Abstract
Objective
To measure the changes in whole blood fatty acid levels in premature infants and evaluate their association with neonatal morbidities.
Study design
Retrospective cohort study of 88 infants born at < 30 weeks of gestation. Serial fatty acid profiles during the first postnatal month and infant outcomes, including chronic lung disease (CLD), retinopathy of prematurity (ROP), and late-onset sepsis, were analyzed. Regression modeling was applied to determine the association between fatty acid levels and neonatal morbidities.
Results
DHA and AA levels declined rapidly in the first postnatal week with a concomitant increase in linoleic acid (LA) levels. Decreased DHA levels were associated with an increased risk of CLD (OR 2.5; 95% CI 1.3 – 5.0). Decreased AA levels were associated with an increased risk of late-onset sepsis (hazard ratio 1.4; 95% CI 1.1 – 1.7). The balance between fatty acids was also a predictor of CLD and late-onset sepsis. An increased LA:DHA ratio was associated with an increased risk of CLD (OR 8.6; 95% CI 1.4 – 53.1) and late-onset sepsis (hazard ratio 4.6; 95% CI 1.5 – 14.1).
Conclusion
Altered postnatal fatty acid levels in premature infants are associated with an increased risk of CLD and late-onset sepsis.
Keywords: fatty acid, docosahexaenoic acid, arachidonic acid, premature infant, infant nutrition, chronic lung disease, late-onset sepsis
Long chain polyunsaturated fatty acids (LCPUFAs) are critical for infant health and neurodevelopment, in particular, the n-3 fatty acid docosahexaenoic acid (DHA), 22:6n-3 and the n-6 fatty acid, arachidonic acid (AA), 20:4n-6. During the third trimester, there is selective placental transfer of these LCPUFAs which substantially increases the levels of these fatty acids in the fetal circulation to meet the demands of the rapidly developing brain and retina.1–3
In addition to their effects on the development of neural tissues, LCPUFAs are also important modulators of inflammation. Studies in animals suggest that the balance of these fatty acids may be important in the development of common neonatal morbidities such as retinopathy of prematurity (ROP)4 and necrotizing enterocolitis (NEC).5
The premature infant is at risk of inadequate accrual of these important fatty acids due to a shortened gestation (early cessation of placental transfer). After delivery, the premature infant becomes dependent on external sources for its nutritional requirements including delivery of LCPUFAs. Additionally, premature infants may be limited in their ability to convert “essential”, precursor fatty acids (i.e., α-Linolenic acid or ALA, 18:3n-3 and Linoleic acid or LA, 18:2n-6) to DHA and AA, respectively, due to reduced levels and activity of desaturase enzymes.6–11 Thus, supplementation of these precursor fatty acids from current soybean oil-based lipid emulsions may not provide normal levels of the downstream fatty acids. 10, 12–16 Therefore, we tested the hypothesis that decreased DHA and AA levels in premature infants are associated with neonatal morbidities.
METHODS
This is a retrospective, cohort study using samples from premature infants serially enrolled in a clinical biorepository in the neonatal intensive care unit (NICU) at Beth Israel Deaconess Medical Center in Boston, MA. Institutional Review Board approval was obtained at Beth Israel Deaconess Medical Center. Exclusion criteria to the clinical biorepository included infants who expired within 48 hours of age, required transfer outside of the hospital immediately after birth, or were born to mothers with limited proficiency in English. Selection criteria for this study included all infants born between 24 and 29 6/7 weeks of gestation from February 2009 to March 2010 (the first full year of the biorepository). A total of 88 infants were selected, which represented 99% of all infants born at less than 30 weeks of gestation (n=89) admitted to the NICU during the study period. Additionally, blood samples were collected at the time of birth from 10 term infants to define birth levels for each of the critical fatty acids at term gestation; an indicator of the levels that the premature infant would have been exposed to throughout advancing gestation if the infant had remained in utero.
Maternal and Infant Clinical Data
Maternal and infant clinical data were collected from the electronic medical record. Maternal data included age, gravida, parity, and race. Baseline infant characteristics included gestational age, birth weight, sex, and route of delivery. Gestational age in completed weeks was determined using the best obstetric estimate as documented in the medical record. The Score for Neonatal Acute Physiology-II (SNAP-II) was used to estimate severity of illness on the day of birth.17
Detailed nutritional data and weights were collected daily for the first 28 postnatal days. Type and volume of intravenous solutions including composition of total parenteral nutrition and soybean oil-based emulsion, e.g., Intralipid™; and, type and volume of enteral feedings were recorded. Total fat intake represents both parenteral and enteral fat intake expressed as grams per kilogram per day (gm/kg/day).
Clinical Outcomes
Chronic lung disease (CLD) was defined as requiring supplemental oxygen at 36 weeks’ postmenstrual age. If discharge or transfer occurred near 36 weeks postmenstrual age and the infant was still receiving oxygen, the infant was classified as having CLD (n=2, 2 days and 8 days prior to their 36 weeks postmenstrual age). The examining ophthalmologist determined the presence of any stage of ROP by serial eye exams beginning at 4 to 6 weeks of age. Early-onset sepsis was defined as a positive blood culture within the first three postnatal days. Late-onset sepsis was defined as a positive blood culture any time after the third postnatal day. The presence of any intraventricular hemorrhage or cysts in the periventricular region (periventricular leukomalacia) was based on head ultrasound. Necrotizing enterocolitis was defined by the presence of pneumatosis on abdominal radiograph or by the clinical spectrum of bloody stools, abnormal abdominal exams, and a change in clinical status resulting in withholding of enteral feedings and provision of systemic antibiotics for 10 days or greater.
