Abstract
The survival of erythrocytes (RBC) is shortened in uremia, and it has been shown that calcium influx into RBC evoked crenation and increased their rigidity. The high blood levels of parathyroid hormone (PTH) may augment entry of calcium into RBC and hence affect their integrity. We examined the effect of PTH on osmotic fragility of human RBC and investigated the mechanisms through which PTH interacts with RBC.
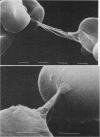
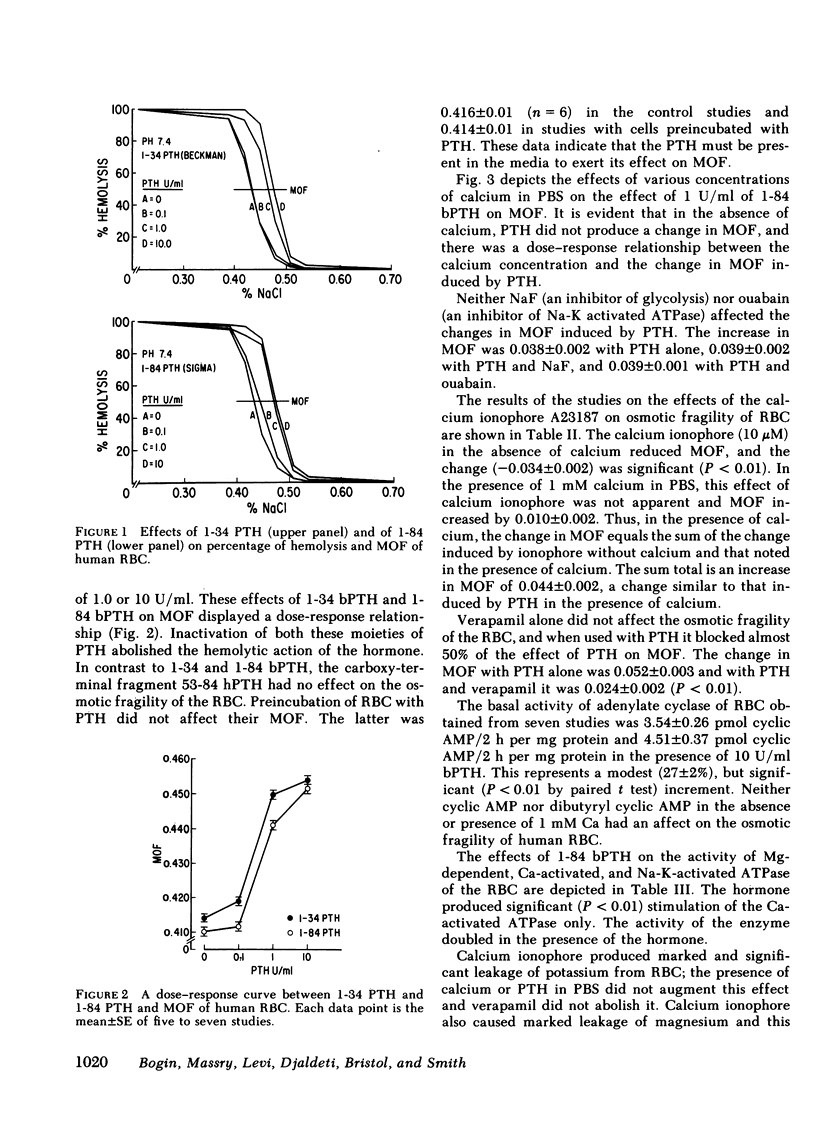
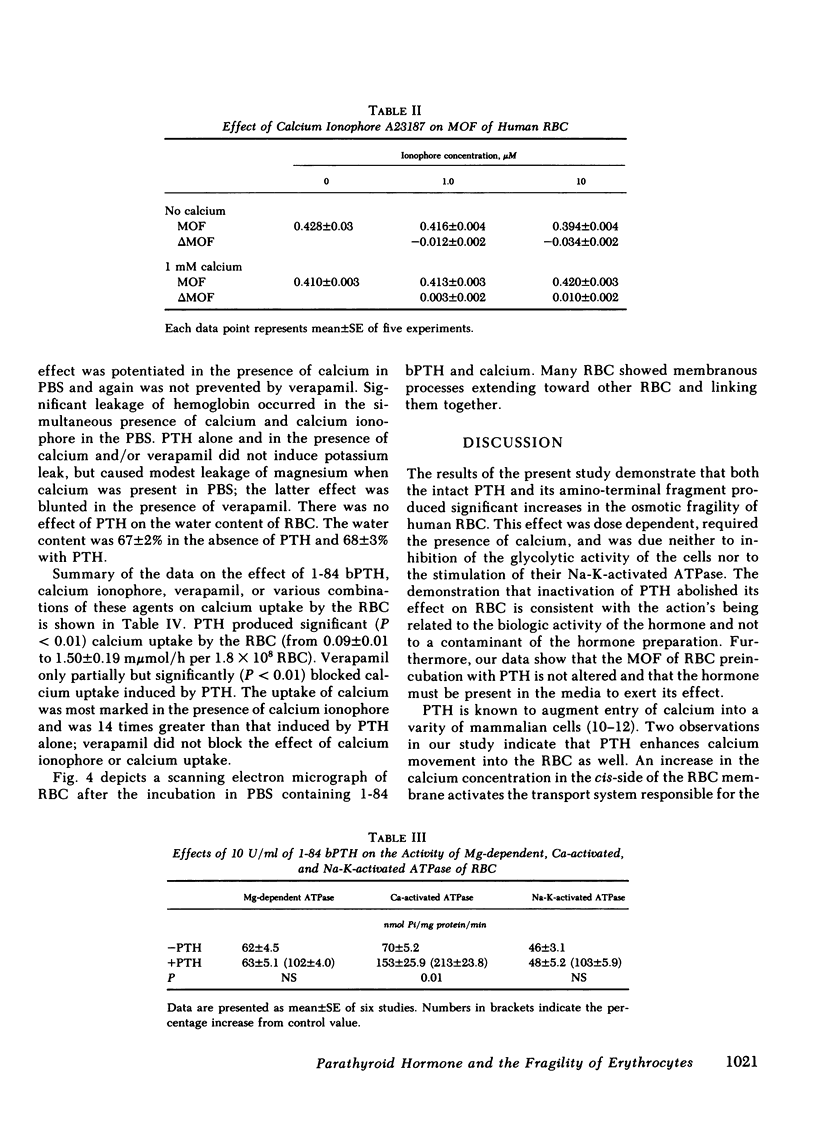
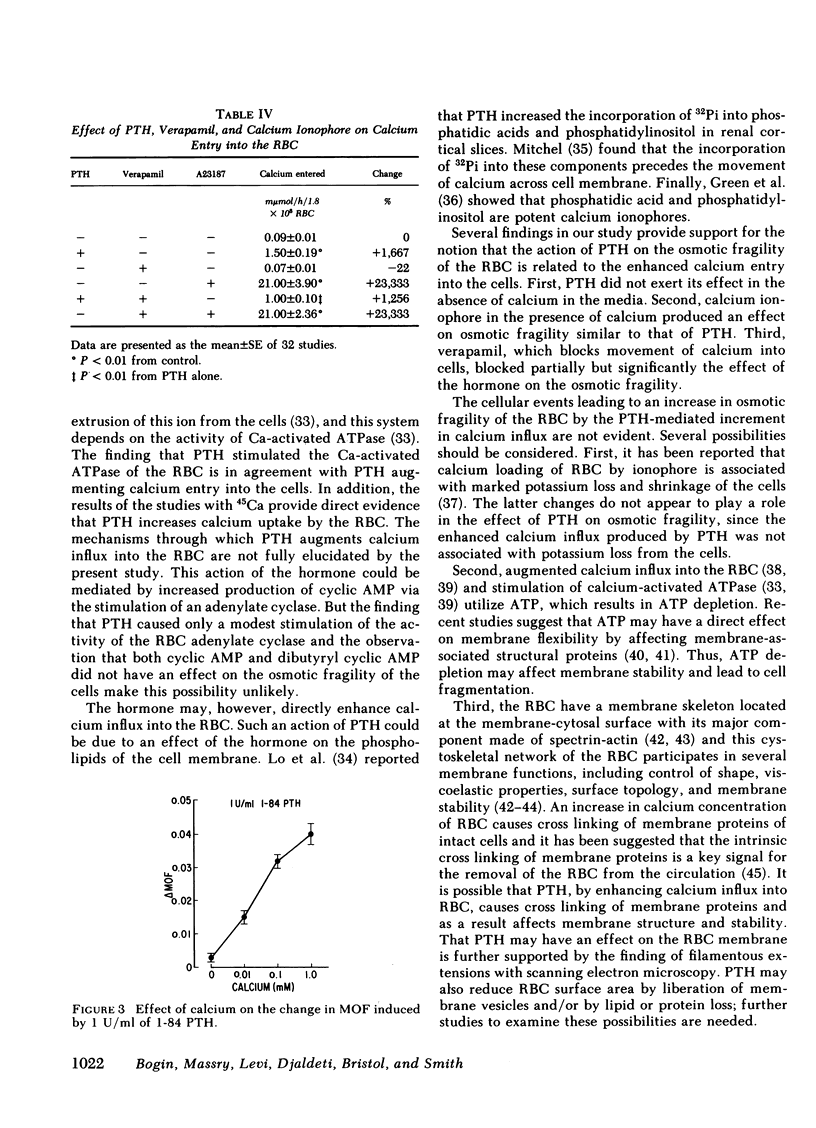
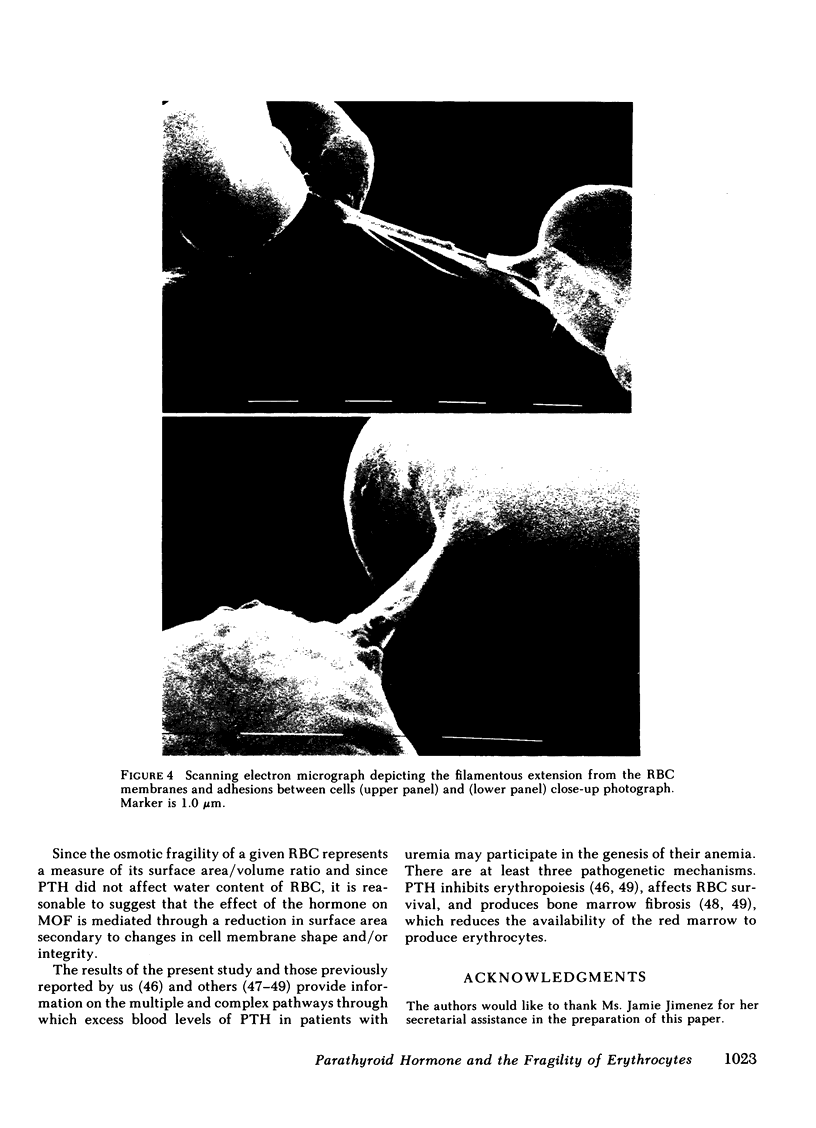
Both the amino-terminal (1-34) PTH and the intact (1-84) PTH, but not the carboxy-terminal (53-84) PTH, produced significant increases in osmotic fragility. This effect was abolished by prior inactivation of the hormone. There was a dose-response relationship between both moieties of PTH and the increase in osmotic fragility. This action of PTH required calcium, was mimicked by calcium ionophore, and was partially blocked by verapamil. PTH caused significant influx of 45Ca into RBC, which was not associated with potassium leak. The hormone did not affect water content of RBC. Scanning electron microscopy revealed that the incubation of RBC with PTH was associated with the appearance of membrane filamentous extensions, which anchor RBC together.
