphasia recovery so that studies developing behavioral (e.g., intonation therapy or constraint-induced aphasia therapy) or non-invasive interventions (e.g., rTMS or tDCS) can target the brain areas relatively important for good recovery.(Baker et al., 2010; Chrysikou and Hamilton, 2011; Richter et al., 2008; Szaflarski et al., 2008a; Szaflarski et al., 2011b; Vines et al., 2011) Of particular interest is how damage to the adult brain may mediate the trajectories of post-stroke aphasia recovery. Thus, the goal of the current study was to investigated the differences in clinical characteristics, basic patterns of aphasia testing performance, extent of damage to anatomical structures and fMRI language activation patterns between chronic LMCA stroke subjects (≥1 year post-stroke) who were classified as having normal (recovered) vs. aphasic language abilities. We hypothesized that while the clinical characteristics of these cohorts may be similar, patients with good recovery would have different language activation patterns with possibly more left-hemispheric (dominant) involvement in language production when compared to the non-recovered group akin to studies in post-stroke motor recovery.(Ward et al., 2003)
2. MATERIAL AND METHODS
2.1 Subjects
As part of studies evaluating post-stroke aphasia recovery, 27 right-handed adults who suffered from LMCA stroke at least one year prior to study enrollment were recruited from the Greater Cincinnati, Ohio community (Table 1). Subjects were categorized into two groups based on their Token Test performance: 9 subjects (4 female) scored ≥41 which is in the normal range (LMCA-R), while 18 subjects (8 female) scored ≤40 which was considered aphasic (LMCA-NR).(De Renzi and Vignolo, 1962) All subjects had no contraindications to undergoing an MRI at 3T. Pre-stroke handedness was determined by the Edinburgh Handedness Inventory (EHI) with a score ≥50 indicating right-handedness.(Oldfield, 1971) History of hypertension, diabetes mellitus, high cholesterol, coronary artery disease, myocardial infarction and atrial fibrillation were also documented. SAS, version 9.3 (Statistical Analysis System, SAS Institute, Cary, NC) was used to perform independent sample t-tests (two-tailed) and Fisher’s Exact Test to characterize group differences in demographic and clinical variables, respectively. This study was approved by the University of Cincinnati Institutional Review Board, and all subjects provided written informed consent before participating in the study.
Table 1.
Demographic, clinical and performance variables for the 9 LMCA-R (recovered) and 18 LMCA-NR (not-recovered) stroke subjects.
| LMCA-R | LMCA-NR | p-value | |
|---|---|---|---|
| Age | 52 (13) | 56 (12) | 0.48 |
| Age at stroke | 50 (13) | 51 (13) | 0.84 |
| Years since stroke | 2.1 (2.1) | 4.9 (3.1) | 0.01 |
| Edinburgh Handedness Inventory score | 83 (19) | 96 (12) | 0.10 |
| Token Test score | 43 (1) | 23 (12) | <0.001 |
| Clinical History | |||
| Hypertension | 3 (33.3) | 5 (27.8) | 1.0 |
| Diabetes Mellitus | 0 (0) | 3 (16.7) | 0.53 |
| High Cholesterol | 1 (11.1) | 10 (55.6) | 0.04 |
| Coronary Artery Disease | 0 (0) | 2 (11.1) | 0.54 |
| Myocardial Infarction | 0 (0) | 1 (5.6) | 1.0 |
| Atrial Fibrillation | 1 (11.1) | 1 (5.6) | 1.0 |
| Comorbidity Loada | 0.6 | 1.2 | 0.78 |
| Language Assessments | |||
| Boston Naming Test | 54 (9) | 32 (19) | <0.001 |
| Semantic Fluency Test | 41 (18) | 17 (12) | 0.004 |
| Controlled Oral Word Association Test | 26 (17) | 7 (5) | 0.01 |
| Peabody Picture Vocabulary Test | 206 (19) | 198 (16) | 0.27 |
| Complex Ideation subtest of Boston Diagnostic Aphasia Examination | 11 (2) | 7 (3) | <0.001 |
| FMRI Task | |||
| Semantic Decision accuracy | 64.4 (18.4) | 47.6 (17.1) | 0.04 |
| Tone Decision accuracy | 76.7 (36.3) | 52.2 (28.7) | 0.10 |
Data reported as mean (SD) except for clinical variables, which are reported as frequency (percentages).
Comorbidity load is defined as the average number of clinical comorbidities per subject in each group.
2.2 Language testing
A battery of language assessments was administered to all subjects prior to MRI. These included the Boston Naming Test, Second edition (BNT) (Kaplan et al., 1983) to assess semantic retrieval and word-finding abilities, the Semantic Fluency Test (SFT) (Kozora and Cullum, 1995; Lezak, 1995) and Controlled Oral Word Association Test (COWAT) (Lezak, 1995) to assess verbal fluency (i.e., generate words for a given category and letter, respectively, in one minute), the Peabody Picture Vocabulary Test, Fourth edition (PPVT) (Dunn and Dunn, 1997) to test vocabulary, and the Complex Ideation subtest of the Boston Diagnostic Aphasia Examination (BDAE) (Goodglass and Kaplan, 1972) to test comprehension and ability to recall information related to 12 items that the examiner presents orally (i.e., answer yes/no and short story questions). Independent samples t-tests (two-tailed) were performed to characterize language function differences between LMCA-R and LMCA-NR groups.