Whole Blood Sample Collection and Selection for Fatty Acid Analysis
Discarded whole blood samples were collected daily, when available, throughout the infant’s stay in the neonatal intensive care unit and stored at −80°C until analyzed. Five time points were examined: birth (sample drawn on the day of birth or postnatal day 1) and postnatal weeks 1, 2, 3, and 4. Samples to represent each postnatal week were selected closest to the time point of interest +/− 3 days. 82 of the 88 (93%) infants contributed at least one sample from the biorepository; 68% of the cohort provided a birth sample; and 90% provided at least one blood sample at week 1 or later.
Fatty Acid Isolation and Quantification of Fatty Acid Profiles from Whole Blood
Fatty acids from whole blood were isolated and methylated using a modified Folch method as previously described.18 Gas chromatography-mass spectroscopy analysis of fatty acids was performed on a Hewlett-Packard Series II 5890 chromatograph coupled to an HP-5971 mass spectrometer equipped with a supelcowax SP-10 capillary column. Peak identification was based upon comparison of both retention time and mass spectra of unknown peak to that of known standards within the GC-MS database library. FAME mass was determined by comparing areas of unknown FAMEs to that of a fixed concentration of 17:0 internal standard. Individual fatty acids are expressed as percent of the total fatty acid mass (mol%).
Statistical Analyses
Population characteristics are expressed as either means ± standard deviations (SD), medians and interquartile ranges (IQR), or a proportion (%) as determined by the variable type. Fatty acid levels are expressed as mean mol % ± standard error of the mean (SE). Comparisons of mean fatty acid levels were analyzed using the Student t-test.
Logistic regression models were used to determine the association (odds ratios with 95% confidence intervals) between fatty acid levels or fatty acid ratios and the most common neonatal morbidities of CLD and ROP (expressed as dichotomous outcomes). The Cox proportional hazards model with time-varying covariates was used to evaluate the association (hazard ratio with 95% confidence intervals) between fatty acid levels or fatty acid ratios and late-onset sepsis given the need to account for time-varying fatty acid exposures prior to the diagnosis of sepsis. Both models were adjusted for the potential confounding factors of gestational age, intrauterine growth restriction (birth weight z-score < −2), severity of illness, sex, and fat intake; in addition, for both models, the fatty acid ratios were log transformed to allow for parametric testing. All analyses were performed using STATA statistical software, version 11 (StataCorp) and SAS statistical software, version 9.1 (SAS, Inc.).
RESULTS
Baseline Infant and Maternal Characteristics
The mean gestational age was 27.1 weeks, mean birth weight was 957 grams, and 58% of the cohort was male (Table I). 74% of infants were delivered by Cesarean section. The mean maternal age was 31.2 years with a median gravida status of 2 and a parity of 0.
Table 1.
Infant and Maternal Characteristics and Neonatal Outcomes
| Characteristics | |
|---|---|
| Infant Characteristics | |
|
| |
| Gestational age, weeks, mean ± SD (range) | 27.1 ± 1.8 (24–29) |
| Birthweight, grams, mean ± SD (range) | 957 ± 272 (350–1515) |
| Gender, male, n (%) | 51 (58) |
| Cesarean section, n (%) | 65 (74) |
|
| |
| Maternal Characteristics | |
|
| |
| Age, years, mean ± SD (range) | 31.2 ± 6.9 (16–45) |
| Gravida, median (IQR) | 2 (1–8) |
| Para, median (IQR) | 0 (0–6) |
| Race (%) | |
| White | 49 |
| Black | 22 |
| Other | 21 |
| Unknown | 8 |
|
| |
| Neonatal Outcomes | n (%) |
|
| |
| Mortality | 10 (11) |
| ROP* | 33 (45) |
| CLD* | 38 (48) |
| Sepsis | 18 (20) |
| Intraventricular hemorrhage | 31 (35) |
| Periventricular leukomalacia | 3 (3) |
| NEC | 5 (6) |
N=88
some infants with unknown outcome due to early transfer or death, % = of those with known outcome; ROP 73/88 or 83% of entire cohort with known outcome; CLD 80/88 or 91% of entire cohort with known outcome
Weekly Infant Nutritional Intake and Growth
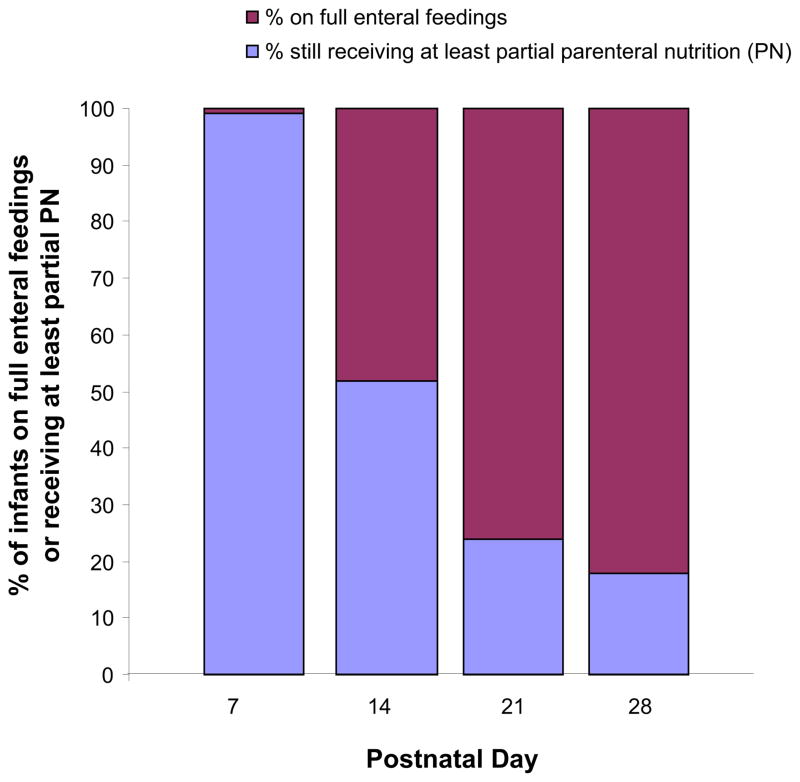
All infants were started on total parenteral nutrition on the day of birth and with lipid emulsion via a separate infusion by postnatal day two (Table II; available at www.jpeds.com). Enteral feedings were administered by gavage, typically begun by 3 days of age, with steady advancement in the daily total volume delivered. During advancement of enteral feedings, the total amount of parenteral nutrition and lipid emulsion administered was progressively decreased (Figure 1; available at www.jpeds.com). The median day at which full volume feedings were attained (a minimum of 120 mL/kg/day) was 14.5 days. At two weeks of age, over half of the infants were still receiving some parenteral nutrition to meet their nutritional goals. The weekly nutritional intake and growth were similar to that reported in a large cohort of premature infants of similar gestational age.19
Table 2.