Inhibition of glycolytic activity of RBC with NaF or inhibition of Na-K-activated ATPase with ouabain did not abolish the effect of PTH on osmotic fragility. PTH did not stimulate RBC Na-K-activated ATPase or Mg-dependent ATPase but caused marked and significant stimulation of Ca-activated ATPase. The basal activity of the RBC adenylate cyclase was low and PTH produced only a modest stimulation of this enzyme. Both cyclic AMP and dibutyryl cyclic AMP had no effect on osmotic fragility.
The data indicate that: (a) the RBC is a target organ for PTH, (b) the hormone increases osmotic fragility of RBC, and (c) this effect of PTH is due to enhanced calcium entry into RBC. We suggest that the increased calcium influx may affect the spectrin-actin of the cytoskeletal network of the RBC and may alter the stability and integrity of the cell membrane. This action of PTH on the RBC could be, at least in part, responsible for the shortened survival of RBC in uremia, and assign a new role for PTH in the pathogenesis of the anemia of uremia.
Full text
PDF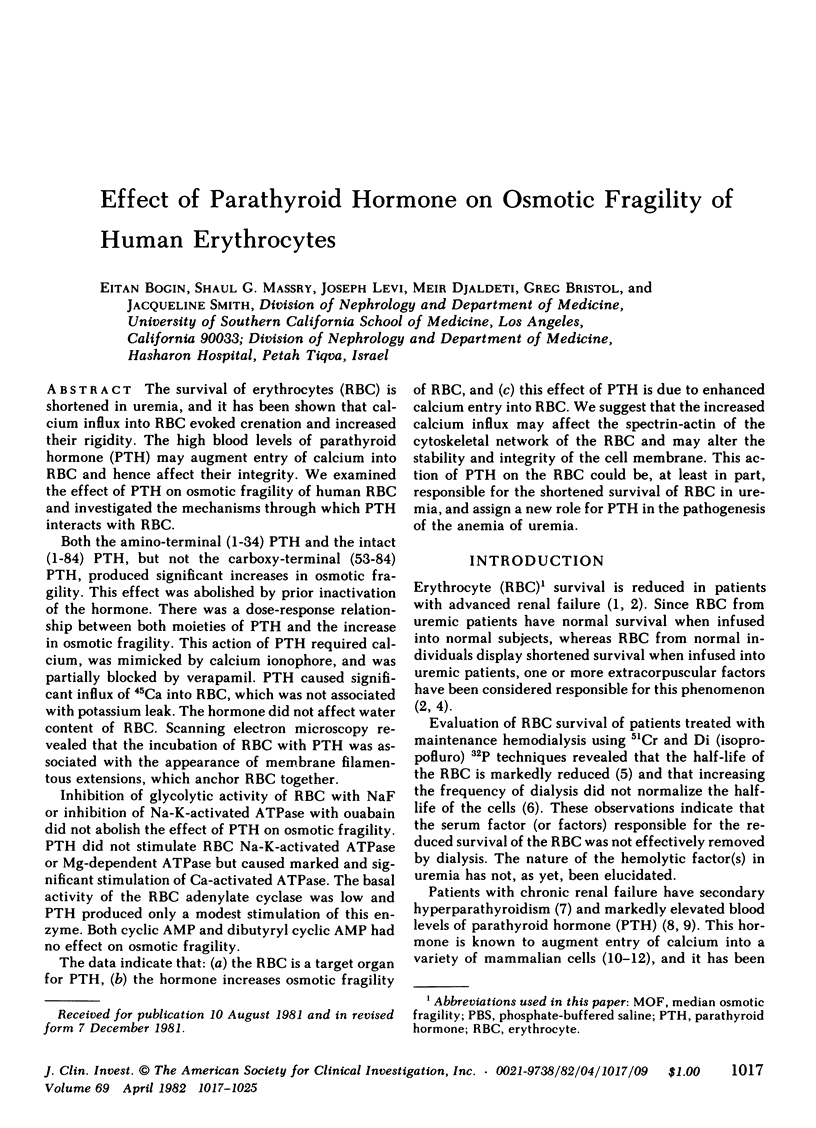




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arieff A. I., Massry S. G. Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Invest. 1974 Feb;53(2):387–392. doi: 10.1172/JCI107571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow J. W., Fine B. S., Zimmerman L. E. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968 Nov;66(5):812–824. doi: 10.1016/0002-9394(68)92795-5. [DOI] [PubMed] [Google Scholar]
- Bernstein D. S., Pletka P., Hattner R. S., Hampers C. L., Merrill J. P. Effect of total parathyroidectomy and uremia on the chemical composition of bone, skin and aorta in the rat. Isr J Med Sci. 1971 Mar;7(3):513–514. [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Parathyroid hormone in plasma in adenomatous hyperparathyroidism, uremia, and bronchogenic carcinoma. Science. 1966 Nov 18;154(3751):907–909. doi: 10.1126/science.154.3751.907. [DOI] [PubMed] [Google Scholar]
- Bilezikian J. P., Aurbach G. D. A beta-adrenergic receptor of the turkey erythrocyte. I. Binding of catecholamine and relationship to adenylate cyclase activity. J Biol Chem. 1973 Aug 25;248(16):5577–5583. [PubMed] [Google Scholar]
- Birchmeier W., Singer S. J. On the mechanism of ATP-induced shape changes in human erythrocyte membranes. II. The role of ATP. J Cell Biol. 1977 Jun;73(3):647–659. doi: 10.1083/jcb.73.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Chausmer A. B., Sherman B. S., Wallach S. The effect of parathyroid hormone on hepatic cell transport of calcium. Endocrinology. 1972 Mar;90(3):663–672. doi: 10.1210/endo-90-3-663. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dunn M. J. Red blood cell calcium and magnesium: effects upon sodium and potassium transport and cellular morphology. Biochim Biophys Acta. 1974 May 30;352(1):97–116. doi: 10.1016/0005-2736(74)90182-5. [DOI] [PubMed] [Google Scholar]
