Abstract
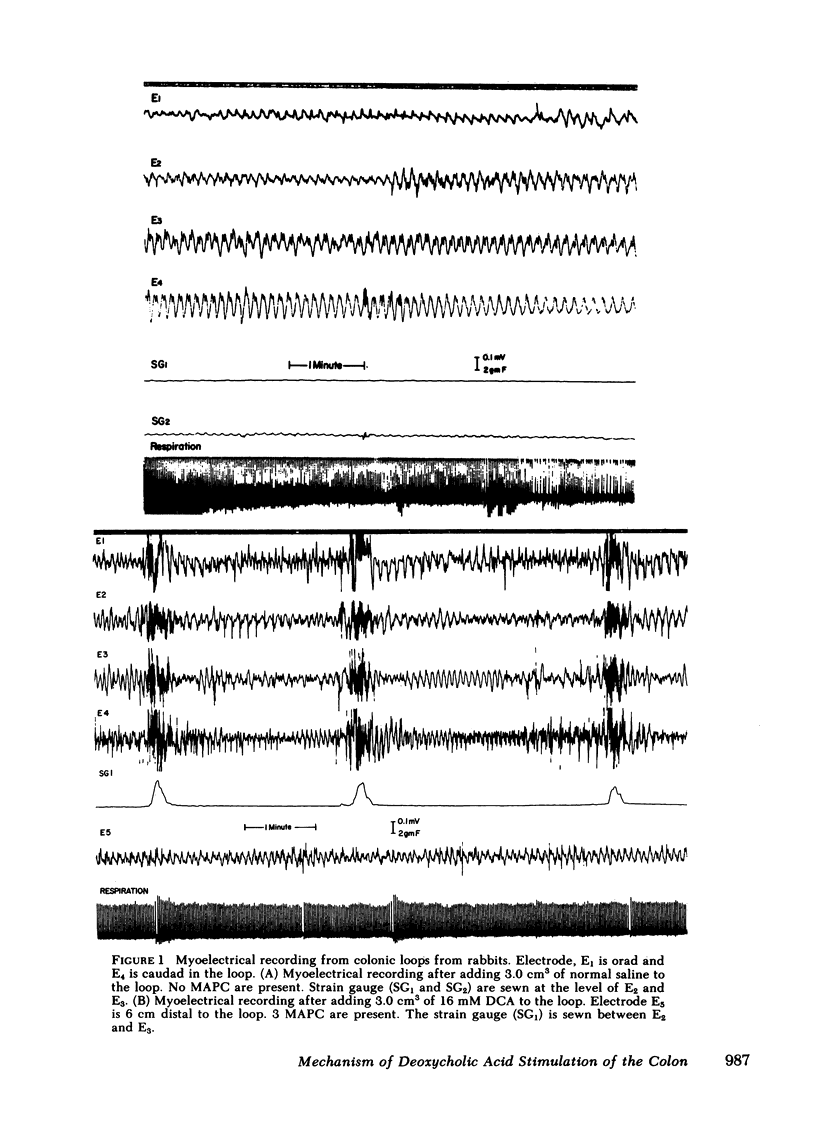
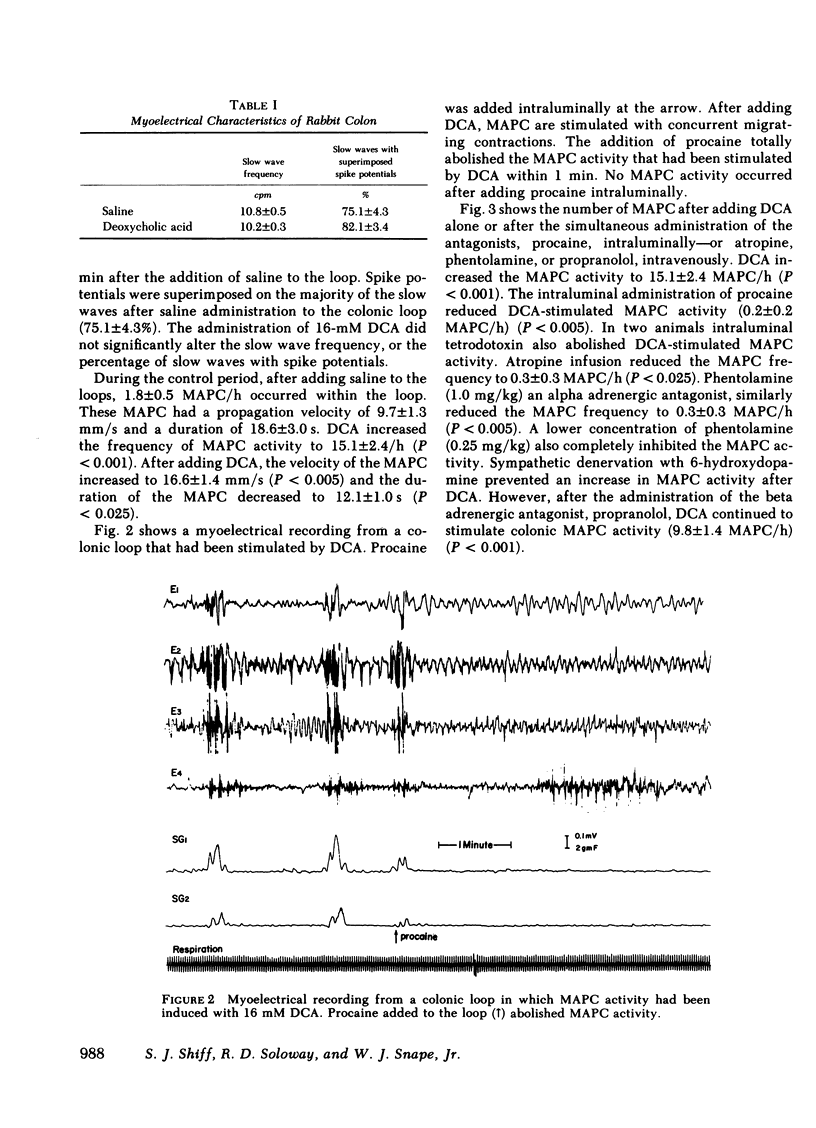
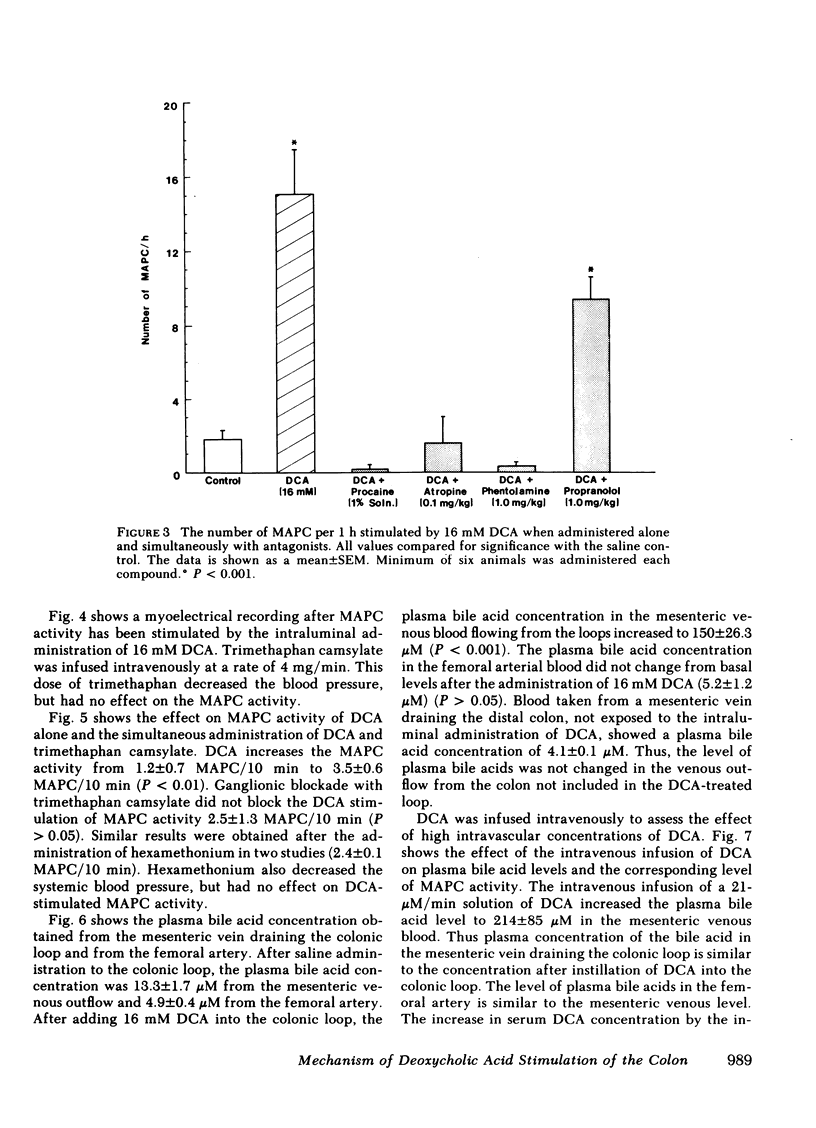
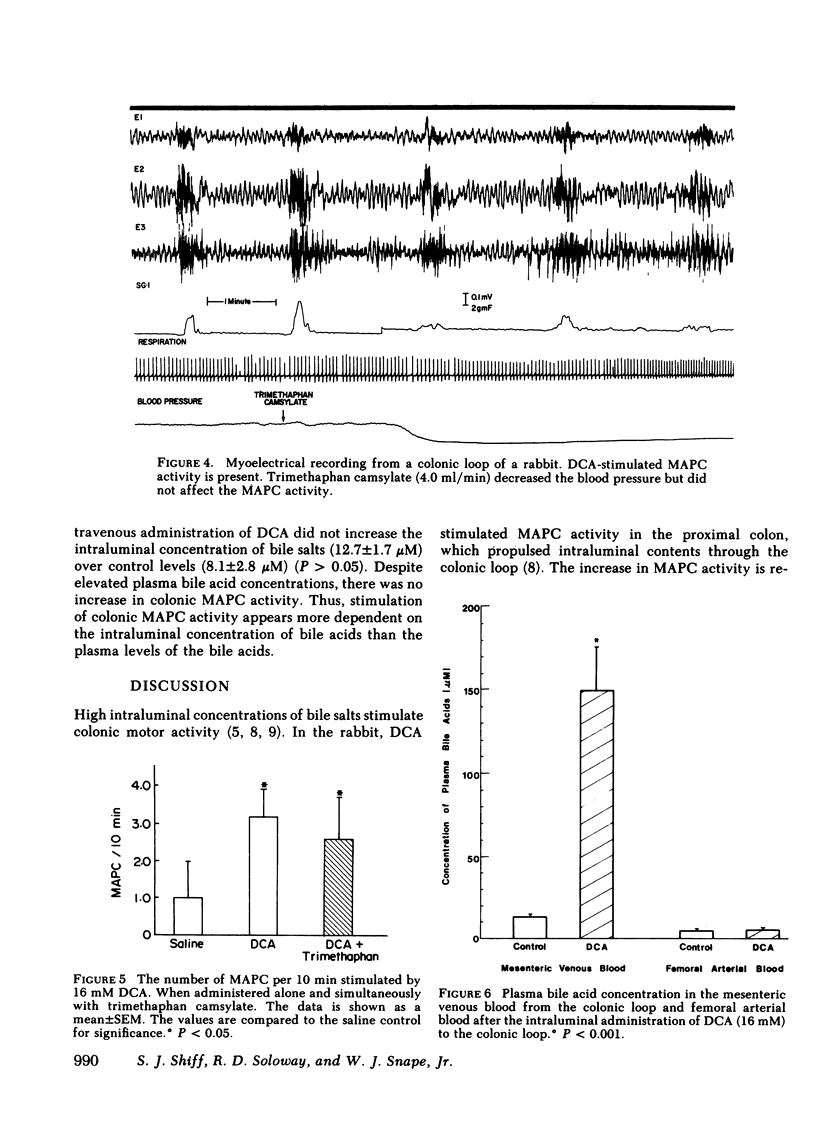
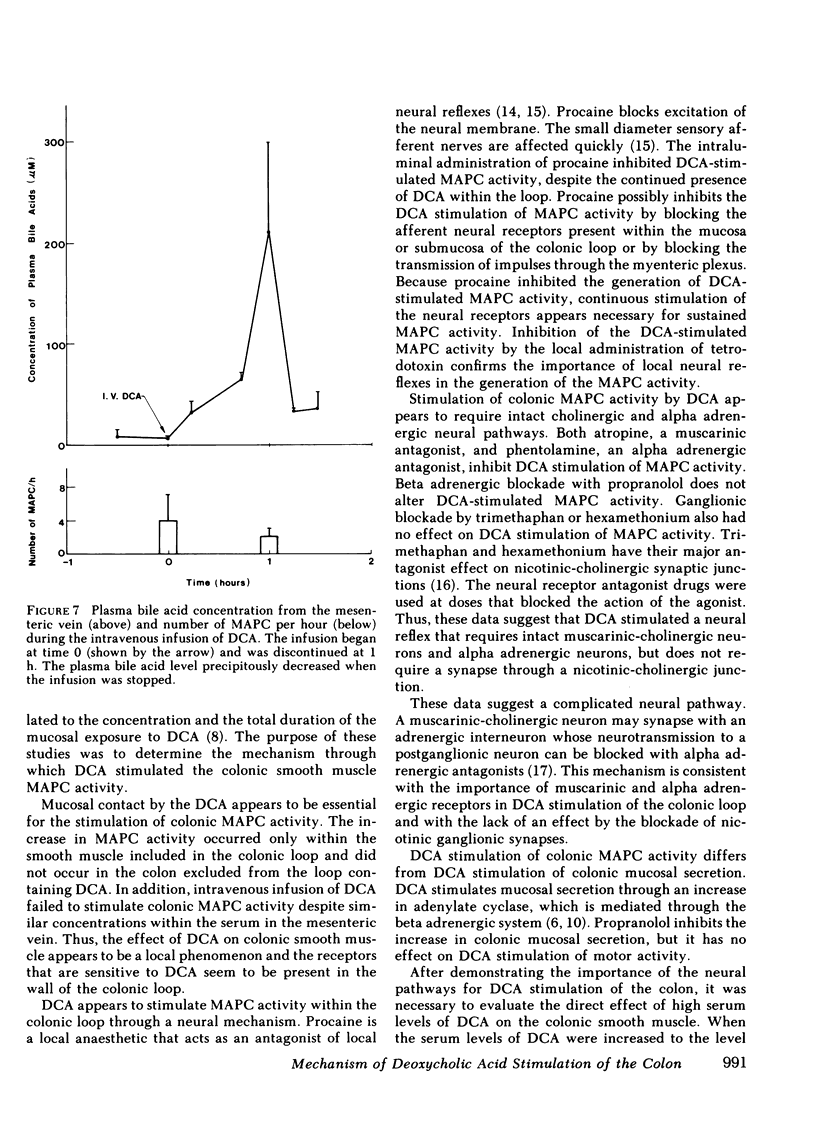
Previous studies showed that deoxycholic acid (DCA) stimulated migrating action potential complexes (MAPC) in the colon. The aim of this study was to clarify the mechanism of DCA-stimulated colonic motility. Myoelectrical and contractile activity were measured in New Zealand White rabbits from a loop constructed in the proximal colon. During the control period, slow waves were present at a frequency of 10.8 +/- 0.5 cycle/min and there were 1.5 +/- 0.5 MAPC/ h. After adding DCA (16 mM) to the loop the slow wave activity was unchanged. However, MAPC increased to 15.1 +/- 2.4 MAPC/h (P less than 0.001). MAPC activity was not stimulated in the colonic smooth muscle outside the loop. The intraluminal addition of procaine or tetrodotoxin