Weekly Nutritional Intake and Growth
| Nutritional Intake Variables | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|
| Total volume (mL/kg/day), mean ± SD | 153 ± 22 | 145 ± 16 | 143 ± 13 | 141 ± 14 |
| Diet | ||||
| % of infants receiving some parenteral nutrition | 99 | 52 | 24 | 18 |
| % of infants on full enteral feedings* | 1 | 48 | 76 | 82 |
| % of infants fed only breast milk | 38 | 61 | 66 | 59 |
| % of infants fed breast milk and formula | 39 | 27 | 22 | 27 |
| % of infants fed only formula | 23 | 12 | 12 | 14 |
| Fat Intake (gm/kg/day), mean ± SD | ||||
| Total fat intake | 3.7 ± 2.0 | 5.2 ± 1.9 | 6.6 ± 2.1 | 6.7 ± 2.4 |
| IntraLipid | 2.3 ± 2.0 | 0.8 ± 1.2 | 0.4 ± 1.2 | 0.3 ± 0.8 |
| Enteral | 1.4 ± 1.6 | 4.5 ± 2.7 | 6.2 ± 2.8 | 6.4 ± 3.0 |
| Growth Velocity (gm/kg/day), mean ± SD | −10.3 ± 10.8 | 15.6 ± 6.5 | 17.3 ± 7.5 | 18.0 ± 6.1 |
Median day to full volume feedings (IQR) = 14.5 (12 – 18); SD, standard deviation
Figure 1.
Proportion of infants on full enteral feedings versus those still receiving at least partial parenteral nutrition
Clinical Outcomes
The mortality rate for the entire cohort was 11% (Table I). ROP was present in 45% of the cohort, CLD 48%, sepsis (early or late) 20%, intraventricular hemorrhage 35%, periventricular leukomalacia 3%, and necrotizing enterocolitis 6%. Late-onset sepsis occurred in 16 of 88 (18%) infants, 1 (6%) had late-onset sepsis in the first postnatal week; 5 (31%) in the second postnatal week; 6 (38%) in the third postnatal week; and, 4 (25%) in the fourth postnatal week. In general, and as expected, the prevalence of neonatal morbidities decreased with advancing gestational age (data not shown). These rates are within ranges previously reported in the literature.20
Critical Fatty Acid Levels at Birth
At birth, the mean mol% ± SE (standard error) for LA was 7.0 ± 0.2, 17.2 ± 0.2 for AA, and 6.9 ± 0.2 for DHA with little variation across the gestational age span for our cohort (Table III; available at www.jpeds.com). The mean values of each of these fatty acids were similar to values obtained from a cohort (n=10) of term infants (gestational age ≥ 37 weeks of gestation) recruited during the same time period. The fact that these levels were similar across the gestational age ranges and to our term cohort suggests that these mean values reflect levels in the blood of the developing fetus throughout the third trimester had the infant not been born prematurely; i.e., reflecting in utero levels and thus providing a range of values in which the premature infant ideally should be maintained during the postnatal period.
Table 3.
Mean fatty acid levels near the time of birth (day 0 or 1) in Preterm (n=60)* and Term (n=10) Infants, expressed as mol%.
| Linoleic Acid (18:2) | Arachidonic Acid (20:4) | Docosahexaenoic Acid (22:6) | ||
|---|---|---|---|---|
|
| ||||
| n | Mean level expressed as mol% ± SE | |||
| Preterm Infants | 60 | 7.0 ± 0.2 | 17.2 ± 0.2 | 6.9 ± 0.2 |
|
| ||||
| By gestational age, week | ||||
| 24 | 9 | 6.8 ± 0.5 | 17.1 ± 0.5 | 6.3 ± 0.3 |
| 25 | 3 | 5.8 ± 0.8 | 18.3 ± 0.9 | 7.6 ± 0.3 |
| 26 | 7 | 6.2 ± 0.6 | 16.2 ± 0.7 | 6.4 ± 0.4 |
| 27 | 11 | 7.4 ± 0.5 | 17.0 ± 0.6 | 6.4 ± 0.5 |
| 28 | 10 | 7.3 ± 0.4 | 17.1 ± 0.3 | 7.4 ± 0.6 |
| 29 | 20 | 7.2 ± 0.4 | 17.7 ± 0.3 | 7.3 ± 0.4 |
|
| ||||
| Term Infants | 10 | 7.9 ± 0.4 | 15.7 ± 0.3 | 7.6 ± 0.6 |
SE, standard error of the mean
60 of the 88 infants provided a birth sample
Alterations in Critical Fatty Acid Levels during the Postnatal Period
Rapid changes in the mean levels of DHA, LA, and AA were observed within the first postnatal week and persisted over the subsequent three weeks (Figure 2). The mean DHA level fell by approximately 40% from 6.9 mol% at birth to 4.4 mol% at week 1, and subsequently remained steady at 4.0 to 4.2 mol% for the remainder of the postnatal period. The mean LA level increased from 7.0 mol% at birth to 18.8 mol% at week 1 and remained level over the subsequent 4 weeks. These postnatal fatty acid changes reflect the infusion of a lipid emulsion which contains very small amount of DHA and AA, but, high concentrations of LA (Table IV; available at www.jpeds.com). AA levels declined by 30% from 17.2 mol% at birth to 11.8 mol% at week 1 and remained constant at 11.4 to 11.6 mol% for the remaining three postnatal weeks. The change in DHA levels from birth (Figure 2, A) and the changes in LA and AA levels from birth (Figure 2, B) reflect two distinct patterns: (1) the initial rapid change from birth to postnatal week 1, and, (2) the relative stability or plateau in levels from week 1 to week 4. Thus for statistical purposes, all levels obtained at week 1 and after were averaged to represent the mean values observed in the follow-up period.