2.3 Semantic decision/tone decision task
The semantic decision/tone decision (SD/TD) task used in this study as the fMRI language paradigm was adapted from Binder et al. (1996) and programmed using DirectRT (Version 2008; Empirisoft, www.empirisoft.com).(Binder et al., 1996) A detailed description of the SD/TD task is provided in our recent publications.(e.g., (Donnelly et al., 2011; Kim et al., 2011; Szaflarski and Allendorfer, 2012)) Briefly, subjects heard a series of tone sequences (six 30-second TD control blocks) or a series of animal names (five 30-second SD test blocks) and were instructed to make a 2-choice decision based on properties of the stimuli using a response box held in their left hand. Since the events that occur during the TD control and SD test blocks are operationally similar, contrasting these two conditions allows us to investigate brain activation related to semantic processing. Subjects were presented first with a TD control block, followed by alternating SD and TD blocks. Two runs of the SD/TD task were performed during fMRI. As later described, data from the first TD block were not included in analysis and the remaining five TD and five SD blocks of each run were statistically modeled. Performance on the TD and SD blocks was determined using the proportion of correct responses over the two runs. We have previously shown excellent repeatability of this task in identifying cortical language areas in healthy controls and patients with ischemic LMCA stroke confirming the suitability of this task for mapping of language functions as intended in this study.(Eaton et al., 2008)
The SD/TD fMRI task is known to activate several modules and subnets involved in semantic decision.(Kim et al., 2011) Overall, the auditorily presented information (i.e., the name of an animal) is first encoded to generate a new mental image and stored as such (verbal encoding and mental imagery module) – a process that involves integration of interactions between cuneus, lingual gyrus and hippocampal/parahippocampal structures. Next, the semantic decision module is activated with involvement of reasoning, semantic memory retrieval and later the semantic decision itself, processes which all involve inferior frontal gyri, posterior cingulate and amygdala/hippocampus. Finally, the process of language comprehension, syntactic processing, and speech production are activated (left fronto-temporo-parietal node). Maintaining a high level of attention is necessary for this entire process to occur – thus, activation in the anterior cingulate area is frequently observed with his fMRI task.(Kim et al., 2011; Szaflarski et al., 2008b)
2.4 Magnetic resonance imaging
Neuroimaging was performed at the Imaging Research Center at Cincinnati Children’s Hospital Medical Center using an 8-channel phased array head coil on a Phillips 3T MRI system. Subjects laid supine on the scanner bed, were fitted with non-ferromagnetic headphones (Avotec, Inc.) for auditory stimuli presentation, and given a button box in their left hand to record responses. After subjects were positioned in the scanner, pre-scan procedures (radio frequency coil calibration and shimming) were performed. Then, a high-resolution T1-weighted anatomical MRI (repetition time 8.1 msec, echo time 3.7 msec, field of view 25.0 cm × 21.1 cm × 18.0 cm, matrix 252 × 211, flip angle 8°, slice thickness 1 mm) was acquired to localize brain activation maps, followed by a multi-echo reference scan acquired to correct for high-field geometric distortions and ghosting artifacts.(Schmithorst et al., 2001) A gradient-echo echo planar imaging sequence (repetition time 2000 msec, echo time 38 msec, field of view 24.0 cm × 24.0 cm, matrix 64 × 64, 32 axial slices, slice thickness 4 mm) was used to acquire T2*-weighted fMRI while subjects performed two runs of the SD/TD task (165 scans and duration of 5 min 30 sec per run).
2.5 MRI data analysis
As in our previous studies, we used in-house software (Cincinnati Children’s Hospital Image Processing Software; https://irc.cchmc.org/software/cchips.php) programmed in Interactive Data Language (www.ittvis.com) to reconstruct scanner images, applying the multi-echo reference scan to correct for ghosting and geometric signal distortions.(Schmithorst et al., 2001; Szaflarski et al., 2012; Szaflarski et al., 2004) The MRI/fMRI data were then processed, analyzed and visualized using AFNI (http://afni.nimh.nih.gov).(Cox, 1996)
Each subject’s stroke lesion was manually traced on his or her anatomical MRI in native space by a trained neuro-anatomist (Fig. 1). The residual volume (cm3) of each subject’s stroke lesion was calculated using the number of voxels within the lesion. We examined the relationship between lesion size and intra- and extra-scanner language performance using Pearson’s correlation coefficient. Some analysis software programs including AFNI require a brain extraction step in which the algorithm uses signal intensity to identify cortical edges of the brain and remove the skull from the image before spatial normalization. However, low signal intensity within a stroke lesion can result in the removal of not only the lesion area but also surrounding areas after this brain extraction step. This, in turn, may result in an inadequate spatial normalization of the anatomical scan (Fig. 1A). One strategy to address this issue is the use of lesion masks to aid in the process of spatial normalization.(Meinzer et al., 2012) Therefore, we applied individual lesion masks to the anatomical MRIs prior to performing the brain extraction algorithm, followed by spatial normalization into Talairach space (Talairach and Tournoux, 1988) using the ICBM452 brain template (www.loni.ucla.edu) in AFNI (Fig. 1B). The transformation matrix from the spatial normalization step was also applied to the lesion mask and used to create a map of lesion overlap between the subjects in each of the two groups (Fig. 2). Visual inspection of the images and lesion masks in Talairach space was performed by the same neuro-anatomist to confirm the correctness of the transformation.
Figure 1.
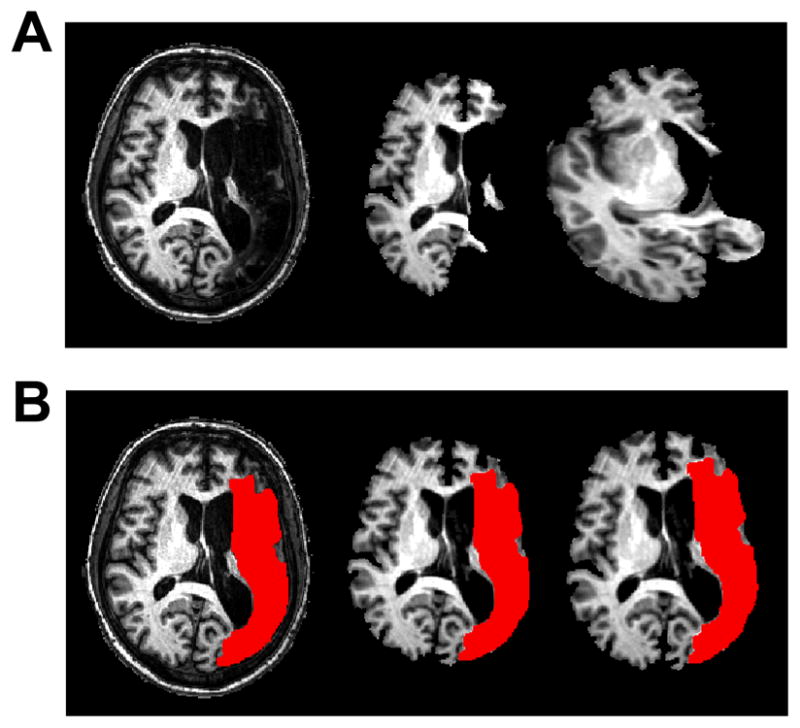
Example of using the lesion mask for the brain extraction and normalization steps. (A) Standard brain extraction of lesioned anatomical MRI (left) results in removal of viable tissue beyond the stroke lesion (middle) and subsequent misregistration to standardized space (right). (B) The lesion mask (in red) was traced on each subject’s anatomical MRI (left) and applied to achieve good results during the brain extraction algorithm (middle) and subsequent normalization to standardized space (right). Images are presented in radiological convention (left=right).
