Abstract
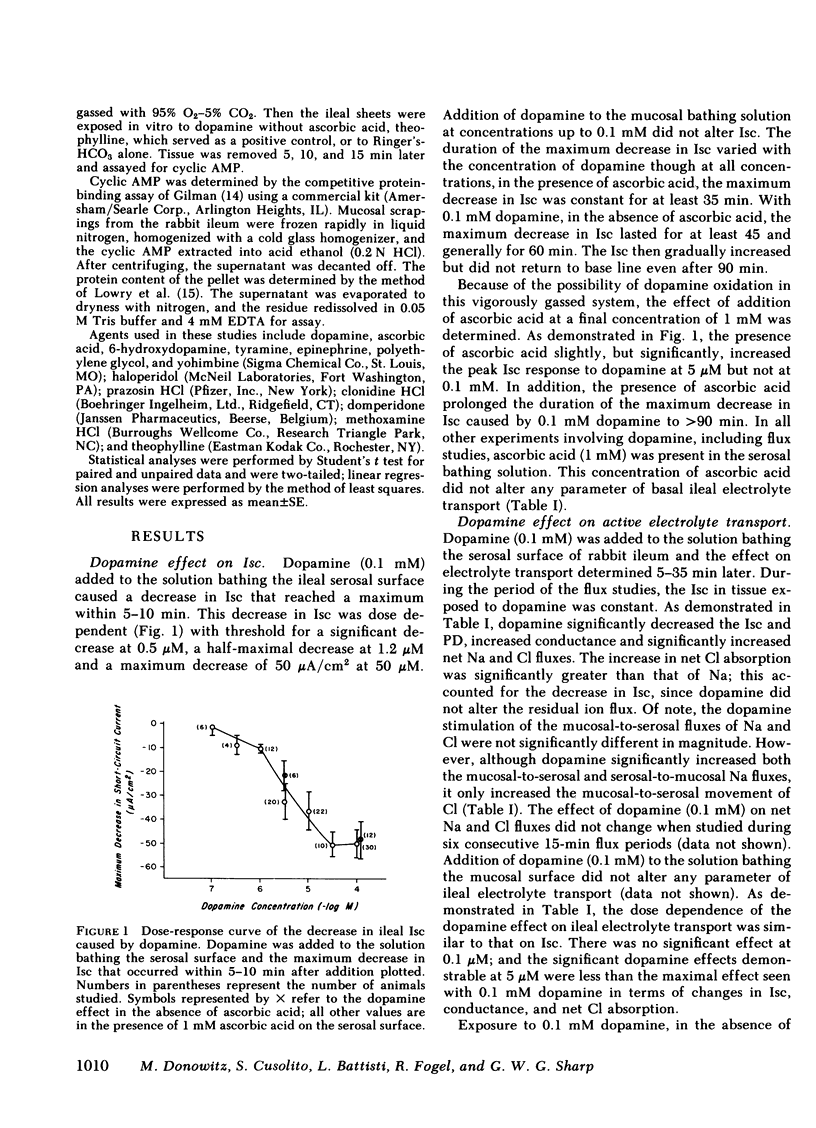
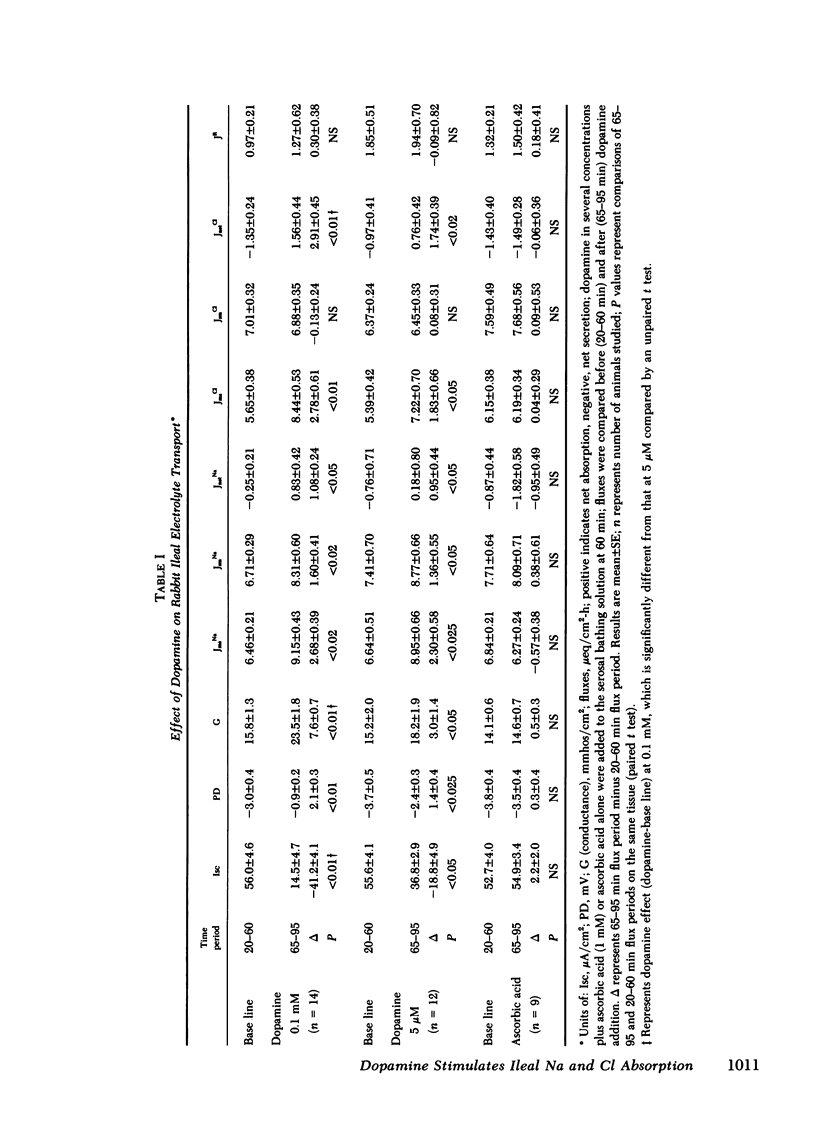
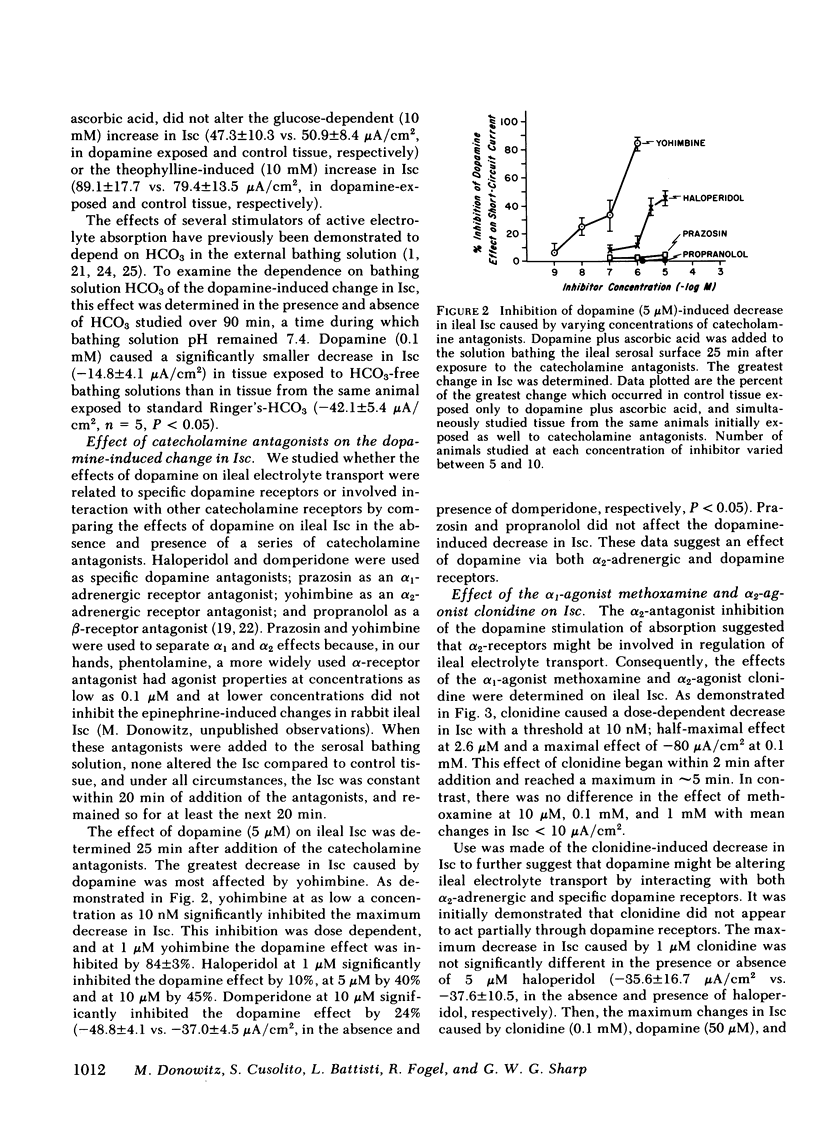
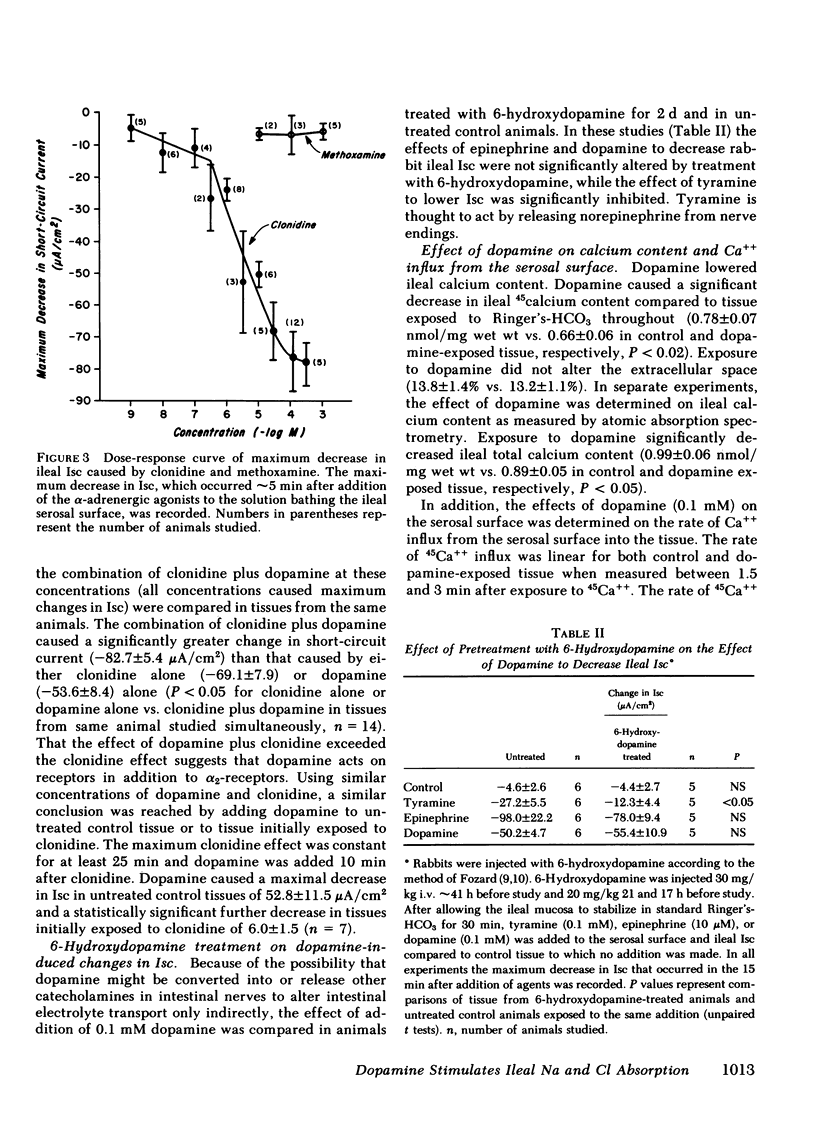
The effects of dopamine on active intestinal ion transport have been evaluated. An epithelial sheet preparation of rabbit ileum was used in vitro with the Ussing chamber-voltage clamp technique. Dopamine, in the presence of 1 mM ascorbic acid, added to the serosal bathing solution caused a dose-dependent decrease in short-circuit current, with a half-maximal effect at 1.2 μM and maximal effect of −50 μA/cm2 at 50 μM; dopamine decreased the potential difference, and increased the conductance and net Na and net Cl absorption. There was no effect on the residual ion flux. Dopamine did not alter the change in short-circuit current caused by mucosal glucose (10 mM) or serosal theophylline (10 mM). Mucosal dopamine had no effect. The effect of dopamine on short-circuit current was inhibited by the dopamine antagonists haloperidol and domperidone and the α2-adrenergic antagonist yohimbine; there was no effect of the α1-antagonist prazosin and the β-antagonist propranolol. In addition, the α2-adrenergic agonist clonidine, but not the α1-agonist methoxamine caused a dose-dependent decrease in short-circuit current. The ileal effects of dopamine did not occur via conversion into norepinephrine or release of norepinephrine from the peripheral nerves since “peripheral sympathectomy” with 6-hydroxydopamine did not alter the dopamine-induced