Figure 2.
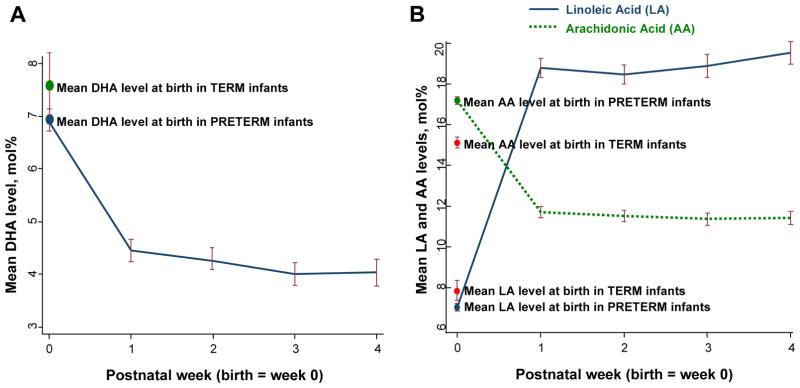
A, DHA levels in preterm infants decrease soon after birth and plateau by the first postnatal week. B, LA levels in preterm infants increase soon after birth, and AA levels decrease soon after birth; both LA and AA plateau by the first postnatal week (B).
Table 4.
Fatty acid composition of IntraLipid emulsion*
| Major fatty acid constituents** | mol% ± SE |
|---|---|
| 16 | 15 ± 0.2 |
| 18 | 8 ± 0.8 |
| 18:1n-9 (oleic acid) | 24 ± 0.4 |
| 18:2n-6 (linoleic acid) | 44 ± 0.5 |
| 18:3n-3 (α-linolenic acid) | 4 ± 0.2 |
|
| |
| Other fatty acid constituents of interest | |
|
| |
| 20:4n-6 (arachidonic acid) | 0.4 ± 0.008 |
| 22:6n-3 (docosahexaenoic acid) | 0.4 ± 0.03 |
SE, standard error of the mean
Fatty acid composition as determined by gas chromatography- mass spectroscopy.
The listed major fatty acids consitituents represent 95% of the entire IntraLipid emulsion composition.
In addition to a 2.7-fold increase in the essential fatty acid 18:2n-6 (LA), both the initial precursor of the n-3 pathway (18:3n-3; α-linolenic acid), and the n-9 pathway (18:1n-9; oleic acid) were also increased (Table V; available at www.jpeds.com) by 19.2 and 1.1-fold, respectively, reflecting the composition of the soybean oil-based lipid emulsion administered during the first two postnatal weeks (Table IV). There was a reduction in the levels of the remaining downstream fatty acids in the n-6 pathway including 20:3n-6, 20:4n-6 (AA), 22:4n-6, and 22:5n-6. In the n-3 pathway, there was an increase in 20:5n-3 (EPA) and 22:5n-3 compared with birth levels, 1.7 and 1.5-fold respectively; but a decrease in the downstream product, 22:6n-3 (DHA) by 1.6-fold.
Table 5.
Fold change in fatty acid levels from birth to the follow-up period (postnatal week 1 through week 4).
| Fatty Acid Biosynthetic Pathways | Fatty Acid | Levels, mean mol% ± SE | Fold Change | ||
|---|---|---|---|---|---|
| Birth | Follow-up | ||||
| Enzyme | n-6 | ||||
|
| |||||
| 18:2 | Linoleic Acid (LA) | 7.0 ± 0.2 | 18.9 ± 0.3 | 2.7 | |
| Δ6 desaturase | ↓ | ||||
| 18:3 | γ-Linoleic Acid | 0.1 ± 0.008 | 0.3 ± 0.01 | 3.1 | |
| elongase | ↓ | ||||
|
|
|||||
| 20:3 | Dihomo-γ-Linoleic Acid | 2.4 ± 0.07 | 1.8 ± 0.04 | −1.3 | |
| Δ5 desaturase | ↓ | ||||
| 20:4 | Arachidonic Acid (AA) | 17.2 ± 0.2 | 11.6 ± 0.2 | −1.5 | |
| elongase | ↓ | ||||
| 22:4 | Adrenic Acid | 2.8 ± 0.08 | 1.7 ± 0.04 | −1.7 | |
| Δ4 desaturase | ↓ | ||||
| 22:5 | n6- Docosapentaenoic Acid | 1.5 ± 0.05 | 0.7 ± 0.02 | −2.0 | |
|
| |||||
| n-3 | |||||
|
| |||||
| 18:3 | α-Linolenic Acid (ALA) | 0.1 ± 0.005 | 0.9 ± 0.05 | 19.2 | |
| Δ6 desaturase, elongase, Δ5 desaturase | ↓ | ||||
| 20:5 | Eicosapentaenoic Acid (EPA) | 0.2 ± 0.02 | 0.3 ± 0.01 | 1.7 | |
| elongase | ↓ | ||||
| 22:5 | Docosapentaenoic Acid | 0.6 ± 0.04 | 0.8 ± 0.03 | 1.5 | |
| Δ4 desaturase | ↓ | ||||
|
|
|||||
| 22:6 | Docosahexaenoic Acid (DHA) | 6.9 ± 0.2 | 4.2 ± 0.1 | −1.6 | |
|
| |||||
| n-9 | |||||
|
| |||||
| 18:1 | Oleic Acid | 15.8 ± 0.3 | 18.1 ± 0.2 | 1.1 | |
| Δ6 desaturase, elongase, Δ5 desaturase | ↓ | ||||
|
|
|||||
| 20:3 | Mead Acid | 0.7 ± 0.05 | 0.4 ± 0.02 | −2.0 | |
|
| |||||
| Fatty Acid by Class (%) | Birth | Follow-up | Fold Change | ||
|
| |||||
| Saturates | 38 | 35 | −0.9 | ||
| Monounsaturates | 22 | 23 | 1.1 | ||