Figure 2.
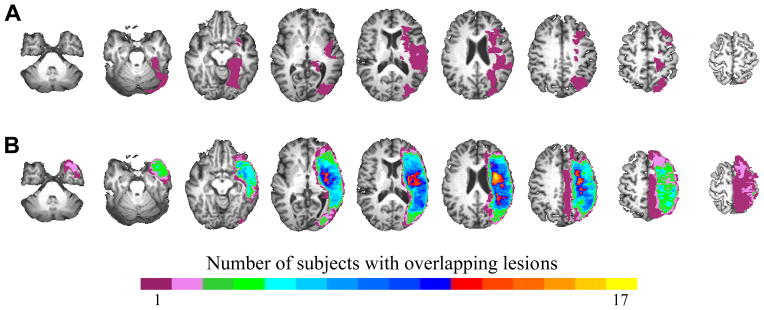
Group composite maps depicting lesion overlap between subjects in (A) LMCA patients with Token Test ≥41 (LMCA-R; n=9) and (B) LMCA patients with Token Test ≤40 (LMCA-NR; n=18). Images are presented in radiological convention (left=right) and lesion maps are overlaid onto one of the stroke subject’s anatomical scan in Talairach space with slices ranging from z=−34 to z=+62 (left to right).
Processing of fMRI data involved removal the first TD control block (15 scans) of each run to allow for MR signal equilibration, followed by co-registration of the anatomical MRI image and the first image of the first SD/TD fMRI run. An image co-registration algorithm was then used to correct for subject head movements and to align all other fMRI images from both runs to the first fMRI image of the first run.(Cox and Jesmanowicz, 1999) The transformation matrix used to normalize the anatomical MRI was applied to convert the functional images into Talairach space, followed by resampling of functional images to a 4 × 4 × 4 mm3 voxel resolution and application of a 6 mm Gaussian smoothing kernel. A binary mask was created to remove extraneous signal outside of the brain. For each subject, their spatially normalized lesion mask was resampled to the resolution of the functional images and used to exclude signal within the stroke lesion from statistical analyses.
2.6 MRI statistical analysis
For each participant, each block type (TD and SD) was modeled as a 30-second block function using the 3dDeconvolve program in AFNI. Motion-correction parameters were used as covariates in single-subject analysis to account for subject head motion and reduce motion-related signal artifacts in fMRI results.(Evans et al., 2010) Linear, quadratic and cubic polynomials were fit to each of the two runs to account for low-frequency signal drift. A general linear model (GLM) approach (Worsley and Friston, 1995) was used to determine the difference in activation between SD and TD blocks for each subject and estimate brain activation related to semantic processing. For group-level analyses, a one-sample t-test of the GLM results was performed for each LMCA group to determine the overall patterns of activation during SD/TD. A two-sample t-test assessed differences in activation between groups on the SD/TD task. Activation clusters on group maps were considered significant at a corrected p<0.05 (voxelwise threshold of p<0.05 and a cluster threshold of at least 65 contiguous voxels in which the faces of neighboring voxels must touch) as determined by Monte Carlo simulation in AFNI. Given evidence that BOLD fMRI signal to noise is reduced in individuals who are aged or diseased (D’Esposito et al., 2003) we employed a less stringent voxelwise threshold in our analyses.
2.7 Relating task activation with language performance
We performed Pearson’s correlation analysis to investigate the relationship between language-related activation differences between groups and language performance, with p<0.05 considered significant. Clusters that showed significant between-group differences were defined as regions of interest (ROIs). For each ROI, the average t-score was extracted from each subject’s GLM map and used in correlation analysis with language assessments scores (BDAE, BNT, SFT, COWAT, and PPVT) and SD accuracy.
3. RESULTS
3.1 Demographics and language performance
Table 1 provides a summary of the demographic and performance variables. LMCA-R and LMCA-NR were similar in mean age (52 vs. 56, p=0.46), pre-stroke EHI (83 vs. 96, p=0.1), the proportion of males and females (both 44.4% female) and age at time of stroke (50 vs. 51, p=0.84). There were no differences between groups in their incidence of comorbid conditions (all p>0.5), with the exception of a greater number of LMCA-NR with a history of high cholesterol (p=0.04). Additionally, we found that the overall health of the two groups with regards to their comorbidity load (i.e., the average number of clinical comorbidities per subject in each group) was not significantly different (p=0.78). As expected, LMCA-NR showed decreased accuracy on SD blocks (p=0.036) and poorer performance on the BNT, SFT, COWAT and BDAE (all p≤0.01) when compared with LMCA-R. Further, the performance accuracy of the LMCA-R subjects on the SD portion of the SDTD task was similar to healthy controls as assessed in previous studies utilizing this fMRI task.(Donnelly et al., 2011; Kim et al., 2011; Szaflarski and Allendorfer, 2012) There were no significant differences between groups on accuracy on the TD blocks (p=0.1) or on PPVT performance (p=0.27). [0]These results indicate that the LMCA-NR patients have overall more severe expressive/word-finding than receptive deficits when compared to the LMCA-R patients. Stroke volumes were different between groups (p≤0.001) with median (range) for recovered of 9.2 (2.2–26.5) cm3 and for not recovered of 74.0 (5.1–206.0) cm3 (Fig. 2). Finally, we did not observe an effect of age at stroke on post-stroke language performance (all measures |r|≤0.31; p≥0.11).
