Abstract
The Polygonum orientale L. extracts were investigated for antibacterial activity against Clavibater michiganense subsp. sepedonicum (Spieckermann & Kotthoff) Davis et al., the causal agent of a serious disease called bacterial ring rot of potato. The results showed that the leaf extracts of P. orientale had significantly (p<0.05) greater antibacterial activity against C. michiganense subsp. sepedonicum than root, stem, flower extracts in vitro. According to the results of single factor experiments and L273(13) orthogonal experiments, optimum extraction conditions were A1B3C1, extraction time 6 h, temperature 80°C, solid to liquid ratio 1∶10 (g:mL). The highest (p<0.05) antibacterial activity was observed when pH was 5, excluding the effect of control. The extracts were stable under ultraviolet (UV). In vivo analysis revealed that 50 mg/mL of P. orientale leaf extracts was effective in controlling decay. Under field conditions, 50 mg/mL of P. orientale leaf extracts also improved growth parameters (whole plant length, shoot length, root length, plant fresh weight, shoot fresh weight, root fresh weight, dry weight, and number of leaves), in the 2010 and 2011 two growing seasons. Further solvent partition assays showed that the most active compounds were in the petroleum ether fractionation. Transmission electron microscopy (TEM) showed drastic ultrastructural changes caused by petroleum ether fractionation, including bacterial deformation, electron-dense particles, formation of vacuoles and lack of cytoplasmic materials. These results indicated that P. orientale extracts have strong antibacterial activity against C. michiganense subsp. sepedonicum and a promising effect in control of bacterial ring rot of potato disease.
Introduction
Clavibater michiganense subsp. sepedonicum (Spieckermann & Kotthoff) Davis et al., is a causal agent of a serious disease called bacterial ring rot of potato. This disease has occurred in major potato-growing areas on all continents except Australia [1]–[2], and yield loss in China was up to 60% [3]–[4]. The name of bacterial ring rot of potato originates from the characteristic “ring rot” symptom (destruction of vascular ring) visible after cutting of infected tuber. C. michiganense subsp. sepedonicum is a highly biotrophic pathogen preferring colonization of the vascular system, particularly the xylem vessels [1]. Colonization of these tissues leads to blocking of the natural transport of water and nutrients followed by wilting of infected leaves and stems. Chemical bactericides e.g. quaternary ammonia, bleach, chlorine dioxide, copper sulfate, potassium permanganate, iodine and phenol groups, are the most commonly used methods for controlling bacterial ring rot of potato [5]–[7]. However, these chemicals have the potential to exert toxic effects on humans and wildlife as well as to cause environment pollution [8]. They also lead to the selection of resistant bacterial populations [9]. In addition, chemical bactericides may not readily be biodegradable and tend to persist for years in environment [10]–[13]. Plants produce a wide variety of physiologically active substances, flavonoids, tannins, alkaloids, saponins sterols, and volatile essential oils [14]. These secondary metabolites are more biodegradable than chemical bactericides and have various functions, including antibacterial activity [15]–[21]. There are few reports available in the literature on the biological prevention and control of C. michiganense subsp. sepedonicum using plant extracts.
Polygonum orientale L. is a fast-growing robust annual herb that is widely distributed in China [22]. It is a traditional Chinese medicinal herb and has been used to treat various diseases, such as fractures, muscle injuries, rheumatism and pain from tissue swelling [23]–[24]. This plant has a porous caudex system, and it can produce large quantities of biomass [25], which may offer a good basis for the production of antibacterial substances. However, no attempts have been made for the management of C. michiganense subsp. sepedonicum by using P. orientale extracts.
The objectives of present study are (1) to evaluate in vitro antibacterial activity of P. orientale extracts against C. michiganense subsp. sepedonicum, and to optimize extraction of P. orientale that can give maximal antibacterial activity. (2) to test the effect of pH and UV on antibacterial activity in P. orientale extracts. (3) to study in vivo effect of P. orientale extracts, and growth parameters of potatoes under field conditions. (4) to partition the P. orientale extracts and determine which fractionation showed the highest antibacterial activity. (5) to determine whether petroleum ether fractionation of P. oriental extracts lead to cell damage of C. michiganense subsp. sepedonicum carrying out with TEM.