- Goldstein D. A., Chui L. A., Massry S. G. Effect of parathyroid hormone and uremia on peripheral nerve calcium and motor nerve conduction velocity. J Clin Invest. 1978 Jul;62(1):88–93. doi: 10.1172/JCI109118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. E., Fry M., Blondin G. A. Phospholipids as the molecular instruments of ion and solute transport in biological membranes. Proc Natl Acad Sci U S A. 1980 Jan;77(1):257–261. doi: 10.1073/pnas.77.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado R., Arieff A. I., Massry S. Muscle water and electrolytes in uremia and the effects of hemodialysis. J Lab Clin Med. 1977 Feb;89(2):322–331. [PubMed] [Google Scholar]
- JOSKE R. A., MCALISTER J. M., PRANKERD T. A. Isotope investigations of red cell production and destruction in chronic renal disease. Clin Sci. 1956 Nov;15(4):511–522. [PubMed] [Google Scholar]
- Kirkpatrick F. H., Hillman D. G., La Celle P. L. A23187 and red cells: changes in deformability, K+, Mg-2+, Ca-2+ and ATP. Experientia. 1975 Jun 15;31(6):653–654. doi: 10.1007/BF01944610. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick F. Spectrin: current understanding of its physical, biochemical, and functional properties. Life Sci. 1976 Jul 1;19(1):1–17. doi: 10.1016/0024-3205(76)90368-4. [DOI] [PubMed] [Google Scholar]
- Kominami N., Lowrie E. G., Ianhez L. E., Sakren A., Hampers C. L., Merrill J. P., Lange R. D. The effect of total nephrectomy on hematopoiesis in patients undergoing chronic hemodialysis. J Lab Clin Med. 1971 Oct;78(4):524–532. [PubMed] [Google Scholar]
- LOGE J. P., LANGE R. D., MOORE C. V. Characterization of the anemia associated with chronic renal insufficiency. Am J Med. 1958 Jan;24(1):4–18. doi: 10.1016/0002-9343(58)90357-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi J., Bessler H., Hirsch I., Djaldetti M. Increased RNA and heme synthesis in mouse erythroid precursors by parathyroid hormone. Acta Haematol. 1979;61(3):125–129. doi: 10.1159/000207644. [DOI] [PubMed] [Google Scholar]
- Lo H., Lehotay D. C., Katz D., Levey G. S. Parathyroid hormone-mediated incorporation of 32P-orthophosphate into phosphatidic acid and phosphatidylinositol in renal cortical slices. Endocr Res Commun. 1976;3(6):377–385. doi: 10.3109/07435807609073911. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Liu S. C., Palek J. Release of spectrin-free vesicles from human erythrocytes during ATP depletion. I. Characterization of spectrin-free vesicles. J Cell Biol. 1977 Jun;73(3):548–560. doi: 10.1083/jcb.73.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Peacock M., Kleeman C. R. Turnover of endogenous parathyroid hormone in uremic patients and those undergoing hemodialysis. Trans Am Soc Artif Intern Organs. 1972;18(0):416–422. doi: 10.1097/00002480-197201000-00103. [DOI] [PubMed] [Google Scholar]
