Abstract
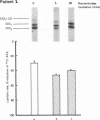
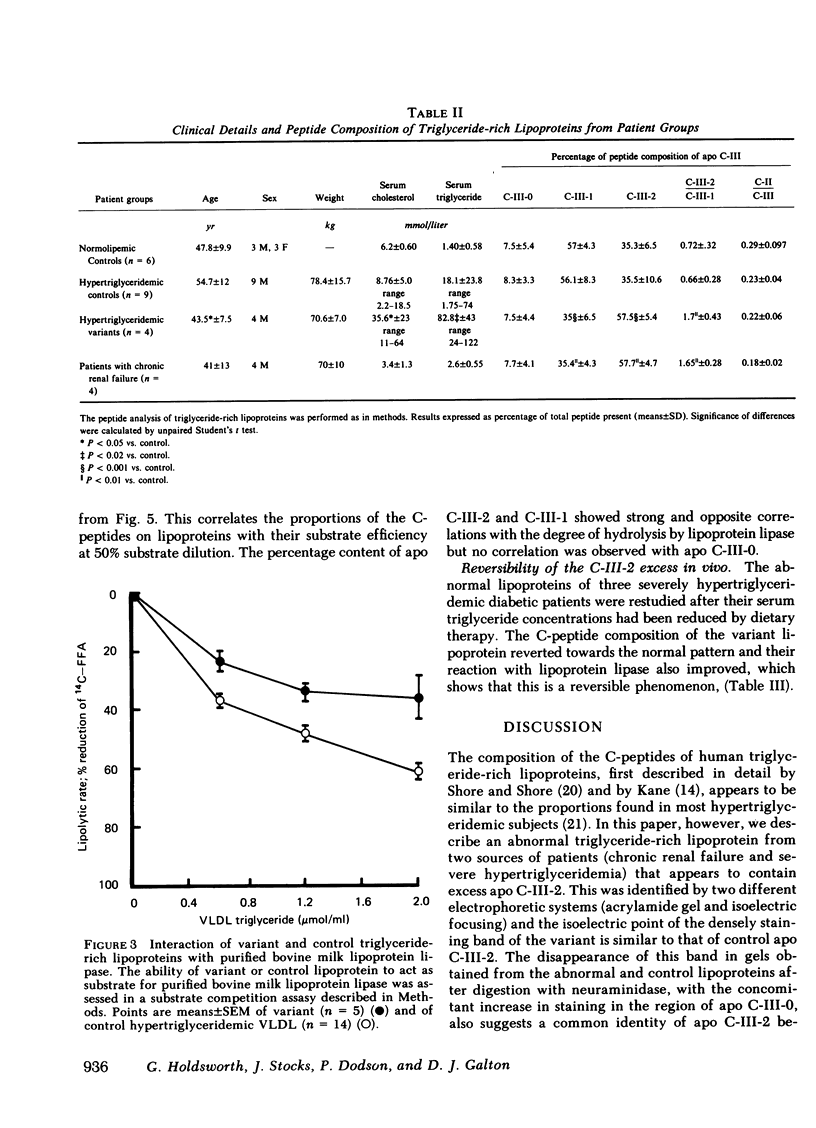
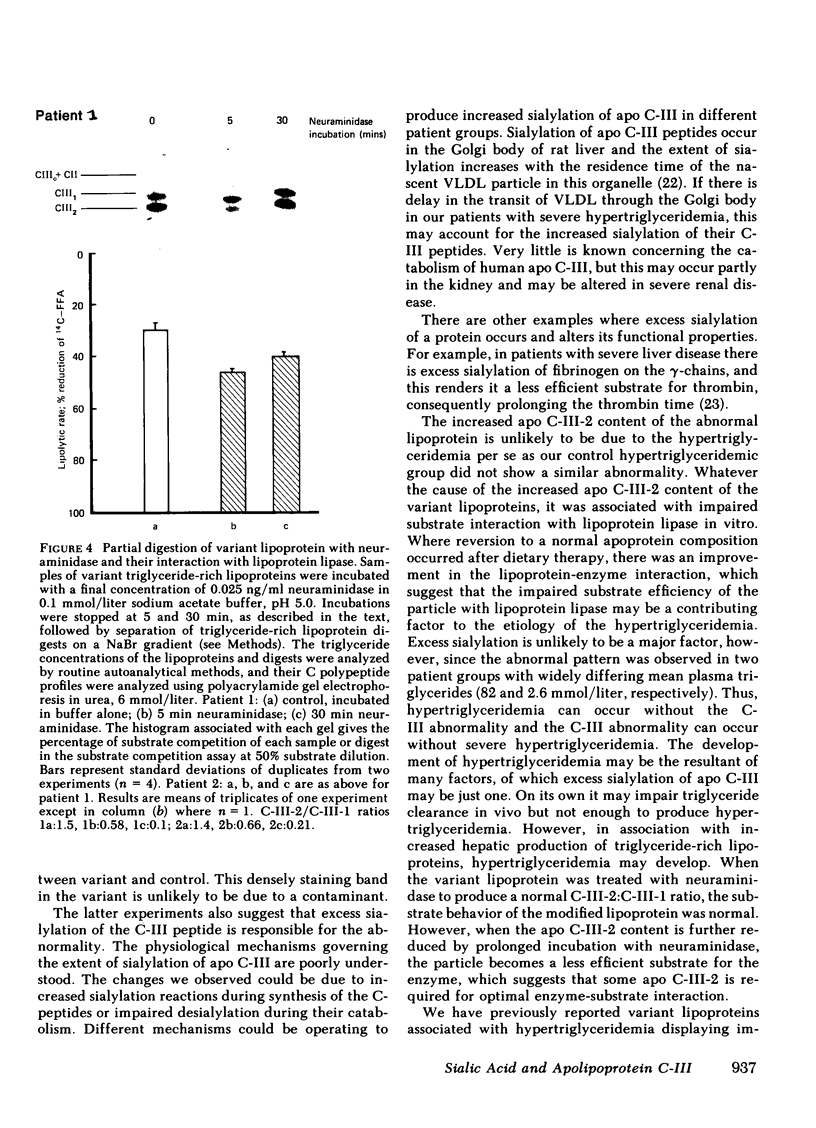
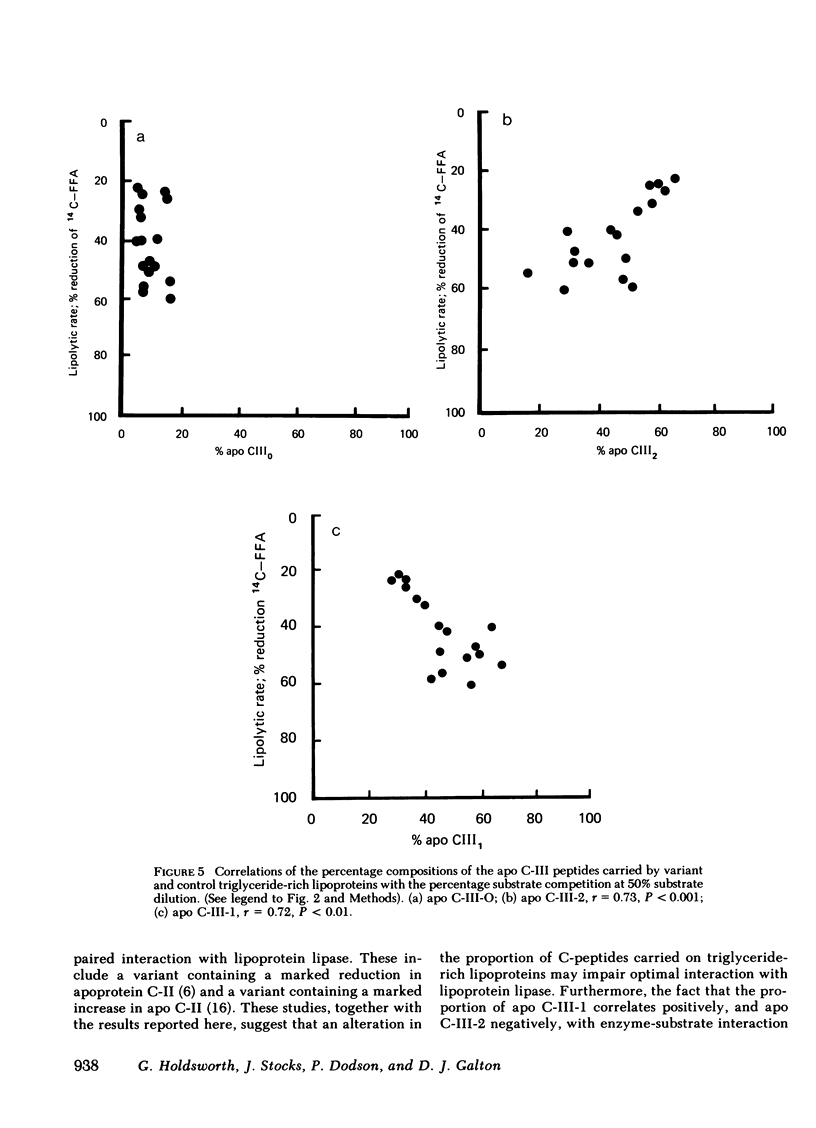
An abnormal triglyceride-rich lipoprotein has been isolated from some patients with chronic renal failure or severe hypertriglyceridemia. The abnormal lipoprotein was characterized by an increased content of apolipoprotein (apo) C-III-2 (57.5% of total apo C-III peptides compared with 35.5% for controls, P less than 0.001) as characterized by isoelectric focusing and scanning densitometry. As determined by a substrate competition assay, the abnormal lipoprotein was a less efficient substrate for purified bovine milk lipoprotein lipase than control lipoproteins. Neuraminidase digestion of abnormal or control lipoprotein resulted in a reduction of the apo C-III-2 band with a corresponding increase in the region of apo C-III-0, which suggests that the increased content of apo C-III-2 in the abnormal is due to excessive sialylation of the C-III peptide. Limited incubation of the abnormal lipoproteins with neuraminidase caused a partial loss of sialic acid and resulted in a triglyceride-rich lipoprotein with a normal C-III-2:C-III-1 ratio. This preparation displayed normal substrate interaction with lipoprotein lipase. Three severely hypertriglyceridemic patients with the abnormal lipoprotein showed a marked reduction in serum triglyceride concentration, which is associated with a reversion to a normal C-peptide profile after dietary therapy. The results suggest that the extent of sialylation of the apo C-III