Results
In vitro Antibacterial Activity of P. orientale Extracts against C. michiganense Subsp. Sepedonicum
The results presented in Figure 1 showed that root, stem, leaf, flower and whole plant extracts of P. orientale were all effective in inhibiting the growth of C. michiganense subsp. sepedonicum, compared to control (p<0.05, Figure 1). The leaf extracts of P. orientale showed the significantly (p<0.05) highest antibacterial activity, followed by flower extracts, whole plant extracts, root extracts and stem extracts (Figure 1). Therefore, all subsequent assays were performed with leaf extracts.
Figure 1. Antibacterial activities of root, stem, leaf, flower and whole plant extracts of P. orientale.
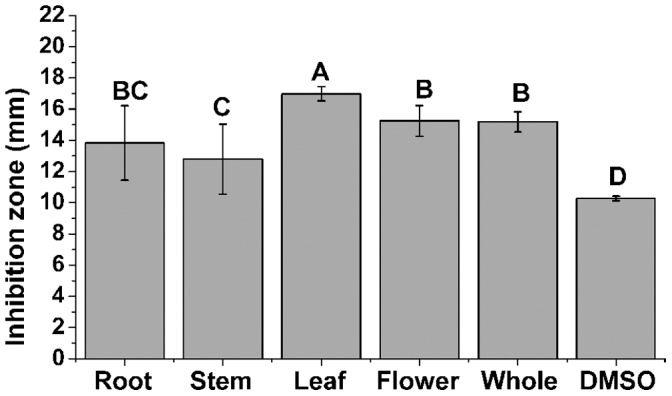
DMSO was used as control. A negative result was defined as an inhibition zone of 10 mm. Greater than 10 mm indicated positive result of the presence of antibacterial substance. Different letters indicated significant differences (p<0.05, ANOVA and Duncan’s multiple range test). Bars represent the means ± standard deviation (S.D.). Each was replicated nine times.
Optimization Study
Single factor experiments
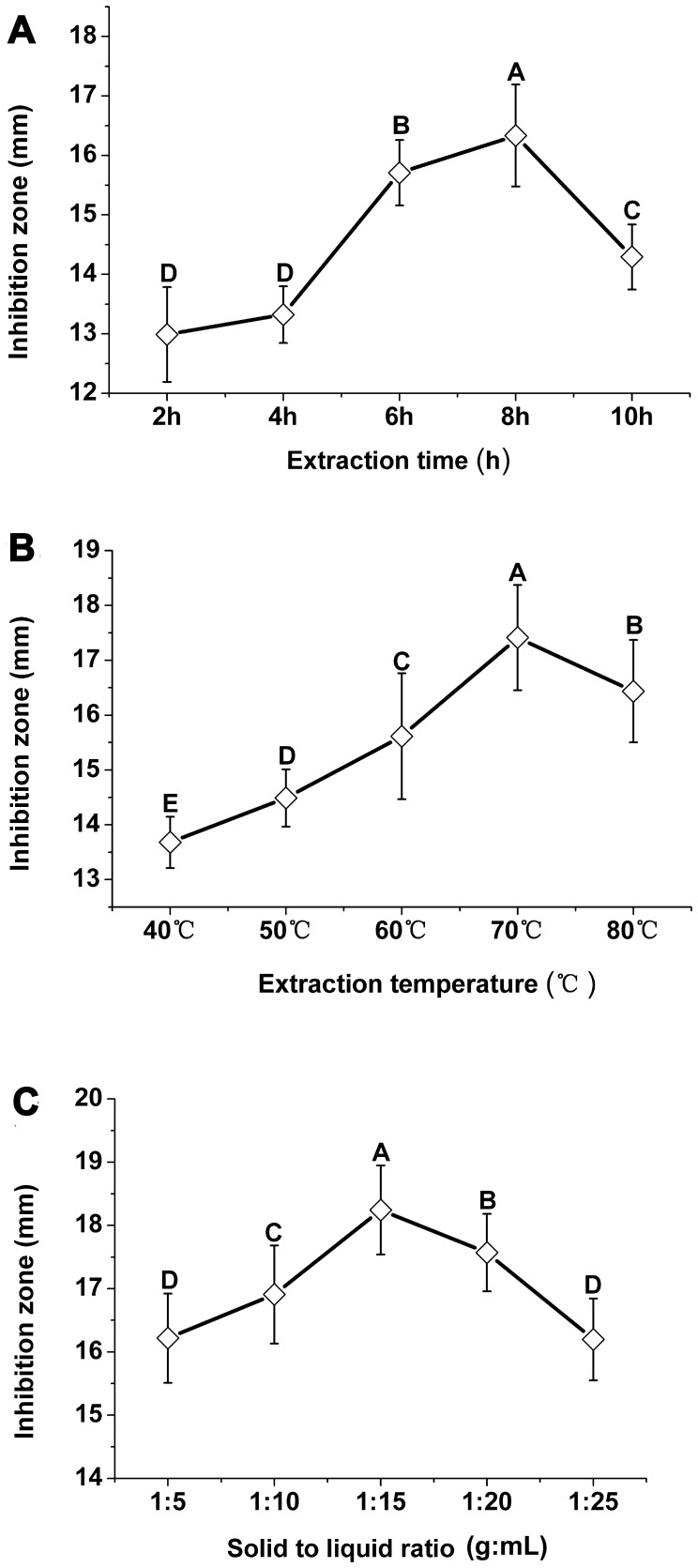
Extraction time (h), extraction temperature (°C), solid to liquid ratio (g:mL) were assessed individually (Figure 2). Figure 2 A depicted the effect of different extraction time on the antibacterial activity in P. orientale leaf extracts. The antibacterial activity increased with extraction time extended. The highest (p<0.05) inhibition zone value was observed at 8 h. Thereafter, antibacterial activity decreased gradually. One-way analysis of variance (ANOVA) shows that the best extraction times with significant (p<0.05) difference were Level 3 (6 h), Level 4 (8 h), Level 5 (10 h), and selected them for orthogonal experimental design in Table 1. Increase in temperature led to greater antibacterial activity in extracts (Figure 2 B), and the highest antibacterial activity with significant (p<0.05) difference was observed at 70°C. However, increasing temperature did not improve the antibacterial activity at 80°C. ANOVA shows that the best extraction temperatures with significant (p<0.05) difference were Level 3 (60°C), Level 4 (70°C), Level 5 (80°C), and selected them for orthogonal experimental design in Table 1. Antibacterial activity of P. orientale increased with an increasing solid to liquid ratio. Maximum (p<0.05) extraction yield of antibacterial substances was achieved at 1∶15 ratio, then antibacterial activity decreased with increasing ratio (Figure 2 C). ANOVA shows that the best solid to liquid ratios with significant (p<0.05) difference were Level 2 (1∶10), Level 3 (1∶15), Level 4 (1∶20), and selected them for orthogonal experimental design in Table 1.