3.2 Whole-brain analysis of fMRI activation
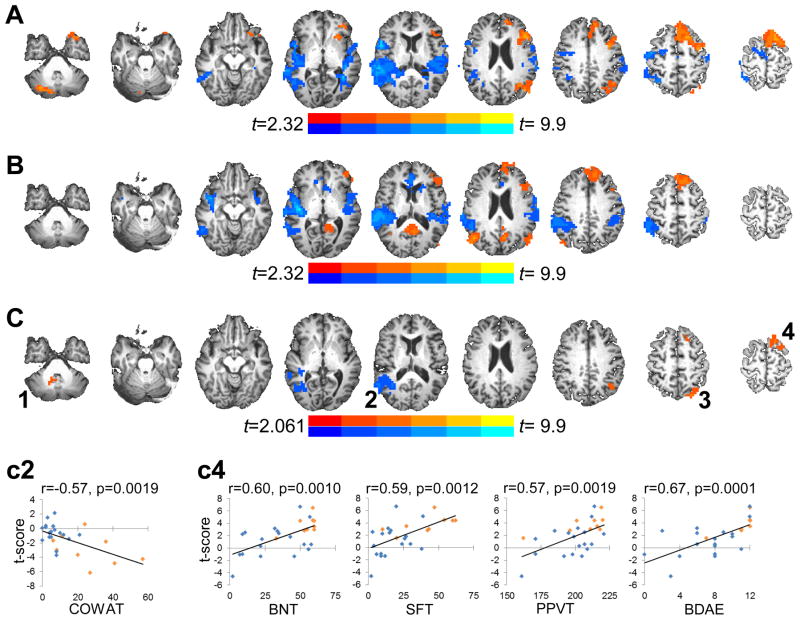
SD/TD task activation patterns were similar for LMCA-R (Fig. 3A) and LMCA-NR (Fig. 3B) with increased left hemisphere recruitment of the inferior/middle frontal gyrus, superior frontal gyrus, and inferior parietal lobule/supramarginal gyrus during semantic decision compared to tone decision (orange clusters) and increased bilateral (right>left) activation in temporal auditory processing regions during tone decision compared to semantic decision (blue clusters). LMCA-R showed additional activation related to semantic processing in the right cerebellar hemisphere, while LMCA-NR showed increased activation for semantic decision bilaterally in posterior cingulate and posterior parietal regions.
Figure 3.
Group composite activation maps for the SD/TD task for (A) LMCA-R and (B) LMCA-NR patients. Both groups show overall typical BOLD activation patterns during semantic processing with increased activation (in orange) in medial frontal, left lateral frontal and left/bilateral parietal regions and decreased activation (in blue) in bilateral temporal regions. (C) Group differences in BOLD activation during semantic processing. Compared to LMCA-NR, LMCA-R showed greater increases in activation (in orange) in the bilateral (right>left) cerebellum (1), left superior parietal lobule (3) and the left superior frontal gyrus (4). LMCA-R also showed a greater decrease in activation (in blue) than LMCA-NR in the right superior temporal gyrus (2). Activation clusters in all images are significant after correction for multiple comparisons at a corrected p<0.05 (voxelwise p<0.05, minimum cluster of 65 contiguous voxels). Images are presented in radiological convention (left=right) and group activation maps are overlaid onto one of the stroke subject’s anatomical scan in Talairach space with slices ranging from z=−34 to z=+62 (left to right). The clusters in (C) showing significant group activation differences were defined as regions of interest (ROIs 1–4) for use in correlation analyses; for each ROI, the average t-score was extracted from each subject’s GLM map. (c2) shows significant associations (Bonferroni corrected) between fMRI activation in ROI 2 and Controlled Oral Word Association Test (COWAT) performance. (c4) shows significant associations (Bonferroni corrected) between fMRI activation in ROI 4 and language performance on the Boston Naming Test (BNT), Semantic Fluency Test (SFT), Peabody Picture Vocabulary Test (PPVT), and the Complex Ideation subtest of the Boston Diagnostic Aphasia Examination (BDAE). On the scatterplots, data points in orange are LMCA-R subjects and in blue are LMCA-NR subjects.
Direct group comparison of semantic decision portion of the SD/TD task related activation revealed significant differences in recruitment of four brain regions (Fig. 3C, 1–4). When compared to LMCA-NR, LMCA-R showed greater increases in activation in the bilateral (right>left) cerebellar tonsil (84 voxels, centroid at 3, −50, −41), left superior parietal lobule (84 voxels, centroid at −32, −68, 49) and the left superior frontal gyrus (67 voxels, centroid at −17, 20, 62), and greater decreases in activation in the right superior temporal gyrus (254 voxels, centroid at 47, −53, 11).
3.3 Relating lesion size and task activation with language performance
We examined the relationship between lesion size and language performance using Pearson’s correlation coefficient. Lesion size showed negative associations with performance on the BDAE (r=−0.56, p=0.003), the SFT (r=−0.51, p=0.007), the COWAT (r=−0.50, p=0.009), and the BNT (r=−0.38, p=0.05). Lesion size was not significantly correlated with PPVT performance (r=−0.22, p=0.28) or SD accuracy (r=−0.21, p=0.28). After applying Bonferroni correction for 6 correlations (lesion size × 6 performance variables) alpha was set at p<0.0083; the correlations with BDAE and SFT remained significant. Finally, the relationships between lesion location and linguistic performance were not assessed due to substantial lesion variability and a relatively small group sizes but the group lesion distribution patterns appeared to be similar based on visual comparison (Fig. 2). But, it is clear that the activations associated with recovery noted in Fig. 3C, when superimposed on lesion analysis from Fig. 2, show an overlap with the lesion distribution from the LMCA-NR in only 1–2 subjects while there is overlap between the activations and lesion location in only 1 LCMA-R patient. Since the activation-lesion overlap exists in only small minority of patients it appears that lesion size plays a greater role in language performance than lesion location.
Correlation analyses were also performed to investigate the relationship between language-related activation on the semantic decision portion of the SD/TD task and language performance. Comparing task activation between LMCA-R and LMCA-NR groups revealed four regions that were differentially recruited, which were defined as ROIs (Fig. 3C, 1–4). The average t-score was extracted from each single-subject GLM map for each ROI and used in correlation analyses. Activation in the bilateral cerebellum (ROI 1) showed positive associations with performance on the BNT (r=−0.41; p=0.03) and SFT (r=−0.51; p=0.006) and positive trends with performance on the remaining language assessments and SD accuracy (0.33<r<0.37; 0.05<p<0.09). BOLD signal changes observed in the right superior temporal gyrus (ROI 2) showed negative associations with SFT, COWAT, BDAE and SD performance (all r<−0.42; all p<0.05), and a negative trend with performance on the BNT (r=−0.35; p=0.073) but not on the PPVT (r=−0.17; p=0.4). Task-related activation in the left superior parietal lobule (ROI 3) showed significant positive associations with performance on the BNT, SFT, COWAT, PPVT and BDAE (all r>0.39; all p<0.05), but not with SD accuracy (r=0.21, p=0.29). Finally, activation in the left superior frontal gyrus showed significant positive associations with all of the language assessments (all r>0.54; all p<0.005) and with SD accuracy (r=0.40, p=0.036). After applying Bonferroni correction for 24 correlations (4 ROIs × 6 performance variables) alpha was set at p<0.0021, and the following remained significant: associations between activation in the right superior temporal gyrus (ROI 2) and COWAT performance (Fig. 3c2), and associations between activation in the left superior frontal gyrus (ROI 4) and performance on the BDAE, BNT, SFT, and PPVT (Fig. 3c4).