change in ileal short-circuit current. The dopamine effects were not associated with a change in basal ileal cyclic AMP content but were associated with a decrease in total ileal calcium content as measured by atomic absorption spectrometry and as estimated by 45Ca++ uptake. The decrease in calcium content could be attributed to a dopamine-induced decrease in 45Ca++ influx from the serosal surface. Because of the presence of dopamine in ileal mucosa and these effects on ileal electrolyte transport, it is possible that dopamine may be involved in the physiologic regulation of active intestinal electrolyte absorption.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AULSEBROOK K. A. INTESTINAL ABSORPTION OF GLUCOSE AND SODIUM: EFFECTS OF EPINEPHRINE AND NOREPINEPHRINE. Biochem Biophys Res Commun. 1965 Jan 18;18:165–169. doi: 10.1016/0006-291x(65)90734-5. [DOI] [PubMed] [Google Scholar]
- Anuras S. Effect of dopamine on opossum duodenal smooth muscle. Gastroenterology. 1981 Jan;80(1):51–54. [PubMed] [Google Scholar]
- Berthelsen S., Pettinger W. A. A functional basis for classification of alpha-adrenergic receptors. Life Sci. 1977 Sep 1;21(5):595–606. doi: 10.1016/0024-3205(77)90066-2. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Binder H. J., Dobbins J. W. Somatostatin stimulates sodium and chloride absorption in the rabbit ileum. Gastroenterology. 1980 Jun;78(6):1559–1565. [PubMed] [Google Scholar]