Figure 2. Effect of extraction time (A), extraction temperature (B), and solid to liquid ratio (C).
Different letters indicated significant differences (p<0.05, ANOVA and Duncan’s multiple range test). Bars represent the means ± standard deviation (S.D.). Each was replicated nine times.
Table 1. Factors and levels of orthogonal experiment of P. orientale leaf extraction.
| Levels | Factors | ||
| Extraction time (A) | Extraction temperature(B) | Solid to liquid ratio(C) | |
| 1 | 6h | 60°C | 1∶10 (g:mL) |
| 2 | 8h | 70°C | 1∶15 (g:mL) |
| 3 | 10h | 80°C | 1∶20 (g:mL) |
Optimization of extraction conditions
Orthogonal experimental design, the main method of fractional factorial design, can effectively screen out key variables by several representative experiments [26]–[27]. From experimental results, it was inferred that antibacterial activity of P. orientale was influenced by both different factors at different levels and their interactions. The term L27 (313) of an orthogonal array implies 27 groups of experiments (Table 2). This array handles up to three factors at three levels each. The subscripts 1, 2, and 3 represent the value of a designed factor at levels 1, 2, and 3 respectively. In other words, these subscripts designate each special trial run of the experiment. For example, in the first row of Table 2 (following the indicated subscripts), the factor level of factor A (which is assigned to the first column of the array) is 1, and the level of factors B and C are 1 as well. The first trial run of this experiment will be designed as a level set {1, 1, 1} for factors A, B, C according to Table 1. Therefore the first experiment was carried under extraction time 6 h, temperature 60°C, solid to liquid ratio 1∶10 (g:mL) conditions. The other experiments would perform in the same way, and the experimental results of the orthogonal design were shown in Table 2. Factors that influence antibacterial activity of P. orientale were listed in a decreasing order as follow: C>A>B (Table 2). The individual levels within each factor were ranked as Figure 3: A: 1>2>3; B: 3>1>2; C: 1>2>3.