4. DISCUSSION
This study focuses specifically on the cortical correlates of good vs. poor post-stroke language recovery in a large sample of adults with LMCA stroke. While several studies have previously documented the post-stroke language activation patterns, contrasts between recovered and not-recovered patients have not been made. Contrasting these patients may provide vital information regarding not only post-stroke recovery but also neuroimaging characteristics predictive of better recovery and may help guide development of future interventions. Thus, our analyses contrasting these patient cohorts show that LMCA-R patients with normal or close to normal language functions at least 1 year after stroke show a return to typical fMRI activation patterns when compared to the results of fMRI data obtained in previous studies that included healthy controls and this language task.(Donnelly et al., 2011; Kim et al., 2011; Szaflarski and Allendorfer, 2012) Second, we observe that compensatory reorganization of language function in LMCA-NR patients and shifts to the right hemispheric brain regions are a less effective mode of language function recovery. This notion is further supported by the finding of an increasing association between the strength of non-dominant BOLD signal shift and decreasing performance (Fig 3c2). Third, the increasing strength of the observed left hemispheric fMRI signal increases is associated with performance improvements as tested with standardized linguistic measures. Fourth, performance on some of the linguistic tests is associated with stroke volume. Finally, there is an association between cerebellar activation and linguistic performance. All these findings require further discussion.
4.1 Left-hemispheric contributions to post-stroke aphasia recovery
Our findings of the more pronounced left hemispheric BOLD signal increases (frontal and temporo-parietal) in patients who recovered when compared to those who have not recovered are in agreement with results of other studies in which authors examined the cortical correlates of post-stroke recovery.(e.g., (Raja Beharelle et al., 2010; Saur et al., 2006; Saur et al., 2010; Szaflarski et al., 2011a)) Thus, the presented results support the notion that following stroke in adulthood it is the left hemisphere that is necessary for language function preservation/recovery rather than a shift to the non-dominant homologues as observed in some of the previous studies in pediatric population with prenatal or early post-natal stroke.(Jacola et al., 2006; Tillema et al., 2008) The distribution of the observed activations in the left frontal and left temporo-parietal regions is similar to that of the “Wernicke-Geschwind module” in healthy controls examined with this fMRI task.(Kim et al., 2011) This is the most left-lateralized module of the language network as observed in the healthy subjects with known bidirectional connections via white matter tracts.(Catani et al., 2005) This node of the language network is postulated to be involved in language comprehension, syntactic processing and speech production.(Kim et al., 2011) Thus, it is not surprising to find correlations between activations in this module and performance on a majority of the linguistic measures from our battery (semantic and verbal fluency, vocabulary, and comprehension/recall; Fig. 3). Overall, our findings are in agreement with previous studies which either evaluated the temporal correlates of post-stroke language recovery (Saur et al., 2006), correlated behavioral measures with language outcomes (Allendorfer et al., 2012a; Fridriksson et al., 2012; Heiss et al., 1999; Rosen et al., 2000; van Oers et al., 2010), or assessed pre/post-intervention language activation distribution.(Fridriksson et al., 2012; Richter et al., 2008; Szaflarski et al., 2011b) But, our findings are different from the results of other studies of post-stroke aphasia in adults.(Abo et al., 2004; Elkana et al., 2011b; Thulborn et al., 1999)
Thulborn et al., reported a subject (Case 2) who had fMRI before and after LMCA stroke and improvement after therapy associated with increasing activation in the right temporal area,(Thulborn et al., 1999) which is in contrast to our finding of declining language performance with increasing right temporal activation (Fig 3c2). Of note is that this subject had left hemispheric childhood head trauma and subsequent epilepsy originating from the left temporal area which both have likely contributed to more bilateral language distribution as documented previously by Wada test or fMRI in patients with epilepsy and early injury.(Rasmussen and Milner, 1977; Springer et al., 1999; Szaflarski et al., 2008b) Thus, the right-hemispheric shift is consistent with the observed atypical language lateralization in patients with relatively early onset of brain injury and epilepsy (Springer et al., 1999) and is not necessarily in disagreement with our results. Another study that showed post-stroke language recovery associated with right-hemispheric activation used an unmonitored fMRI task.(Abo et al., 2004) While widespread right hemispheric activations were observed in 2 subjects multiple factors could’ve affected their results including thresholding and use of a task with a prominent component of articulation (overt repetition of words) which is known to prominently activate right more than left hemispheric regions in subjects recovering from aphasia.(Abo et al., 2004; Allendorfer et al., 2012a) Finally, a small study by Elkana et al., tested language lateralization in 7 patients with relatively early (pre-teen and teenage) LMCA stroke and found associations between the degree of recovery and increases in right hemispheric activation.(Elkana et al., 2011b) In view of the results of the studies of post-stroke recovery from pre/peri-natal stroke the partial shift to the right hemispheric regions observed by Elkana et al. is not surprising as age may have an effect on post-stroke recovery based on the “Kennard Principle”.(Kennard, 1942; Knoflach et al., 2012; Staudt et al., 2001; Staudt et al., 2002; Staudt et al., 2008; Tillema et al., 2008)
4.2 Right-hemispheric contributions to post-stroke aphasia recovery
Right hemispheric activations are frequently considered an impediment to post-stroke language recovery in adults. In fact, already the early fMRI studies of post-stroke language recovery hinted that right-hemispheric shifts of activation in right-handed subjects might contribute to the less efficient and only partial/incomplete recovery.(Cao et al., 1999; Rosen et al., 2000) Recently, even studies in patients recovered from pre/peri-natal LMCA stroke question the ability of the right hemisphere to take over all language functions.(Anderson et al., 2011b; Raja Beharelle et al., 2010) This is despite data from children who received left hemispherectomy for the management of epilepsy which showed clear shift of language functions to the right hemisphere and decreases in the typically right-hemispheric functions including visuo-spatial measures (Liegeois et al., 2008; Mariotti et al., 1998) and despite previous data from pre/peri-natal LMCA stroke children with recovered language functions and activations in the right hemisphere.(Jacola et al., 2006; Staudt et al., 2001; Staudt et al., 2002; Tillema et al., 2008) Our findings of increasing deactivations (negative BOLD signal; Fig. 3) in the right hemisphere with decreasing linguistic performance confirm the results of these studies and suggest that with damage to the adult brain, switching to the right hemisphere may not be the most efficacious way for the brain to deal with the post-stroke recovery of language functions. While the right hemisphere has been shown in healthy controls to be important for improved linguistic performance (Donnelly et al., 2011; Just et al., 1996) and is known to be important for more automatic language functions,(Code, 1997; Searleman, 1977) in the case of adult post-stroke recovery function restitution in the left hemisphere may be much more important than function substitution in the non-dominant for language hemisphere.(Rothi and Horner, 1983) Certainly, with early or chronic injury a switch to the non-dominant homologues is possible, but this mechanism of post-stroke language recovery in adults may not be readily available. Thus, restitution of the peri-stroke areas may be of utmost importance for language recovery.
