Abstract
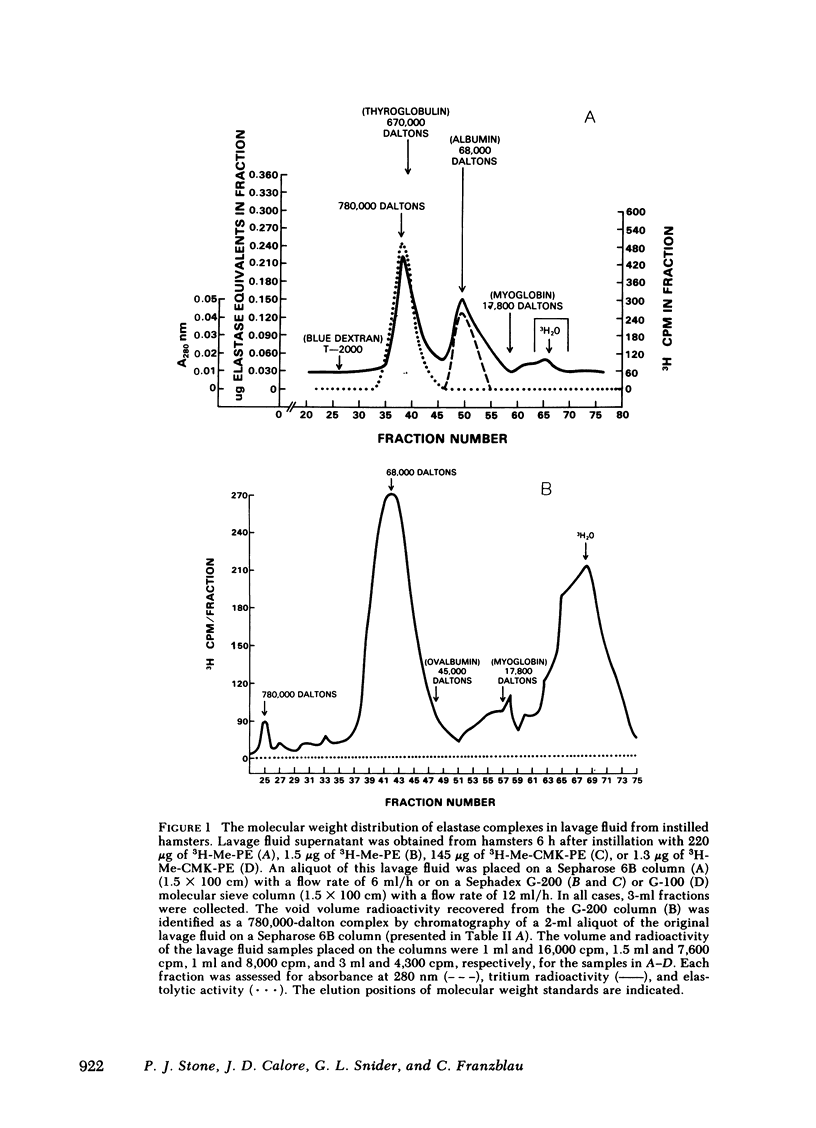
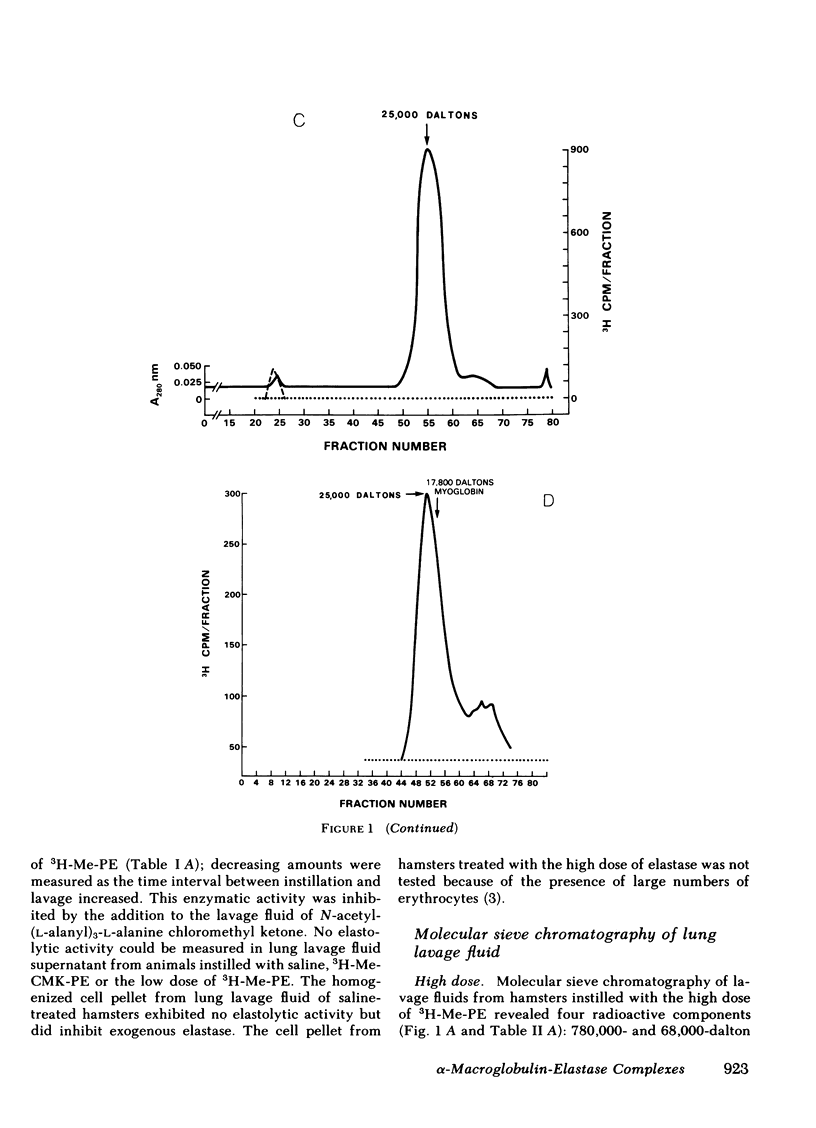
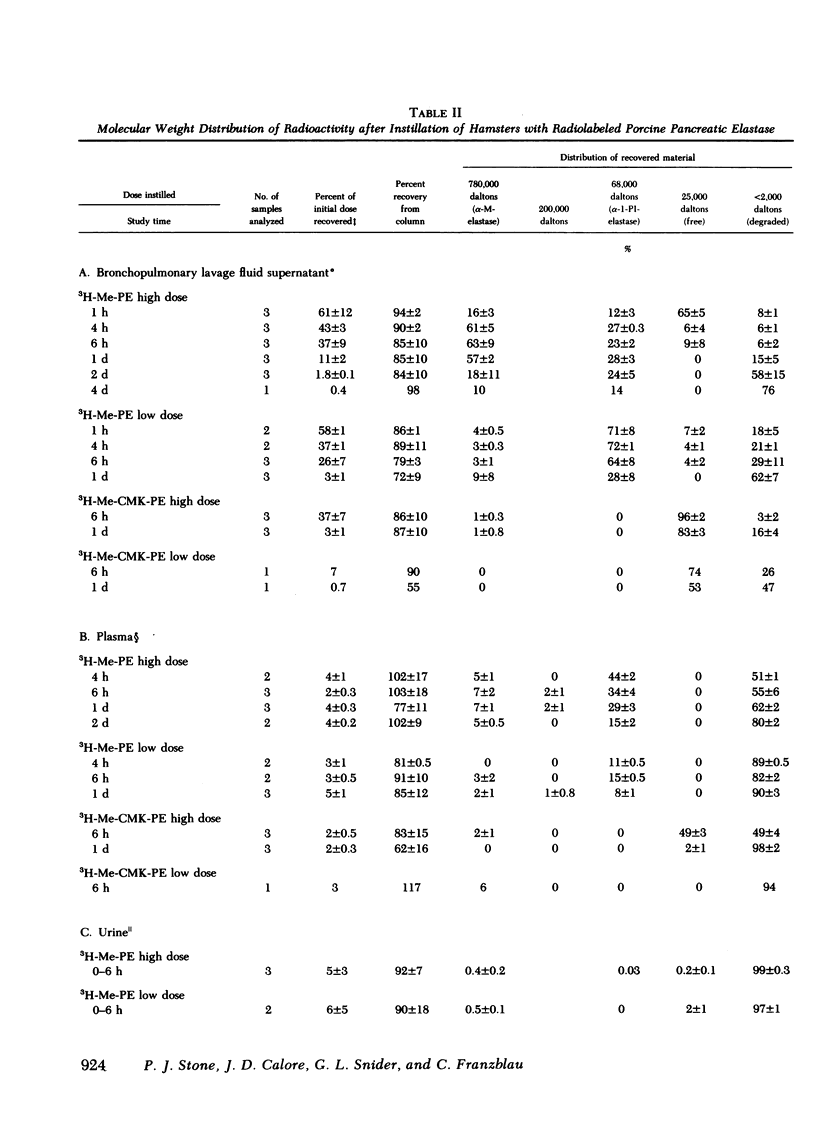
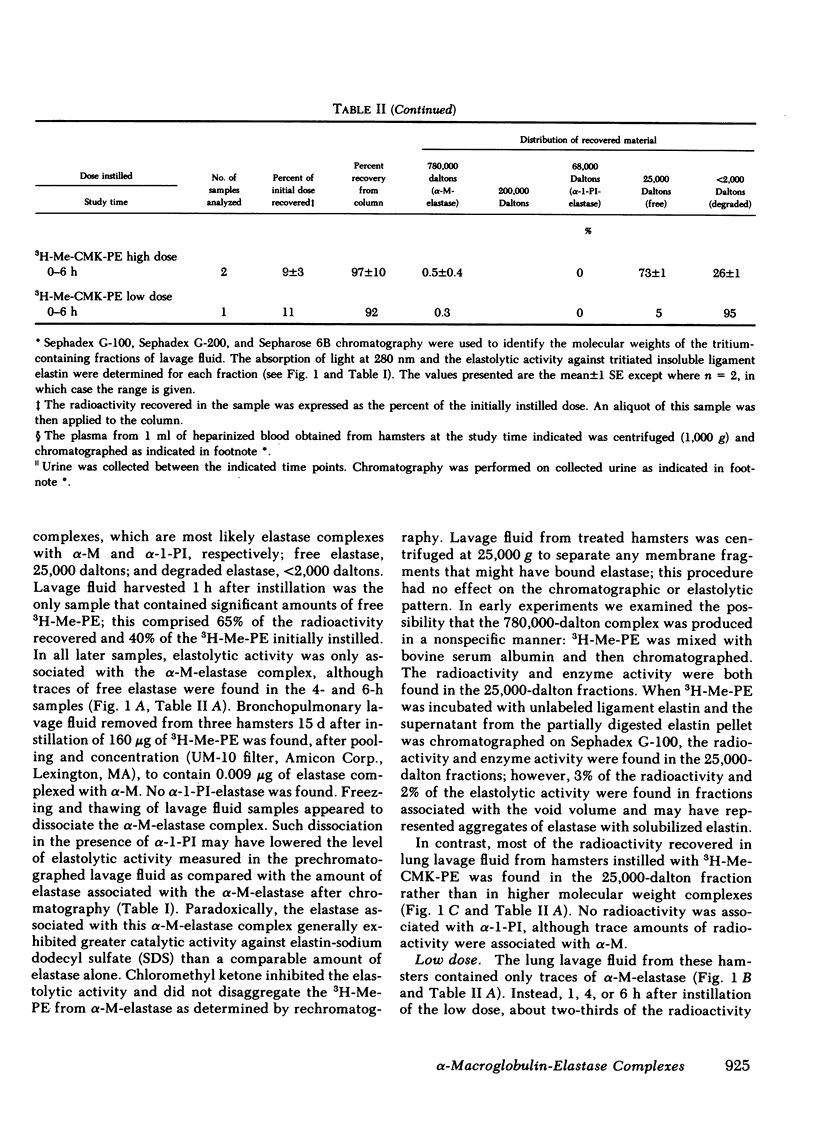
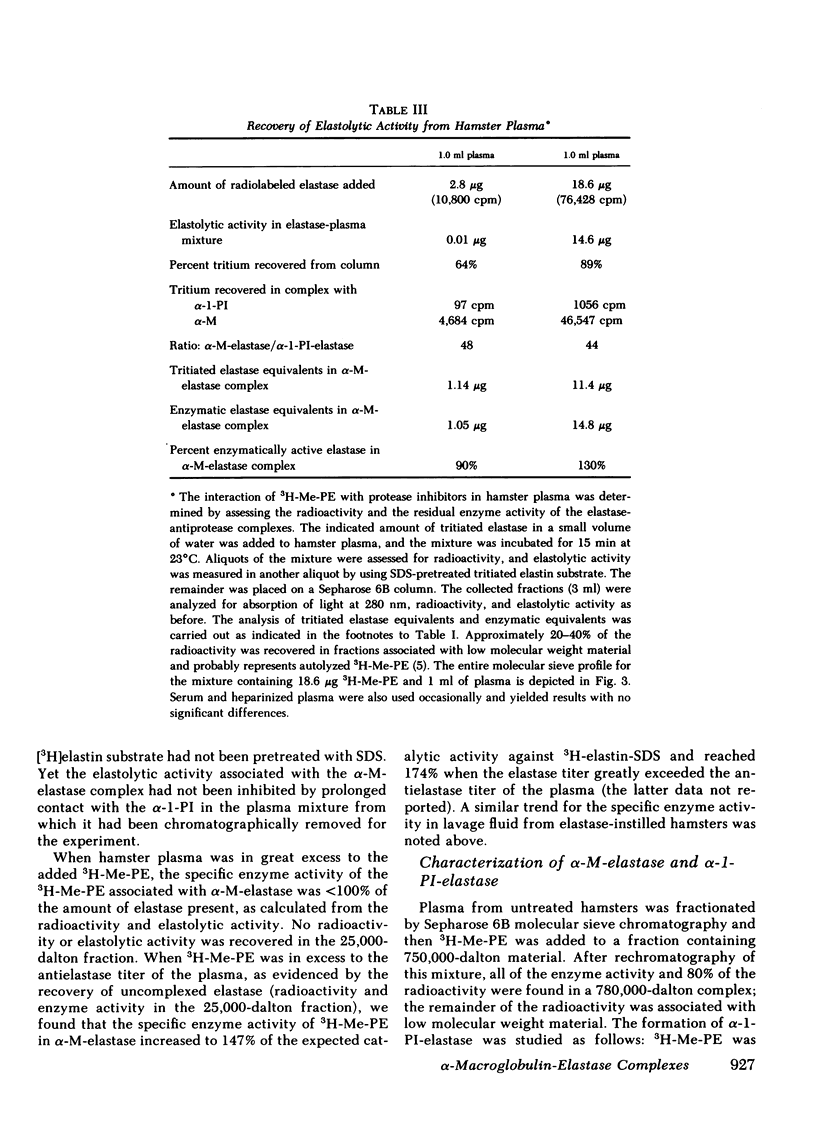
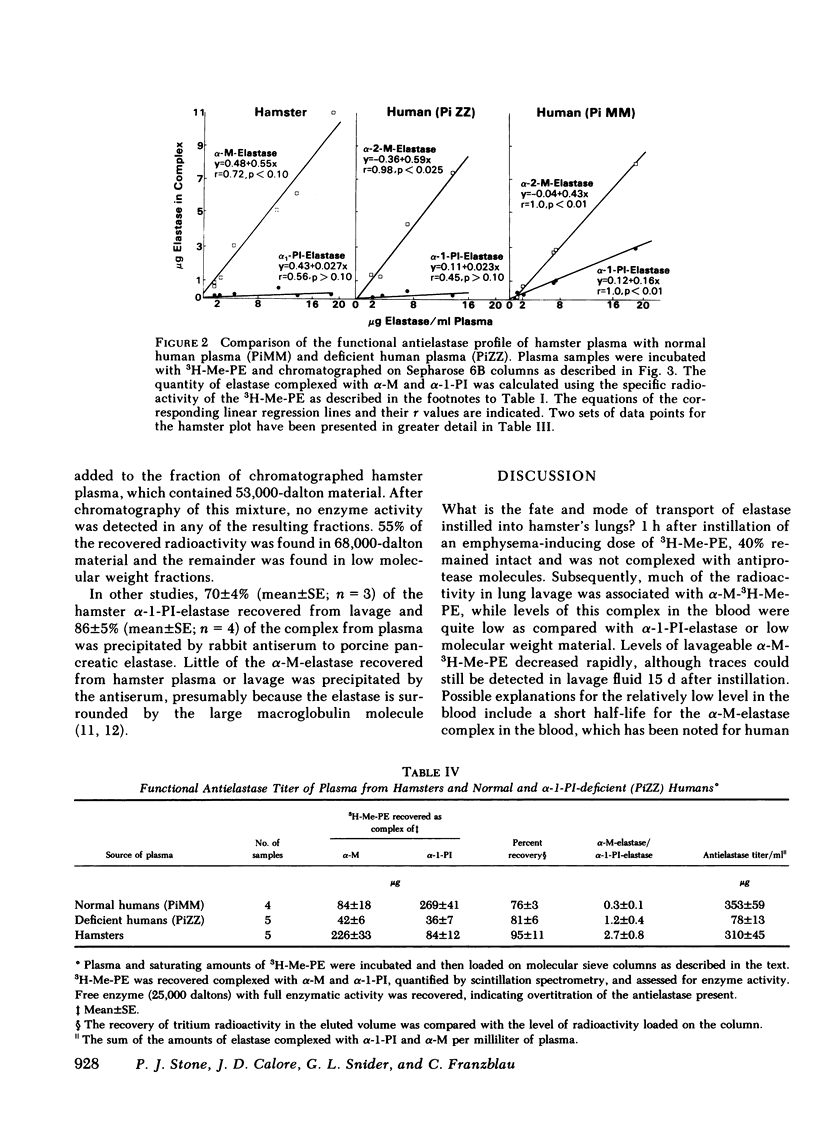
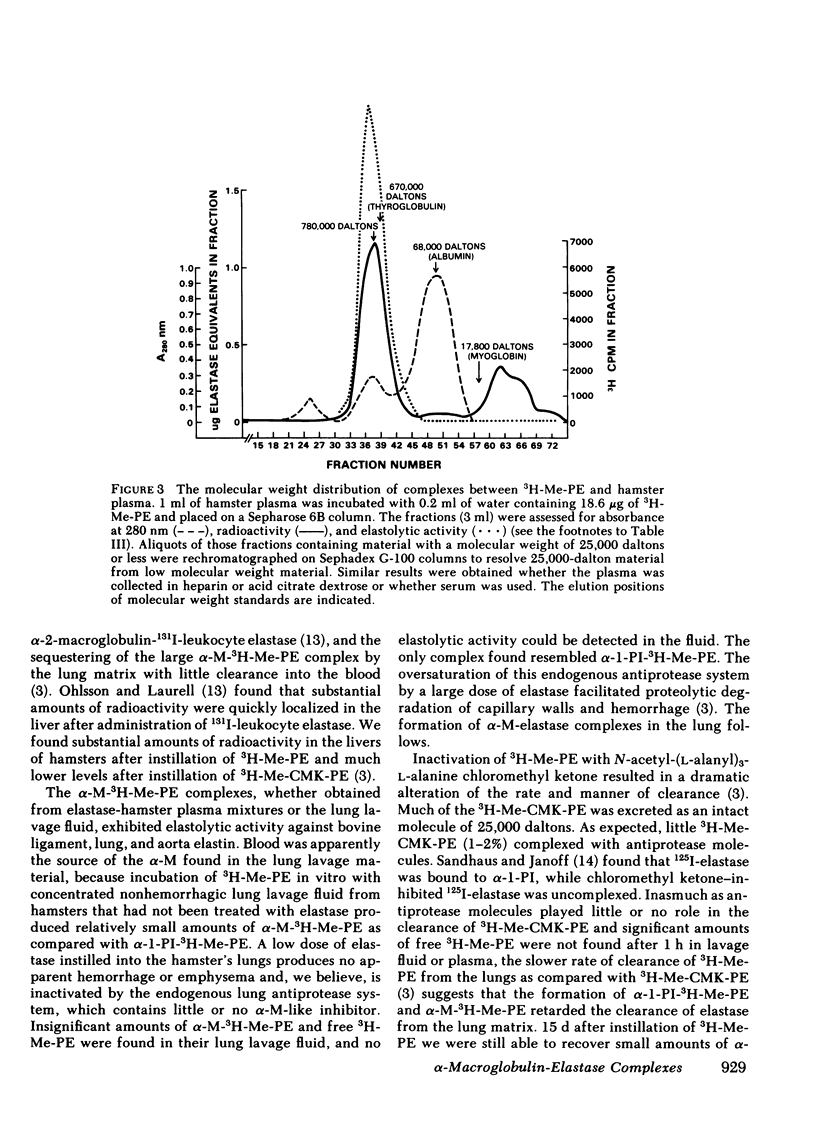
Radiolabeled, enzymatically active or chloromethyl ketone-inactivated porcine pancreatic elastase was endotracheally instilled into hamsters. Gel filtration of the bronchopulmonary lavage fluid revealed two major radioactive fractions: one, eluting at 780,000 daltons, corresponding to an alpha-macroglobulin-pancreatic elastase complex, and another, at 68,000 daltons, corresponding to an alpha-1-protease inhibitor-pancreatic elastase complex. Elastolytic activity was recovered in the bronchopulmonary lavage fluid up to 4 d after elastase instillation and was associated with the alpha-macroglobulin-pancreatic elastase complex. Small amounts of this complex were recovered 14 d