Table 2. Orthogonal experiment L27 (313) and intuitive analysis.
| ExperimentNO. | Factors | Inhibition zone (mm) | ||
| Extractiontime (A) | Extraction temperature (B) | Solid toliquid ratio (C) | C.michiganense subsp. sepedonicum a | |
| 1 | 1 (6h) | 1 (60°C) | 1 (1∶10, g:mL) | 15.57±2.53 |
| 2 | 1 (6h) | 1 (60°C) | 2 (1∶15, g:mL) | 14.11±1.63 |
| 3 | 1 (6h) | 1 (60°C) | 3 (1∶20, g:mL) | 15±1.91 |
| 4 | 1 (6h) | 2 (70°C) | 1 (1∶10, g:mL) | 16.97±3.01 |
| 5 | 1 (6h) | 2 (70°C) | 2 (1∶15, g:mL) | 14.38±1.72 |
| 6 | 1 (6h) | 2 (70°C) | 3 (1∶20, g:mL) | 13.85±1.11 |
| 7 | 1 (6h) | 3 (80°C) | 1 (1∶10, g:mL) | 17.35±1.32 |
| 8 | 1 (6h) | 3 (80°C) | 2 (1∶15, g:mL) | 16.16±0.96 |
| 9 | 1 (6h) | 3 (80°C) | 3 (1∶20, g:mL) | 14.97±0.92 |
| 10 | 2 (8h) | 1 (60°C) | 1 (1∶10, g:mL) | 15.62±1.45 |
| 11 | 2 (8h) | 1 (60°C) | 2 (1∶15, g:mL) | 15.16±3.08 |
| 12 | 2 (8h) | 1 (60°C) | 3 (1∶20, g:mL) | 12.92±1.53 |
| 13 | 2 (8h) | 2 (70°C) | 1 (1∶10; g:mL) | 15.95±2.83 |
| 14 | 2 (8h) | 2 (70°C) | 2 (1∶15, g:mL) | 15.1±1.94 |
| 15 | 2 (8h) | 2 (70°C) | 3 (1∶20, g:mL) | 11.45±1.90 |
| 16 | 2 (8h) | 3 (80°C) | 1 (1∶10, g:mL) | 16.33±2.33 |
| 17 | 2 (8h) | 3 (80°C) | 2 (1∶15, g:mL) | 14.87±1.07 |
| 18 | 2 (8h) | 3 (80°C) | 3 (1∶20, g:mL) | 15.86±3.42 |
| 19 | 3 (10h) | 1 (60°C) | 1 (1∶10, g:mL) | 18.49±0.77 |
| 20 | 3 (10h) | 1 (60°C) | 2 (1∶15, g:mL) | 14.06±1.63 |
| 21 | 3 (10h) | 1 (60°C) | 3 (1∶20, g:mL) | 13.24±1.39 |
| 22 | 3 (10h) | 2 (70°C) | 1 (1∶10, g:mL) | 15.4±1.53 |
| 23 | 3 (10h) | 2 (70°C) | 2 (1∶15, g:mL) | 15.36±2.66 |
| 24 | 3 (10h) | 2 (70°C) | 3 (1∶20, g:mL) | 13.17±2.48 |
| 25 | 3 (10h) | 3 (80°C) | 1 (1∶10, g:mL) | 15.06±1.80 |
| 26 | 3 (10h) | 3 (80°C) | 2 (1∶15, g:mL) | 14.3±1.62 |
| 27 | 3 (10h) | 3 (80°C) | 3 (1∶20, g:mL) | 12.1±0.97 |
| K 1j b | 15.37 | 14.91 | 16.30 | ∑402.8 |
| K 2j | 14.81 | 14.63 | 14.83 | |
| K 3j | 14.58 | 15.22 | 13.62 | |
| R c | 0.79 | 0.59 | 2.68 | |
| O d | A1 | B3 | C1 | |
DMSO was used as control. A negative result was defined as an inhibition zone of 10 mm. Greater than 10 mm indicated positive result of the presence of antibacterial substance (S.D.). Each value was mean and standard deviation of four replications.