- Dobbins J., Racusen L., Binder H. J. Effect of D-alanine methionine enkephalin amide on ion transport in rabbit ileum. J Clin Invest. 1980 Jul;66(1):19–28. doi: 10.1172/JCI109830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Charney A. N., Heffernan J. M. Effect of serotonin treatment on intestinal transport in the rabbit. Am J Physiol. 1977 Jan;232(1):E85–E94. doi: 10.1152/ajpendo.1977.232.1.E85. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Charney A. N. Propranolol prevention of cholera enterotoxin-induced intestinal secretion in the rat. Gastroenterology. 1979 Mar;76(3):482–491. [PubMed] [Google Scholar]
- Donowitz M., Tai Y. H., Asarkof N. Effect of serotonin on active electrolyte transport in rabbit ileum, gallbladder, and colon. Am J Physiol. 1980 Dec;239(6):G463–G472. doi: 10.1152/ajpgi.1980.239.6.G463. [DOI] [PubMed] [Google Scholar]
- Fain J. N., García-Sáinz J. A. Role of phosphatidylinositol turnover in alpha 1 and of adenylate cyclase inhibition in alpha 2 effects of catecholamines. Life Sci. 1980 Apr 14;26(15):1183–1194. doi: 10.1016/0024-3205(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Field M., McColl I. Ion transport in rabbit ileal mucosa. 3. Effects of catecholamines. Am J Physiol. 1973 Oct;225(4):852–857. doi: 10.1152/ajplegacy.1973.225.4.852. [DOI] [PubMed] [Google Scholar]
- Field M., Sheerin H. E., Henderson A., Smith P. L. Catecholamine effects on cyclic AMP levels and ion secretion in rabbit ileal mucosa. Am J Physiol. 1975 Jul;229(1):86–92. doi: 10.1152/ajplegacy.1975.229.1.86. [DOI] [PubMed] [Google Scholar]