peptide carried on triglyceride-rich lipoproteins may be critical for their interaction with lipoprotein lipase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-On H., Roheim P. S., Eder H. A. Serum lipoproteins and apolipoproteins in rats with streptozotocin-induced diabetes. J Clin Invest. 1976 Mar;57(3):714–721. doi: 10.1172/JCI108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Breckenridge W. C., Little J. A., Steiner G., Chow A., Poapst M. Hypertriglyceridemia associated with deficiency of apolipoprotein C-II. N Engl J Med. 1978 Jun 8;298(23):1265–1273. doi: 10.1056/NEJM197806082982301. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Baginsky M. L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972 Jan 31;46(2):375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- Carlson L. A., Ballantyne D. Changing relative proportions of apolipoproteins CII and CIII of very low density lipoproteins in hypertriglyceridaemia. Atherosclerosis. 1976 May-Jun;23(3):563–568. doi: 10.1016/0021-9150(76)90016-2. [DOI] [PubMed] [Google Scholar]
- Cramp D. G., Robertson G. The fluorometric assay of triglyceride by a semiautomated method. Anal Biochem. 1968 Oct 24;25(1):246–251. doi: 10.1016/0003-2697(68)90097-3. [DOI] [PubMed] [Google Scholar]
- Durrington P. N., MacIver J. E., Holdsworth G., Galton D. J. Severe hypertriglyceridemia associated with pancytopenia and lipoprotein lipase deficiency. Ann Intern Med. 1981 Feb;94(2):211–212. doi: 10.7326/0003-4819-94-2-211. [DOI] [PubMed] [Google Scholar]