after instillation. When less than 1% (1.5--1.7 micrograms) of the usual dose of elastase was instilled into hamsters, the major radioactive complex was alpha-1-protease inhibitor-pancreatic elastase complex, and little or no elastolytic activity was found in the lavage fluid. In contrast to the instillation of 220 micrograms of elastase, no disease or hemorrhagic reaction was detected with this low dose, and without hemorrhage only insignificant amounts of alpha-macroglobulin-pancreatic elastase complexes were recovered from the lungs. To study the interaction of circulating antiproteases with elastase, hamster plasma was allowed to interact directly with the radiolabeled elastase; alpha-macroglobulin bound much more of the elastase than alpha-1-protease inhibitor, confirming the findings in the lung lavage experiments. The hamster's susceptibility to pancreatic elastase-induced emphysema may depend on the preferential binding of elastase to alpha-macroglobulin, which protects the elastolytic potential, rather than to alpha-1-protease inhibitor, which inactivates elastase. We speculate that if even a fraction of the residual radioactivity found in the hamster lungs as long as 144 d after instillation of elastase represents enzymatically active alpha-macroglobulin-pancreatic elastase complex, this could serve as a source of persistent elastolytic activity, which might explain the progressive nature of the pulmonary lesion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumstark J. S. Studies on the elastase-serum protein interaction. I. Molecular identity of the inhibitors in human serum and direct demonstration of inhibitor-elastase complexes by zone and immunoelectrophoresis. Arch Biochem Biophys. 1967 Mar 20;118(3):619–630. doi: 10.1016/0003-9861(67)90397-9. [DOI] [PubMed] [Google Scholar]