- Meytes D., Bogin E., Ma A., Dukes P. P., Massry S. G. Effect of parathyroid hormone on erythropoiesis. J Clin Invest. 1981 May;67(5):1263–1269. doi: 10.1172/JCI110154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- NATHAN D. G., SCHUPAK E., STOHLMAN F., Jr, MERRILL J. P. ERYTHROPOIESIS IN ANEPHRIC MAN. J Clin Invest. 1964 Nov;43:2158–2165. doi: 10.1172/JCI105089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpart A. K., Lorenz P. B., Parpart E. R., Gregg J. R., Chase A. M. THE OSMOTIC RESISTANCE (FRAGILITY) OF HUMAN RED CELLS. J Clin Invest. 1947 Jul;26(4):636–640. doi: 10.1172/JCI101847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W. Effects of divalent cation ionophore A23187 on potassium permeability of rat erythrocytes. J Biol Chem. 1976 Jun 10;251(11):3489–3494. [PubMed] [Google Scholar]
- Rodan S. B., Rodan G. A., Sha'afi R. I. Demonstration of adenylate cyclase activity in human red blood cell ghosts. Biochim Biophys Acta. 1976 Apr 23;428(2):509–515. doi: 10.1016/0304-4165(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Roth S. I., Marshall R. B. Pathology and ultrastructure of the human parathyroid glands in chronic renal failure. Arch Intern Med. 1969 Oct;124(4):397–407. [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- Sarkadi B. Active calcium transport in human red cells. Biochim Biophys Acta. 1980 Sep 30;604(2):159–190. doi: 10.1016/0005-2736(80)90573-8. [DOI] [PubMed] [Google Scholar]
- Shaw A. B. Haemolysis in chronic renal failure. Br Med J. 1967 Apr 22;2(5546):213–216. doi: 10.1136/bmj.2.5546.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Taylor D., Baker R., Hochstein P. The effect of calcium ionophore A23187 on the ATP level of human erythrocytes. Biochem Biophys Res Commun. 1976 May 23;76(2):205–211. doi: 10.1016/0006-291x(77)90712-4. [DOI] [PubMed] [Google Scholar]
- Wallach S., Bellavia J. V., Schorr J., Schaffer A. Tissue distribution of electrolytes, 47ca and 28mg in experimental hyper- and hypoparathyroidism. Endocrinology. 1966 Jan;78(1):16–28. doi: 10.1210/endo-78-1-16. [DOI] [PubMed] [Google Scholar]
- Weed R. I., LaCelle P. L., Merrill E. W. Metabolic dependence of red cell deformability. J Clin Invest. 1969 May;48(5):795–809. doi: 10.1172/JCI106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg S. G., Lubin A., Wiener S. N., Deoras M. P., Ghose M. K., Kopelman R. C. Myelofibrosis and renal osteodystrophy. Am J Med. 1977 Nov;63(5):755–764. doi: 10.1016/0002-9343(77)90162-0. [DOI] [PubMed] [Google Scholar]
- White J. G. Effects of an ionophore, A23187, on the surface morphology of normal erythrocytes. Am J Pathol. 1974 Dec;77(3):507–518. [PMC free article] [PubMed] [Google Scholar]