- Fozard J. R., Kelly M. J., Small R. C. Proceedings: Chemical sympathectomy of the rabbit with 6-hydroxydopamine. Br J Pharmacol. 1973 Sep;49(1):182P–183P. [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R., Mwaluko G. M. Mechanism of the indirect sympathomimetic effect of 5-hydroxytrypt-amine on the isolated heart of the rabbit. Br J Pharmacol. 1976 May;57(1):115–125. doi: 10.1111/j.1476-5381.1976.tb07661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman J., Rasmussen H., Dobbins J. W. Somatostatin stimulates coupled sodium chloride influx across the brush border of the rabbit ileum. Biochem Biophys Res Commun. 1980 Nov 17;97(1):243–247. doi: 10.1016/s0006-291x(80)80160-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman B. B., Lefkowitz R. J. Alpha-adrenergic receptor subtypes. N Engl J Med. 1980 Jun 19;302(25):1390–1396. doi: 10.1056/NEJM198006193022504. [DOI] [PubMed] [Google Scholar]
- Kachur J. F., Miller R. J., Field M. Control of guinea pig intestinal electrolyte secretion by a delta-opiate receptor. Proc Natl Acad Sci U S A. 1980 May;77(5):2753–2756. doi: 10.1073/pnas.77.5.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W. Multiple classes of dopamine receptors in mammalian central nervous system: the involvement of dopamine-sensitive adenylyl cyclase. Life Sci. 1978 Aug 7;23(5):479–483. doi: 10.1016/0024-3205(78)90157-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nellans H. N., Schultz S. G. Relations among transepithelial sodium transport, potassium exchange, and cell volume in rabbit ileum. J Gen Physiol. 