- Falko J. M., Schonfeld G., Witztum J. L., Kolar J. B., Salmon P. Effects of short-term high carbohydrate, fat-free diet on plasma levels of Apo C-II and Apo C-III and on the Apo C subspecies in human plasma lipoproteins. Metabolism. 1980 Jul;29(7):654–661. doi: 10.1016/0026-0495(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Harlan W. R., Jr, Winesett P. S., Wasserman A. J. Tissue lipoprotein lipase in normal individuals and in individuals with exogenous hypertriglyceridemia and the relationship of this enzyme to assimilation of fat. J Clin Invest. 1967 Feb;46(2):239–247. doi: 10.1172/JCI105526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton R. H., Al-Tamer Y., Mallinson A., Marks V. The use of density gradient centrifugation for the separation of serum lipoproteins. Clin Chim Acta. 1974 Jun 28;53(3):355–360. doi: 10.1016/0009-8981(74)90275-7. [DOI] [PubMed] [Google Scholar]
- Holdsworth G., Taylor K. G., Galton D. J. Lipoprotein lipase activity in human adipose tissue during induced hypertriglyceridaemia. Atherosclerosis. 1979 Jun;33(2):253–258. doi: 10.1016/0021-9150(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Kane J. P. A rapid electrophoretic technique for identification of subunit species of apoproteins in serum lipoproteins. Anal Biochem. 1973 Jun;53(2):350–364. doi: 10.1016/0003-2697(73)90081-x. [DOI] [PubMed] [Google Scholar]
- Martinez J., Palascak J. E., Kwasniak D. Abnormal sialic acid content of the dysfibrinogenemia associated with liver disease. J Clin Invest. 1978 Feb;61(2):535–538. doi: 10.1172/JCI108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestruck A. C., Rubinstein D. The synthesis of apoproteins of very low density lipoproteins isolated from the Golgi apparatus of rat liver. Can J Biochem. 