4.3 Cerebellar contributions to post-stroke aphasia recovery
An observation of an increased BOLD signal in cerebellum during language tasks, especially the SD/TD task is not new. Right cerebellar hemisphere is observed to activate typically with this task whether right- or left-handers are tested (Binder et al., 1997; Szaflarski et al., 2002) with this activation possibly related to semantic discrimination modulated by task difficulty.(Xiang et al., 2003) In fact, there is now a wealth of studies in healthy controls and subjects with cerebellar pathology documenting mainly right cerebellar participation in language and verbal memory processes that are clearly affected by vascular, neoplastic or developmental pathology.(Baillieux et al., 2010; Highnam and Bleile, 2011; Stoodley and Schmahmann, 2009) At the clinical level, comparisons between subjects with lesions and healthy controls usually reveal subtle deficits with relatively minor detectable group differences.(Baillieux et al., 2010; Murdoch, 2010) Patients with cerebellar injury frequently experience decreased verbal fluency and semantic access.(Highnam and Bleile, 2011) Further, a recent study of children with congenital vascular lesions functioning in the normal range showed remodeling of cerebellar language representation with right-sided activation observed in healthy control children and left-sided activation in children who recovered from the perinatal stroke; these authors observed a significant correlation between the strength of the left cerebellar lateralization of the activation and the right-hemispheric shift in the frontal language areas.(Lidzba et al., 2008) Our results are in agreement with and extend the results of those studies; in fact, we observed that overall better language performance was associated with increased activation in the right cerebellum (ROI 1 in Fig. 3C) and in the left frontal regions (ROIs 3–4 in Fig. 3C). This finding of a similar but reversed hemispheric pattern of associations further supports the notion that in adults, post-stroke aphasia recovery is associated with improvements in left-hemispheric peri-stroke functioning and also typical right cerebellar activation. But, in children with brains that are still undergoing development, it is not surprising to see the shift of activation to the contralateral cerebellar hemisphere.
4.4 Post-stroke recovery and lesion volume
Studies in healthy animals have shown that plasticity in motor cortex can be induced via exercise/practice (Nudo et al., 1996) and that the degree of this plasticity and subsequent remapping of the lost functions may be dependent on the lesion volume.(Nudo and Milliken, 1996) In human studies of motor recovery after stroke larger lesions were significantly associated with poorer recovery as assessed with Rankin scores (Beloosesky et al., 1995) or neurological examination/stroke scale.(Brott et al., 1989) Both groups independently postulated that the reason for this volume-outcome relationship is a decreased compensatory ability of the remaining brain tissue. This is certainly in agreement with the results of the above animal study.(Nudo and Milliken, 1996) While the studies of association between volume size and outcome in motor stroke are important and may help in explaining the results of the aphasia studies, an animal model of post-stroke aphasia is currently not available. What we do know about the stroke volume effect on aphasia outcomes are derived from a growing number of studies in patients with post-stroke aphasia.
Initial computed tomography studies of the effects of stroke volume on the post-stroke aphasia recovery consistently indicated a clear volume/outcome relationship between in volumetric studies obtained at 2 months to more than 1 year after incident stroke.(Kertesz et al., 1979; Knopman et al., 1983; Naeser et al., 1981) In these studies, patients with lower stroke volumes exhibited better aphasia recovery and overall better communication abilities at the time of testing. The results of aphasia recovery studies that utilized MRI measurements are less consistent.(Allendorfer et al., 2012a; Heiss et al., 1999; Lazar et al., 2008; Raja Beharelle et al., 2010; van Oers et al., 2010) This may be related to the fact that many modern studies do not examine this relationship or use relatively small sample sizes to examine the functional correlates of post-stroke aphasia recovery with the volumetric analysis being secondary to the analysis of functional recovery as tested with e.g., fMRI. Nevertheless, in the studies where this relationship was observed, the authors speculated that the lesion size may be the limiting factor in long-term recovery (Allendorfer et al., 2012a) and these studies are in support of the notion of a hierarchical model of aphasia recovery.(Hamilton et al., 2011; Heiss and Thiel, 2006) This model posits that complete post-stroke aphasia recovery occurs in patients with small lesions located in the eloquent areas of the dominant hemisphere. Larger (medium-size) lesions in such areas with peri-stroke recovery lead to contralateral homologue disinhibition and only partial but frequently adequate recovery, while still larger lesions with severe LMCA cortex injury cause inadequate recovery despite increased contralateral homologue participation in recovery.(Heiss and Thiel, 2006) Finally, another possible explanation for the presence of this discrepancy between studies is the fact that anatomical measures are usually very crude – while volume can be measured adequately, the brain hypoperfusion and the presence of a related sub-anatomical injury may not be adequately assessed.(Fridriksson et al., 2002; Hillis et al., 2003) Nevertheless, our results are in agreement with some of the studies of post-stroke aphasia recovery and the hierarchical model of post-stroke aphasia recovery, indicating that lesion size needs to be taken into account when assessing rehabilitative potential.