to the colonic loop inhibited the DCA-stimulated increase in MAPC activity (0.2 +/- 0.2 MAPC/h) (P less than 0.005). Intravenous administration of atropine or phentolamine also inhibited MAPC activity that had been stimulated by DCA (P less than 0.005). Pretreatment with 6-hydroxydopamine also inhibited an increase in MAPC activity. Propranolol, trimethaphan camsylate, or hexamethonium had no effect on DCA stimulation of MAPC activity. Although the concentration of bile salt increased in the mesenteric venous outflow from the colonic loop, the intravenous administration of bile salt did not stimulate colonic MAPC activity. These studies suggest: (a) the action of DCA on smooth muscle activity is a local phenomenon, (b) the increase in MAPC activity is dependent on intact cholinergic and alpha adrenergic neurons, and (c) an increase in the concentration of bile salts in the serum is not associated with an increase in colonic MAPC activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Filburn C., Volpe B. T. Bile salt alteration of colonic electrolyte transport: role of cyclic adenosine monophosphate. Gastroenterology. 1975 Mar;68(3):503–508. [PubMed] [Google Scholar]
- Conley D. R., Coyne M. J., Bonorris G. G., Chung A., Schoenfield L. J. Bile acid stimulation of colonic adenylate cyclase and secretion in the rabbit. Am J Dig Dis. 1976 Jun;21(6):453–458. doi: 10.1007/BF01072128. [DOI] [PubMed] [Google Scholar]
- Conley D., Coyne M., Chung A., Bonorris G., Schoenfield L. Propranolol inhibits adenylate cyclase and secretion stimulated by deoxycholic acid in the rabbit colon. Gastroenterology. 1976 Jul;71(1):72–75. [PubMed] [Google Scholar]
- Coyne M. J., Bonorris G. G., Chung A., Conley D., Schoenfield L. J. Propranolol inhibits bile acid and fatty acid stimulation of cyclic AMP in human colon. Gastroenterology. 1977 Nov;73(5):971–974. [PubMed] [Google Scholar]