| Polyunsaturates | 40 | 42 | 1.1 | ||
SE, standard error of the mean
Whole Blood Critical Fatty Acid Levels and Associations with Neonatal Morbidities
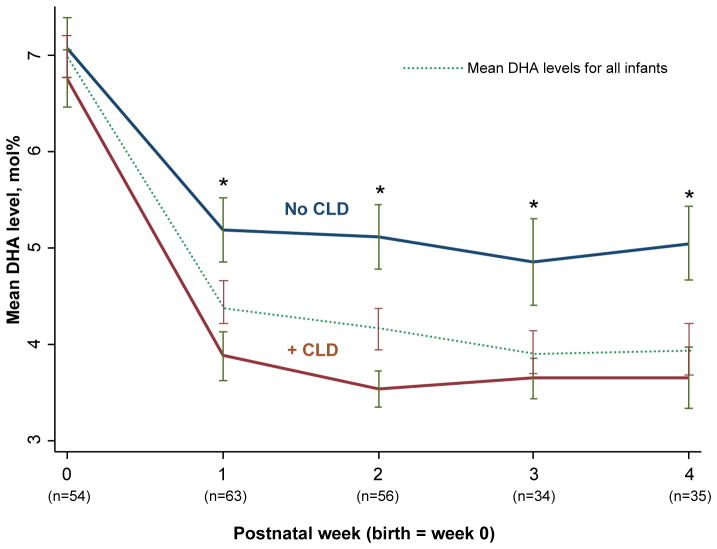
80 of 88 (91%) infants were assessed for the presence of CLD. No differences in mean DHA levels at birth were seen in infants with or without CLD (data not shown). However, in the follow-up period, infants who developed CLD had lower mean DHA levels compared with infants without CLD (Figure 3). To assess the association between DHA levels and the risk of developing CLD, logistic regression modeling was performed while adjusting for potential confounders (Table VI). Lower DHA levels were significantly associated with the development of CLD and remained significant even after adjustment for gestational age, intrauterine growth restriction, severity of illness at birth, sex, and total fat intake during the first postnatal week (p=0.001). For every 1 mol% reduction in whole blood DHA levels, there was a 2.5-fold increase in the odds of developing CLD (CI 1.3 – 5.0). LA and AA birth and follow-up levels were not associated with the development of CLD.
Figure 3.
Mean docosahexaenoic acid (DHA) levels are higher throughout the first postnatal month in infants without chronic lung disease (CLD) compared with levels in infants who developed CLD. * All p values ≤ 0.02 comparing the mean DHA level in each postnatal week in infants with and without CLD. Bars represent the standard error of the mean (SE).
Table 6.
Adjusted logistic regression models for individual critical fatty acid levels and fatty acid ratios on the odds of Chronic Lung Disease (CLD) and Retinopathy of Prematurity and Cox proportional hazard model for individual critical fatty acid levels and fatty acid ratios on the risk of late-onset sepsis*
| CLD | OR (95% CI) | p |
|---|---|---|
| Linoleic Acid | 0.9 (0.7, 1.1) | 0.4 |
| Arachidonic Acid | 0.9 (0.6, 1.3) | 0.6 |
| Docosahexaenoic Acid | 2.5 (1.3, 5.0) | 0.001 |
| LA: DHA | 8.6 (1.4, 53.1) | 0.02 |
| AA: DHA | >999 (12.0, >999) | 0.003 |
| n6: n3 | 156.0 (3.7, >999) | 0.008 |
|
| ||
| ROP | ||
|
| ||
| Linoleic Acid | 0.9 (0.7, 1.1) | 0.3 |
| Arachidonic Acid | 1.0 (0.7, 1.4) | 1.0 |
| Docosahexaenoic Acid | 0.8 (0.4, 1.3) | 0.3 |
| LA: DHA | 0.8 (0.2, 4.0) | 0.8 |
| AA: DHA | 0.2 (0.006, 3.7) | 0.2 |
| n6: n3 | 0.2 (0.008, 4.6) | 0.3 |
|
| ||
| Late-onset sepsis | Hazard ratio (95% CI) | p |
|
| ||
| Linoleic Acid | 0.8 (0.7, 0.96) | 0.02 |
| Arachidonic Acid | 1.4 (1.1, 1.7) | 0.02 |
| Docosahexaenoic Acid | 1.4 (1.0, 2.0) | 0.08 |
| LA: DHA | 4.6 (1.5, 14.1) | 0.007 |
| AA: DHA | 3.1 (0.6, 15.6) | 0.2 |
| n6: n3 | 3.3 (0.6, 16.9) | 0.2 |
OR, Odds ratio; CI, Confidence interval
LA, linoleic acid; DHA, docosahexaenoic acid; AA, arachidonic acid; n6, fatty acids present in the n6 fatty acid pathway; n3, fatty acids present in the n3 fatty acid pathway
All models were adjusted for gestational age, intrauterine growth restriction, severity of illness (SNAP-II), gender, and fat intake. The Odds and Hazard Ratios reflect the change in risk for every unit decrease in mol% for the indicated fatty acid or every unit increase in the fatty acid ratio. Final number of infants for each model was as follows: CLD, n=71; ROP, n=66; late-onset sepsis, n=79.