Kij = (1/9) ∑ mean inhibition zone at factor j (j = A, B, C).
Rij = max { Kij } − min { Kij }, j and i mean factor and setting level here, respectively.
O means the optimum condition. The optimum combination of conditions is A1B3C1.
Figure 3. Effect of each parameter on antibacterial activity of P. orientale.
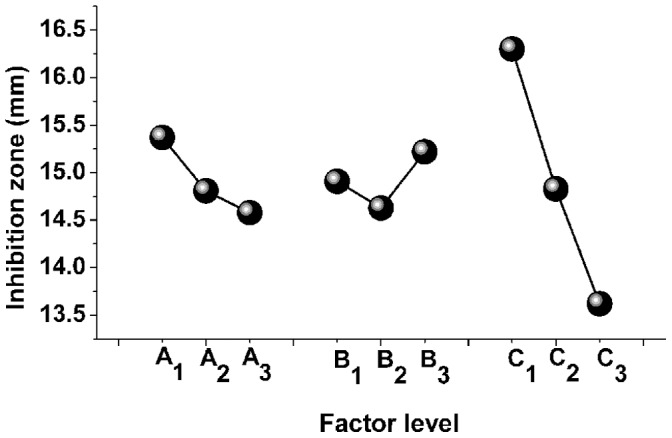
A, extraction time: A1∶6 h, A2∶8 h, A3∶10 h. B, temperature: B1∶60°C, B2∶70°C, B3∶80°C. C, solid to liquid ratio (g:mL): C1∶1:10, C2∶1:15, C3∶1:20.
Because interactions between factors are complex, only low-order interactions were analyzed while high-order (three-, four-, and five-order) interactions were neglected. Table 3 summarize the analysis of variance (ANOVA) of factors and their second-order interactions that affect antibacterial activity. In Table 3, the term “interaction”, indicated by inserting the “×” symbol between the two interacting factors, is used to describe the condition in which the effect of one factor’s influence upon the result is dependent on the condition of the other factor. F-ratio is defined as F = MSF/MSE, where MSF and MSE represent respectively mean square of factors or interactions, mean square of errors. df, SS and MS respectively represent degree of freedom, sum of squares and mean square. If the calculated value F is greater than critical value F α [e.g. F 0.01(2,8) = 8.65], then that factor or interaction is statistically significant. In Table 3, if significant level α = 0.01, then C (Solid to liquid ratio) was statistically significant factor that affect antibacterial activity of P. orientale. Therefore, factor C was regarded as dependent factor in extraction of antibacterial substance. A (Extraction time), B (Temperature) and interactions A×B, A×C, B×C were regarded as independent factors and interactions. Optimum values of these factors for extraction of antibacterial substance from P. orientale were A1B3C1, extraction time 6 h, temperature 80°C, solid to liquid ratio 1∶10 (Table 2). Through optimization test, the inhibition zone was up to 19.54 mm (Figure 4).
Table 3. Results of variance (ANOVA) analysis.
| Source | SS | df | MS | F a | Significanceb |
| A (Extraction time) | 3.03 | 2 | 1.52 | 1.02 | |
| B (Extraction temperature) | 1.60 | 2 | 0.80 | 0.54 | |
| C (Solid to liquid ratio) | 32.58 | 2 | 16.29 | 10.93 | ** |
| A×B (Interaction of extraction time and extraction temperature ) | 8.09 | 4 | 2.02 | 1.36 | |
| A×C (Interaction of extraction time and solid to liquid ratio) | 2.86 | 4 | 0.72 | 0.48 | |
| B×C(Interaction of extraction temperature and solid to liquid ratio) | 2.80 | 4 | 0.70 | 0.47 | |
| Error | 11.90 | 8 | 1.49 | ||
| Total | 62.86 | 26 |
Significant parameter, F 0.05 (2, 8) = 4.46, F 0.05 (4, 8) = 3.84, F 0.01 (2, 8) = 8.65, F 0.01 (4, 8) = 7.01.