- Campbell E. J., White R. R., Senior R. M., Rodriguez R. J., Kuhn C. Receptor-mediated binding and internalization of leukocyte elastase by alveolar macrophages in vitro. J Clin Invest. 1979 Sep;64(3):824–833. doi: 10.1172/JCI109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiman J. J., van Leuven F., van der Schueren B., van den Berghe H. Immunohistochemical localization of human alpha 2Macroglobulin in connective tissue. Cell Tissue Res. 1980;213(2):301–310. doi: 10.1007/BF00234789. [DOI] [PubMed] [Google Scholar]
- Christner P., Weinbaum G., Sloan B., Rosenbloom J. Degradation of tropoelastin by proteases. Anal Biochem. 1978 Aug 1;88(2):682–688. doi: 10.1016/0003-2697(78)90473-6. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Galdston M., Levytska V., Liener I. E., Twumasi D. Y. Degradation of tropoelastin and elastin substrates by human neutrophil elastase, free and bound to alpha2-macroglobulin in serum of the M and Z (Pi) phenotypes for alpha1-antitrypsin. Am Rev Respir Dis. 1979 Mar;119(3):435–441. doi: 10.1164/arrd.1979.119.3.435. [DOI] [PubMed] [Google Scholar]
- Ihrig J., Kleinerman J., Rynbrandt D. J. Serum antitrypsins in animals. Studies of species variations, components, and the influence of certain irritants. Am Rev Respir Dis. 1971 Mar;103(3):377–389. doi: 10.1164/arrd.1971.103.3.377. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem. 1979 May 25;254(10):4022–4026. [PubMed] [Google Scholar]
- Karlinsky J. B., Snider G. L. Animal models of emphysema. Am Rev Respir Dis. 1978 Jun;117(6):1109–1133. doi: 10.1164/arrd.1978.117.6.1109. [DOI] [PubMed] [Google Scholar]
- Lieberman J. Elastase, collagenase, emphysema, and alpha1-antitrypsin deficiency. Chest. 1976 Jul;70(1):62–67. doi: 10.1378/chest.70.1.62. [DOI] [PubMed] [Google Scholar]
- Morse J. O. Alpha1-antitrypsin deficiency (second of two parts). N Engl J Med. 1978 Nov 16;299(20):1099–1105. doi: 10.1056/NEJM197811162992003. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Laurell C. B. The disappearance of enzyme-inhibitor complexes from the circulation of man. Clin Sci Mol Med. 1976 Jul;51(1):87–92. doi: 10.1042/cs0510087. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. Neutral proteases of human granulocytes. III. Interaction between human granulocyte elastase and plasma protease inhibitors. Scand J Clin Lab Invest. 1974 Dec;34(4):349–355. doi: 10.3109/00365517409049891. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Carroll D. L. Reaction of acyl carbazates with proteolytic enzymes. Biochem Biophys Res Commun. 1975 Nov 17;67(2):639–644. doi: 10.1016/0006-291x(75)90860-8. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Tegner H., Kuhn C., Ohlsson K., Starcher B. C., Pierce J. A. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis. 1977 Sep;116(3):469–475. doi: 10.1164/arrd.1977.116.3.469. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Hayes J. A., Franzblau C., Kagan H. M., Stone P. S., Korthy A. L. Relationship between elastolytic acitivity and experimental emphysema-induced properties of papain preparations. Am Rev Respir Dis. 1974 Sep;110(3):254–262. doi: 10.1164/arrd.1974.110.3.254. [DOI] [PubMed] [Google Scholar]
- Snider G. L., Sherter C. B., Koo K. W., Karlinsky J. B., Hayes J. A., Franzblau C. Respiratory mechanics in hamsters following treatment with endotracrael elastase or collagenase. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):206–215. doi: 10.1152/jappl.1977.42.2.206. [DOI] [PubMed] [Google Scholar]
- Stone P. J., Calore J. D., Snider G. L., Franzblau C. The dose-dependent fate of enzymatically active and inactivated tritiated methylated pancreatic elastase administered intratracheally in the hamster. Am Rev Respir Dis. 1979 Sep;120(3):577–587. doi: 10.1164/arrd.1979.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone P. J., Crombie G., Franzblau C. The use of tritiated elastin for the determination of subnanogram amounts of elastase. Anal Biochem. 1977 Jun;80(2):572–577. doi: 10.1016/0003-2697(77)90680-7. [DOI] [PubMed] [Google Scholar]