73 of 88 (83%) infants were assessed for the presence of ROP. No differences in the mean DHA levels were observed at birth for infants who developed ROP compared with infants who did not (data not shown). In contrast to CLD, no significant association between DHA levels nor in AA and LA levels in the follow-up period were associated with the development of ROP (Table VI).
To assess the association between a select fatty acid level and the risk of sepsis, we employed a Cox proportional hazard model with the fatty acid defined as the time-varying covariate, namely, the fatty acid level measured before or during the week of sepsis. The model was adjusted for gestational age, intrauterine growth restriction, severity of illness at birth, sex, and total fat intake during the first postnatal week (Table VI). For every unit decrease in DHA mol% at a given time, there was a 1.4-fold increase in the risk of late-onset sepsis (CI 1.0 – 2.0), though this did not meet statistical significance. Unlike CLD and ROP, lower AA levels and higher LA levels were significantly associated with the development of late-onset sepsis with a hazard ratio of 1.4 (CI 1.1 – 1.7; p=0.02) and 1.3 (CI 1.04 – 1.4; p=0.02), respectively.
No differences were observed in mean DHA levels at birth or during the follow-up period in infants who developed early-onset sepsis (n=2), intraventricular hemorrhage (n=31), periventricular leukomalacia (n=3), or NEC (n=5) (data not shown).
Fatty Acid Ratios and their Association with Neonatal Morbidities
Fatty acids in the n-3 and n-6 pathways compete with each other as substrates for enzyme activity as well as for integration into cellular membranes. Therefore, the ratio of specific n-6 to n-3 fatty acids has functional consequences in addition to individual fatty acid levels in influencing disease.18, 21 We determined the influence of several commonly assessed fatty acids ratios (LA:DHA, AA:DHA, and n-6:n-3) on the development of CLD, ROP and late-onset sepsis using the models described previously. Increased LA:DHA, AA:DHA and n-6:n-3 ratios were associated with CLD although the large confidence intervals for the latter two limit their interpretability (Table VI). An increased LA:DHA ratio was associated with late-onset sepsis (p=0.007), and none of the three ratios was associated with ROP (Table VI).
DISCUSSION
Our data demonstrate that the postnatal decline in DHA and AA levels and an elevated LA:DHA ratio are associated with CLD and late-onset sepsis in premature infants. Changes in the mol% of DHA, AA, and LA are significantly altered within the first postnatal week compared with birth levels which likely reflect a marked deviation from the levels in the blood of the developing fetus throughout the third trimester in utero. The fact that CLD was selectively associated with decreases in DHA and late-onset sepsis with selective decreases in LA and AA levels indicates that the risk of these neonatal diseases is not a result of global changes in blood fatty acid levels due to severity of illness.
Nutritional requirements for infants born before the 30th week of gestation often are not met initially by enteral feedings alone. As a result, premature infants are reliant on a parenteral soybean oil-based lipid emulsion for the delivery of fatty acids during much of the first week and partially into the second postnatal week. The lipid emulsion therapy used for this purpose was developed for adults in whom the essential fatty acid precursors α-linolenic acid (n-3), LA (n-6), and 18:1(n-9) can reliably be converted to downstream critical fatty acids. Thus, the large increase in blood concentration of these three principal fatty acids observed in the follow-up period reflects the levels in the soybean oil-based lipid emulsion. Even after transition to full enteral feedings with breast milk or DHA and AA supplemented premature formula, premature infants are unable to overcome the decline in whole blood DHA or AA levels that occurs after birth. This suggests that premature infants have limited activity of desaturase enzymes, such as Δ6-desaturase, although others have reported that conversion of precursors to downstream fatty acids can occur.22, 23 An alternative explanation is increased utilization of AA and DHA either due to the disease itself or to the severity of illness. However, these alterations in fatty acid levels were evident within the first postnatal week even before the expression of disease such as CLD and late-onset sepsis and would suggest that these diseases are not the primary cause of these postnatal fatty acid changes. Additionally, adjustment for severity of illness (SNAP-II) did not eliminate the association between changes in select fatty acid levels and CLD or late-onset sepsis. Lastly, the association of disease selectively with DHA and AA implies that this is not a result of non-specific oxidation of stored blood samples because this would decrease levels of all LCPUFAs, and implies that these two fatty acids are likely to play a pathogenic role in increasing the risk of these diseases.
Because DHA and AA modulate inflammatory responses, their alterations during the postnatal period in premature infants may contribute to dysregulation of immune and inflammatory responses predisposing the premature infant to conditions such as CLD and sepsis. DHA-derived metabolites such as resolvins decrease neutrophil infiltration and enhance macrophage phagocytosis.24 DHA also downregulates NFκB activity in cells either directly or via increased activation of peroxisome proliferator-activated receptors, thereby reducing cytokine release.16, 25 Lastly, DHA competes with AA for incorporation into cell membranes thus limiting the proinflammatory signaling mediated by AA.16, 24, 25 Therefore, low levels of DHA would be expected to predispose to increased host inflammatory responses such as that seen in CLD. In late-onset sepsis, decreased AA levels may increase the risk by inhibiting the innate immune response through decreased eicosanoids, in particular, leukotrienes, which are known to enhance chemotaxis of leukocytes, neutrophil activation, and activity of natural killer cells.25
Other studies have described alterations in plasma fatty acid levels in premature infants after birth.26–32 In support of our results, measures of fatty acids at variable time intervals after birth in cord blood (which contains both maternal and fetal blood) and in plasma showed an increase in LA with a concomitant decrease in both AA and DHA. The fact that our postnatal fatty acid patterns are similar to changes reported from serial plasma levels by Leaf et al 31, indicate that whole blood levels are appropriate for assessing relative changes in systemic fatty acid levels.