5. CONCLUSIONS
Overall, this study adds to the existing body of evidence in support of the importance of the left-hemispheric brain regions for the post-stroke aphasia recovery. Further, we show that recovered patients who had LMCA aphasia-producing stroke show normal or close-to-normal fMRI activation pattern which is similar to the recovery patterns in patients recovered from a stroke that produced motor deficits.(Ward et al., 2003) Further, we show that shifts of the activation patterns to the non-dominant (right) hemisphere may contribute to or be associated with decreased linguistic abilities after stroke. We have previously shown that there is an age at which the direction of language lateralization in healthy humans changes.(Szaflarski et al., 2006) Based on the available data from prenatal, early postnatal, childhood and adult stroke presented above we expect that a similar switch in relative contributions of both hemispheres to the language recovery after stroke occurs at a certain age which is yet to be determined. Knowing the age of that switch may facilitate choices regarding rehabilitative strategies with the interventions applied to the right or left hemispheres depending on the age of stroke occurrence.
Acknowledgments
This study was supported by R01 HD068488 and R01 NS048281 (both to JPS); it was presented in part at the Annual Conference of the Organization for Human Brain Mapping in Beijing, China.
Contributor Information
Jane B. Allendorfer, Email: allendjb@uab.edu.
Christi Banks, Email: christi.banks@uc.edu.
Jennifer Vannest, Email: jennifer.vannest@cchmc.org.
Scott K. Holland, Email: scott.holland@cchmc.org.
BIBLIOGRAPHY
- Abo M, Senoo A, Watanabe S, Miyano S, Doseki K, Sasaki N, Kobayashi K, Kikuchi Y, Yonemoto K. Language-related brain function during word repetition in post-stroke aphasics. Neuroreport. 2004;15:1891–1894. doi: 10.1097/00001756-200408260-00011. [DOI] [PubMed] [Google Scholar]
- Allendorfer JB, Kissela BM, Holland SK, Szaflarski JP. Different patterns of language activation in post-stroke aphasia are detected by overt and covert versions of the verb generation fMRI task. Med Sci Monit. 2012a;18:CR135–147. doi: 10.12659/MSM.882518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci. 2012b;30:103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011b;134:2197–2221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- Baillieux H, De Smet HJ, Dobbeleir A, Paquier PF, De Deyn PP, Marien P. Cognitive and affective disturbances following focal cerebellar damage in adults: a neuropsychological and SPECT study. Cortex. 2010;46:869–879. doi: 10.1016/j.cortex.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloosesky Y, Streifler JY, Burstin A, Grinblat J. The importance of brain infarct size and location in predicting outcome after stroke. Age Ageing. 1995;24:515–518. doi: 10.1093/ageing/24.6.515. [DOI] [PubMed] [Google Scholar]
- Berthier ML, Pulvermuller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nat Rev Neurol. 2011;7:86–97. doi: 10.1038/nrneurol.2010.201. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46:978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Borod JC, Carper M, Naeser M, Goodglass H. Left-handed and right-handed aphasics with left hemisphere lesions compared on nonverbal performance measures. Cortex. 1985;21:81–90. doi: 10.1016/s0010-9452(85)80017-4. [DOI] [PubMed] [Google Scholar]
- Breier JI, Maher LM, Schmadeke S, Hasan KM, Papanicolaou AC. Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: a case study. Neurocase. 2007;13:169–177. doi: 10.1080/13554790701448200. [DOI] [PubMed] [Google Scholar]
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KM. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–2340. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Hamilton RH. Noninvasive brain stimulation in the treatment of aphasia: exploring interhemispheric relationships and their implications for neurorehabilitation. Restor Neurol Neurosci. 2011;29:375–394. doi: 10.3233/RNN-2011-0610. [DOI] [PubMed] [Google Scholar]
- Code C. Can the right hemisphere speak? Brain Lang. 1997;57:38–59. doi: 10.1006/brln.1997.1833. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Donnelly KM, Allendorfer JB, Szaflarski JP. Right hemispheric participation in semantic decision improves performance. Brain Res. 2011;1419:105–116. doi: 10.1016/j.brainres.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Eaton KP, Szaflarski JP, Altaye M, Ball AL, Kissela BM, Banks C, Holland SK. Reliability of fMRI for studies of language in post-stroke aphasia subjects. Neuroimage. 2008;41:311–322. doi: 10.1016/j.neuroimage.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen JC, Boespflug EL, Lamy M, Allendorfer JB, Chu WJ, Szaflarski JP. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: a review. Top Stroke Rehabil. 2008;15:427–450. doi: 10.1310/tsr1505-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben-Bashat D, Hendler T, Schmidt D, Schweiger A. Cerebral reorganization as a function of linguistic recovery in children: An fMRI study. Cortex. 2011a;47:202–216. doi: 10.1016/j.cortex.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Elkana O, Frost R, Kramer U, Ben-Bashat D, Schweiger A. Cerebral language reorganization in the chronic stage of recovery: A longitudinal fMRI study. Cortex. 2011b doi: 10.1016/j.cortex.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Evans JW, Todd RM, Taylor MJ, Strother SC. Group specific optimisation of fMRI processing steps for child and adult data. Neuroimage. 2010;50:479–490. doi: 10.1016/j.neuroimage.2009.11.039. [DOI] [PubMed] [Google Scholar]
- Fair DA, Choi AH, Dosenbach YB, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. The functional organization of trial-related activity in lexical processing after early left hemispheric brain lesions: An event-related fMRI study. Brain Lang. 2010;114:135–146. doi: 10.1016/j.bandl.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Holland AL, Coull BM, Plante E, Trouard TP, Beeson P. Aphasia severity: Association with cerebral perfusion and diffusion. Aphasiology. 2002;16:859–871. doi: 10.1080/02687030244000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage. 2012;60:854–863. doi: 10.1016/j.neuroimage.2011.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118:40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J. Poststroke recovery. Alternative and Complimentary Therapies. 2010;16:277–280. [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98:118–123. doi: 10.1016/j.bandl.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Highnam CL, Bleile KM. Language in the cerebellum. Am J Speech Lang Pathol. 2011;20:337–347. doi: 10.1044/1058-0360(2011/10-0096). [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Ulatowski JA, Jacobs MA. Change in perfusion in acute nondominant hemisphere stroke may be better estimated by tests of hemispatial neglect than by the National Institutes of Health Stroke Scale. Stroke. 2003;34:2392–2396. doi: 10.1161/01.STR.0000089681.84041.69. [DOI] [PubMed] [Google Scholar]