1976 Jul;54(7):617–628. doi: 10.1139/o76-091. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem. 1971 Dec;44(2):576–588. doi: 10.1016/0003-2697(71)90247-8. [DOI] [PubMed] [Google Scholar]
- Scow R. O., Egelrud T. Hydrolysis of chylomicron phosphatidylcholine in vitro by lipoprotein lipase, phospholipase A2 and phospholipase C. Biochim Biophys Acta. 1976 Jun 22;431(3):538–549. doi: 10.1016/0005-2760(76)90219-8. [DOI] [PubMed] [Google Scholar]
- Shore V. G., Shore B. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry. 1973 Jan 30;12(3):502–507. doi: 10.1021/bi00727a022. [DOI] [PubMed] [Google Scholar]
- Stocks J., Holdsworth G., Dodson P., Galton D. J. An abnormal triglyceride-rich lipoprotein carrying excess apolipoprotein C-II. Atherosclerosis. 1981 Jan-Feb;38(1-2):1–9. doi: 10.1016/0021-9150(81)90097-6. [DOI] [PubMed] [Google Scholar]
- Stocks J., Holdsworth G., Galton D. Hypertriglyceridaemia associated with an abnormal triglyceride-rich lipoprotein carrying excess apolipoprotein C-III-2. Lancet. 1979 Sep 29;2(8144):667–671. doi: 10.1016/s0140-6736(79)92068-3. [DOI] [PubMed] [Google Scholar]
- Vaith P., Assmann G., Uhlenbruck G. Characterization of the oligosaccharide side chain of apolipoprotein C-III from human plasma very low density lipoproteins. Biochim Biophys Acta. 1978 Jun 15;541(2):234–240. doi: 10.1016/0304-4165(78)90396-3. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. 1978 Dec;27(12):1215–1229. doi: 10.2337/diab.27.12.1215. [DOI] [PubMed] [Google Scholar]