Several limitations of previous studies evaluating fatty acid levels in infants included: (1) a small number of premature infants < 30 weeks of gestation, (2) blood samples analyzed at a limited number of time points, and (3) most of these studies were conducted before the routine supplementation of DHA and AA in formula. Though this study cannot assert causation which will require larger prospective clinical studies and concomitant animal models, our study is the largest to date evaluating changes in fatty acid levels in the most vulnerable of premature infants at less than 30 weeks of gestation. In addition, no study to date has analyzed the association between postnatal changes in fatty acid levels and the risk of neonatal morbidities during the postnatal intensive care period, especially during this era of DHA and AA supplemented premature formulas.
Our data indicate that the current nutritional practices in the neonatal intensive care unit are unable to sustain critical fatty acid levels of DHA and AA at values premature infants experience in utero and this inability may, in part, contribute to the development of significant neonatal morbidity. Our data also indicate that nutritional strategies to minimize this postnatal decline must consider two phases of nutritional intake: (1) the early reliance on total parenteral nutrition from birth to the second postnatal week and (2) the transition to enteral feedings after the second postnatal week.
Several studies have evaluated enteral DHA supplementation for premature infants with variable results observed on infant outcomes.33–35 However, there are two major limitations to these studies. First, DHA was given as a supplement only after oral feedings were established as the dominant route of nutrition. Our study highlights that the significant decline in whole blood DHA and AA levels occurs by postnatal day 7, and the infants are unable to regain birth levels even after transition to full enteral feedings. The transition to full enteral feedings in the preterm infant is delayed and may take up to two weeks (the median day to full feedings in our cohort was 14.5 days). Thus the timing of enteral DHA supplementation may be too late to see an effect in these studies.
Second, it is not simply a single LCPUFA that modulates disease but rather the ratio of fatty acids that regulates inflammatory mediators and other downstream modulators of cell and organ physiology.21, 36 In premature infants there is not simply a decrease in DHA and AA levels, but also profound alterations in LA and other fatty acids, some increased, some decreased, indicating that supplementing with a single LCPUFA would unlikely have a major disease modifying effect. Although several investigators have shown an improvement with parenteral emulsions enriched in DHA for certain disease processes,37, 38 LA and AA remain important to the growth and health of the developing infant.7
Our study would indicate that targeting the rapid alterations in critical fatty acids that occur in the premature infant by the end of the first week of life, a time when the infant is relying on most of its nutritional intake by parenteral nutrition including lipid emulsion, may be essential to lowering disease risk. For both intravenous and enteral phases of nutrition, the balance and bioavailability of all critical fatty acids delivered to the premature infant should approximate levels normally present in the fetus during the third trimester of gestation. Lastly, although not specifically examined in the current study because DHA is the most abundant fatty acid in the neonatal brain, further long term studies are needed to determine whether preventing the profound reduction in DHA in conjunction with the other fatty acid alterations in the first postnatal week may reduce the neurocognitive delays exhibited in some former premature infants.39, 40
Acknowledgments
Supported by the Program for Faculty Development and Diversity of Harvard Catalyst, The Harvard Clinical and Translational Science Center, from the National Center for Research Resources (award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers), Charles H. Hood Foundation, the Alden Trust, and the Gerber Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, and its affiliated academic health care centers, the National Center for Research Resources or the National Institutes of Health. None of the funding bodies had any role in the study design or conduct; data collection, management, analysis or interpretation; or preparation, review, or approval of the manuscript.
We thank the neonatal nurses and the staff of the Clinical Research Center at Beth Israel Deaconess Medical Center. We also extend our thanks to Uchenna Ezeibe who helped initially build our clinical biorepository. Finally, we are indebted to the parents of our premature infants who enthusiastically supported our study and shared our hope that we can improve the care of premature infants.
Abbreviations
- AA
Arachidonic acid
- CLD
chronic lung disease
- DHA
docosahexaenoic acid
- LA
linoleic acid
- ROP
retinopathy of prematurity
- LCPUFAs
long chain polyunsaturated fatty acids
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Hum Dev. 1980;4:121–9. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 2.Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr. 2010;30:237–55. doi: 10.1146/annurev.nutr.012809.104742. [DOI] [PubMed] [Google Scholar]
- 3.Kurlak LO, Stephenson TJ. Plausible explanations for effects of long chain polyunsaturated fatty acids (LCPUFA) on neonates. Arch Dis Child Fetal Neonatal Ed. 1999;80:F148–54. doi: 10.1136/fn.80.2.f148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connor KM, SanGiovanni JP, Lofqvist C, Aderman CM, Chen J, Higuchi A, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, Jilling T, Li D, Caplan MS. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2007;61:427–32. doi: 10.1203/pdr.0b013e3180332ca5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demmelmair H, von Schenck U, Behrendt E, Sauerwald T, Koletzko B. Estimation of arachidonic acid synthesis in full term neonates using natural variation of 13C content. J Pediatr Gastroenterol Nutr. 1995;21:31–6. doi: 10.1097/00005176-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30:39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 8.Rubin M, Moser A, Naor N, Merlob P, Pakula R, Sirota L. Effect of three intravenously administered fat emulsions containing different concentrations of fatty acids on the plasma fatty acid composition of premature infants. J Pediatr. 1994;125:596–602. doi: 10.1016/s0022-3476(94)70017-6. [DOI] [PubMed] [Google Scholar]