- Flynn M., Darby C., Hyland J., Hammond P., Taylor I. The effect of bile acids on colonic myoelectrical activity. Br J Surg. 1979 Nov;66(11):776–779. doi: 10.1002/bjs.1800661106. [DOI] [PubMed] [Google Scholar]
- Greengard P., Kebabian J. W. Role of cyclic AMP in synaptic transmission in the mammalian peripheral nervous system. Fed Proc. 1974 Apr;33(4):1059–1067. [PubMed] [Google Scholar]
- KOSTERLITZ H. W., LEES G. M. PHARMACOLOGICAL ANALYSIS OF INTRINSIC INTESTINAL REFLEXES. Pharmacol Rev. 1964 Sep;16:301–339. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan W. O., Smith A. N., Mitchell W. D., Falconer J. D., Eastwood M. A. Bile acids and colonic motility in the rabbit and the human. Gut. 1975 Nov;16(11):894–902. doi: 10.1136/gut.16.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias J. R., Carlson G. M., DiMarino A. J., Bertiger G., Morton H. E., Cohen S. Intestinal myoelectric activity in response to live Vibrio cholerae and cholera enterotoxin. J Clin Invest. 1976 Jul;58(1):91–96. doi: 10.1172/JCI108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuga T., Mitamura K., Mashige F., Imai D. Evaluation of fluorimetrically estimated serum bile acid in liver disease. Clin Chim Acta. 1977 Feb 15;75(1):81–90. doi: 10.1016/0009-8981(77)90502-2. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Siskos P. A., Cahill P. T., Javitt N. B. Serum bile acid analysis: a rapid, direct enzymatic method using dual-beam spectrophotofluorimetry. J Lipid Res. 1977 Sep;18(5):666–671. [PubMed] [Google Scholar]
- Snape W. J., Jr, Shiff S., Cohen S. Effect of deoxycholic acid on colonic motility in the rabbit. Am J Physiol. 1980 Apr;238(4):G321–G325. doi: 10.1152/ajpgi.1980.238.4.G321. [DOI] [PubMed] [Google Scholar]
- Weisberg P. B., Carlson G. M., Cohen S. Effect of Salmonella typhimurium on myoelectrical activity in the rabbit ileum. Gastroenterology. 1978 Jan;74(1):47–51. [PubMed] [Google Scholar]