- Jacola LM, Schapiro MB, Schmithorst VJ, Byars AW, Strawsburg RH, Szaflarski JP, Plante E, Holland SK. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke 1. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodzio K, Gasecki D, Drumm DA, Lass P, Nyka W. Neuroanatomical correlates of the post-stroke aphasias studied with cerebral blood flow SPECT scanning. Med Sci Monit. 2003;9:MT32–41. [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kennard M. Cortical reorganization of motor function: Studies on series of monkeys of various ages from infancy to maturity. Arch Neurol Psychiatry. 1942;48:227–240. [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Kertesz A, McCabe P. Recovery patterns and prognosis in aphasia. Brain. 1977;100(Pt 1):1–18. doi: 10.1093/brain/100.1.1. [DOI] [PubMed] [Google Scholar]
- Kim KK, Karunanayaka P, Privitera MD, Holland SK, Szaflarski JP. Semantic association investigated with functional MRI and independent component analysis. Epilepsy Behav. 2011;20:613–622. doi: 10.1016/j.yebeh.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, Seyfang L, Kiechl S, Willeit J. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Selnes OA, Niccum N, Rubens AB, Yock D, Larson D. A longitudinal study of speech fluency in aphasia: CT correlates of recovery and persistent nonfluency. Neurology. 1983;33:1170–1178. doi: 10.1212/wnl.33.9.1170. [DOI] [PubMed] [Google Scholar]
- Kozora E, Cullum C. Generative naming in normal aging: Total output and qualitative changes using phonemic and semantic constraints. Clin Neuropsychologist. 1995;9:313–320. [Google Scholar]
- Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry. 2008;79:530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can J Neurol Sci. 1995;22:257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lidzba K, Wilke M, Staudt M, Krageloh-Mann I, Grodd W. Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain Lang. 2008;106:204–210. doi: 10.1016/j.bandl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Baldeweg T, Vargha-Khadem F. Speaking with a single cerebral hemisphere: fMRI language organization after hemispherectomy in childhood. Brain Lang. 2008;106:195–203. doi: 10.1016/j.bandl.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Mariotti P, Iuvone L, Torrioli MG, Silveri MC. Linguistic and non-linguistic abilities in a patient with early left hemispherectomy. Neuropsychologia. 1998;36:1303–1312. doi: 10.1016/s0028-3932(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Beeson PM, Cappa S, Crinion J, Kiran S, Saur D, Parrish T, Crosson B, Thompson CK. Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. Neuroimage. doi: 10.1016/j.neuroimage.2012.02.058. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–868. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Hayward RW, Laughlin SA, Zatz LM. Quantitative CT scan studies in aphasia. I. Infarct size and CT numbers. Brain Lang. 1981;12:140–164. doi: 10.1016/0093-934x(81)90010-9. [DOI] [PubMed] [Google Scholar]
- Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors’ quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Raja Beharelle A, Dick AS, Josse G, Solodkin A, Huttenlocher PR, Levine SC, Small SL. Left hemisphere regions are critical for language in the face of early left focal brain injury. Brain. 2010;133:1707–1716. doi: 10.1093/brain/awq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Richter M, Miltner WH, Straube T. Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain. 2008;131:1391–1401. doi: 10.1093/brain/awn043. [DOI] [PubMed] [Google Scholar]
- Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, Dromerick AW, Fiez JA, Corbetta MD. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Horner J. Restitution and substitution: two theories of recovery with application to neurobehavioral treatment. J Clin Neuropsychol. 1983;5:73–81. doi: 10.1080/01688638308401152. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Saur D, Ronneberger O, Kummerer D, Mader I, Weiller C, Kloppel S. Early functional magnetic resonance imaging activations predict language outcome after stroke. Brain. 2010;133:1252–1264. doi: 10.1093/brain/awq021. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searleman A. A review of right hemisphere linguistic capabilities. Psychol Bull. 1977;84:503–528. [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh-Mann I. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krageloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. Neuroimage. 2002;16:954–967. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Staudt M, Ticini LF, Grodd W, Krageloh-Mann I, Karnath HO. Functional topography of early periventricular brain lesions in relation to cytoarchitectonic probabilistic maps. Brain Lang. 2008;106:177–183. doi: 10.1016/j.bandl.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang. 2009;110:149–153. doi: 10.1016/j.bandl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Allendorfer JB. Topiramate and its effect on fMRI of language in patients with right or left temporal lobe epilepsy. Epilepsy Behav. 2012;24(1):74–80. doi: 10.1016/j.yebeh.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Altaye M, Rajagopal A, Eaton K, Meng X, Plante E, Holland SK. A 10-year longitudinal fMRI study of narrative comprehension in children and adolescents. Neuroimage. 2012;63 (3):1188–1195. doi: 10.1016/j.neuroimage.2012.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Ball A, Grether S, Al-Fwaress F, Griffith NM, Neils-Strunjas J, Newmeyer A, Reichhardt R. Constraint-induced aphasia therapy stimulates language recovery in patients with chronic aphasia after ischemic stroke. Med Sci Monit. 2008a;14:CR243–250. [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward DB, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Eaton K, Ball AL, Banks C, Vannest J, Allendorfer JB, Page S, Holland SK. Poststroke aphasia recovery assessed with functional magnetic resonance imaging and a picture identification task. J Stroke Cerebrovasc Dis. 2011a;20:336–345. doi: 10.1016/j.jstrokecerebrovasdis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell CJ, Privitera MD, Holland SK. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008b;12:74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MP. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5:244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Vannest J, Wu SW, DiFrancesco MW, Banks C, Gilbert DL. Excitatory repetitive transcranial magnetic stimulation induces improvements in chronic post-stroke aphasia. Med Sci Monit. 2011b;17:CR132–139. doi: 10.12659/MSM.881446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Tillema JM, Byars AW, Jacola LM, Schapiro MB, Schmithorst VJ, Szaflarski JP, Holland SK. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105:99–111. doi: 10.1016/j.bandl.2007.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers CA, Vink M, van Zandvoort MJ, van der Worp HB, de Haan EH, Kappelle LJ, Ramsey NF, Dijkhuizen RM. Contribution of the left and right inferior frontal gyrus in recovery from aphasia. A functional MRI study in stroke patients with preserved hemodynamic responsiveness. Neuroimage. 2010;49:885–893. doi: 10.1016/j.neuroimage.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Vines BW, Norton AC, Schlaug G. Non-invasive brain stimulation enhances the effects of melodic intonation therapy. Front Psychol. 2011;2:230. doi: 10.3389/fpsyg.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from Wernicke’s aphasia: a positron emission tomographic study. Ann Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH. Involvement of the cerebellum in semantic discrimination: an fMRI study. Hum Brain Mapp. 2003;18:208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]