- 9.Salem N, Jr, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci U S A. 1996;93:49–54. doi: 10.1073/pnas.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uauy R, Dangour AD. Fat and fatty acid requirements and recommendations for infants of 0–2 years and children of 2–18 years. Ann Nutr Metab. 2009;55:76–96. doi: 10.1159/000228997. [DOI] [PubMed] [Google Scholar]
- 11.Uauy R, Mena P, Wegher B, Nieto S, Salem N., Jr Long chain polyunsaturated fatty acid formation in neonates: effect of gestational age and intrauterine growth. Pediatr Res. 2000;47:127–35. doi: 10.1203/00006450-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Al MD, von Houwelingen AC, Badart-Smook A, Hornstra G. Some aspects of neonatal essential fatty acid status are altered by linoleic acid supplementation of women during pregnancy. J Nutr. 1995;125:2822–30. doi: 10.1093/jn/125.11.2822. [DOI] [PubMed] [Google Scholar]
- 13.Amusquivar E, Sanchez M, Hyde MJ, Laws J, Clarke L, Herrera E. Influence of fatty acid profile of total parenteral nutrition emulsions on the fatty acid composition of different tissues of piglets. Lipids. 2008;43:713–22. doi: 10.1007/s11745-008-3180-7. [DOI] [PubMed] [Google Scholar]
- 14.Blank C, Neumann MA, Makrides M, Gibson RA. Optimizing DHA levels in piglets by lowering the linoleic acid to alpha-linolenic acid ratio. J Lipid Res. 2002;43:1537–43. doi: 10.1194/jlr.m200152-jlr200. [DOI] [PubMed] [Google Scholar]
- 15.Portolesi R, Powell BC, Gibson RA. Competition between 24:5n-3 and ALA for Delta 6 desaturase may limit the accumulation of DHA in HepG2 cell membranes. J Lipid Res. 2007;48:1592–8. doi: 10.1194/jlr.M700081-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–9. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 17.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 18.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004;350:560–9. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 19.Martin CR, Brown YF, Ehrenkranz RA, O’Shea TM, Allred EN, Belfort MB, et al. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics. 2009;124:649–57. doi: 10.1542/peds.2008-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 22.Carnielli VP, Wattimena DJ, Luijendijk IH, Boerlage A, Degenhart HJ, Sauer PJ. The very low birth weight premature infant is capable of synthesizing arachidonic and docosahexaenoic acids from linoleic and linolenic acids. Pediatr Res. 1996;40:169–74. doi: 10.1203/00006450-199607000-00029. [DOI] [PubMed] [Google Scholar]
- 23.Innis SM, Sprecher H, Hachey D, Edmond J, Anderson RE. Neonatal polyunsaturated fatty acid metabolism. Lipids. 1999;34:139–49. doi: 10.1007/s11745-999-0348-x. [DOI] [PubMed] [Google Scholar]
- 24.Seki H, Sasaki T, Ueda T, Arita M. Resolvins as regulators of the immune system. Scientific World Journal. 2010;10:818–31. doi: 10.1100/tsw.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–24. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 26.Carlson SE, Rhodes PG, Ferguson MG. Docosahexaenoic acid status of preterm infants at birth and following feeding with human milk or formula. Am J Clin Nutr. 1986;44:798–804. doi: 10.1093/ajcn/44.6.798. [DOI] [PubMed] [Google Scholar]
- 27.Carnielli VP, Pederzini F, Vittorangeli R, Luijendijk IH, Boomaars WE, Pedrotti D, et al. Plasma and red blood cell fatty acid of very low birth weight infants fed exclusively with expressed preterm human milk. Pediatr Res. 1996;39:671–9. doi: 10.1203/00006450-199604000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Carnielli VP, Rossi K, Badon T, Gregori B, Verlato G, Orzali A, et al. Medium-chain triacylglycerols in formulas for preterm infants: effect on plasma lipids, circulating concentrations of medium-chain fatty acids, and essential fatty acids. Am J Clin Nutr. 1996;64:152–8. doi: 10.1093/ajcn/64.2.152. [DOI] [PubMed] [Google Scholar]
- 29.Ghebremeskel K, Leighfield M, Leaf A, Costeloe K, Crawford M. Fatty acid composition of plasma and red cell phospholipids of preterm babies fed on breast milk and formulae. Eur J Pediatr. 1995;154:46–52. [PubMed] [Google Scholar]
- 30.Koletzko B, Sauerwald U, Keicher U, Saule H, Wawatschek S, Bohles H, et al. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diets without or with long-chain polyunsaturated fatty acids. A randomized clinical trial. Eur J Nutr. 2003;42:243–53. doi: 10.1007/s00394-003-0418-2. [DOI] [PubMed] [Google Scholar]
- 31.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA. Factors affecting long-chain polyunsaturated fatty acid composition of plasma choline phosphoglycerides in preterm infants. J Pediatr Gastroenterol Nutr. 1992;14:300–8. doi: 10.1097/00005176-199204000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Sabel KG, Lundqvist-Persson C, Bona E, Petzold M, Strandvik B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis. 2009;8:20. doi: 10.1186/1476-511X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. Am J Clin Nutr. 2010;91:628–34. doi: 10.3945/ajcn.2009.28603. [DOI] [PubMed] [Google Scholar]
- 34.Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301:175–82. doi: 10.1001/jama.2008.945. [DOI] [PubMed] [Google Scholar]
- 35.Beyerlein A, Hadders-Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, et al. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18 months of age--IPD meta-analysis of 4 large clinical trials. J Pediatr Gastroenterol Nutr. 2010;50:79–84. doi: 10.1097/MPG.0b013e3181acae7d. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–8. doi: 10.1016/j.plefa.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Fallon EM, Le HD, Puder M. Prevention of parenteral nutrition-associated liver disease: role of omega-3 fish oil. Curr Opin Organ Transplant. 2010;15:334–40. doi: 10.1097/mot.0b013e3283394879. [DOI] [PubMed] [Google Scholar]
- 38.Gura KM, Duggan CP, Collier SB, Jennings RW, Folkman J, Bistrian BR, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 39.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 40.Makrides M, Smithers LG, Gibson RA. Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:123–33. doi: 10.1159/000281154. [DOI] [PubMed] [Google Scholar]