1976 Oct;68(4):441–463. doi: 10.1085/jgp.68.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. C. Active and passive Ca movements in dog red blood cells and resealed ghosts. Am J Physiol. 1979 Jul;237(1):C10–C16. doi: 10.1152/ajpcell.1979.237.1.C10. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Tapper E. J., Morris S. M. Aspirin-stimulated intestinal electrolyte transport in rabbit ileum in vitro. Gastroenterology. 1979 Jun;76(6):1429–1437. [PubMed] [Google Scholar]
- Ribes G., Siegel E. G., Wollheim C. B., Renold A. E., Sharp G. W. Rapid changes in calcium content of rat pancreatic islets in response to glucose. Diabetes. 1981 Jan;30(1):52–55. doi: 10.2337/diab.30.1.52. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Martres M. P., Schwartz J. C. Three classes of dopamine receptor (D-2, D-3, D-4) identified by binding studies with 3H-apomorphine and 3H-domperidone. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(2):89–102. doi: 10.1007/BF00499251. [DOI] [PubMed] [Google Scholar]
- Tapper E. J., Bloom A. S., Lewand D. L. Endogenous norepinephrine release induced by tyramine modulates intestinal ion transport. Am J Physiol. 1981 Sep;241(3):G264–G269. doi: 10.1152/ajpgi.1981.241.3.G264. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton K. G., Liepmann P., Baldessarini R. J. Inhibition of dopamine-stimulated adenylate cyclase activity by phenoxybenzamine. Eur J Pharmacol. 1978 Nov 15;52(2):231–234. doi: 10.1016/0014-2999(78)90211-x. [DOI] [PubMed] [Google Scholar]