** indicated more significant difference.
Figure 4. Antibacterial activity of P. orientale leaf extracts against C. michiganense subsp. sepedonicum.

Using optimum combination of extraction conditions (extraction time 6 h, temperature 80°C, solid to liquid ratio (g:mL) 1∶10, left). DMSO was used as control (right).
Effect of pH and UV on the Antibacterial Activity
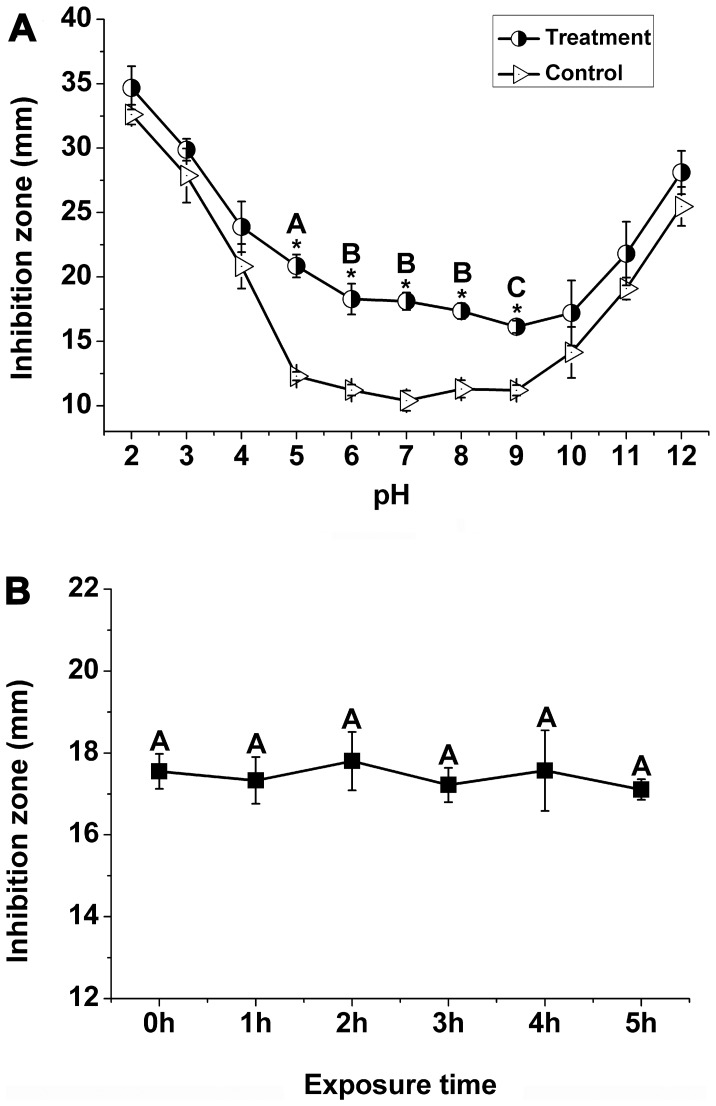
As shown in Figure 5 A, there were no statistically significant (p<0.05) differences between test sets and control sets in the range from pH2 to pH4, and pH10 to pH12. The result implied that, antibacterial activity was enhanced under strongly acidic and alkaline conditions (pH2 to pH4, and pH10 to pH12), but this might be the role of acid and alkali, rather than P. orientale leaf extracts. Based on t-test, we got the result that there were statistically significant (p<0.05) differences between test sets and control sets in the range from pH 5 to pH 9. In other words, antibacterial activity of P. orientale leaf extracts was not impacted by the control in the range from pH 5 to pH 9. In order to exclude the effect of control, we only compared the antibacterial activity of test sets when pH values were between 5 and 9, based on ANOVA. The data showed that maximum efficiency of antibacterial activity was observed when pH was 5 (p<0.05), but it decreased rapidly when pH values were between 6 and 9.
Figure 5. Effect of pH (A) and UV (B) on antibacterial activity in P. orientale leaf extracts.
For pH effect, t-test was carried out to determine significant (p<0.05) differences between test sets and control sets. * indicated significant differences. ANOVA was carried out to determine significant (p<0.05) differences between test sets at different pH values ranging from 5 to 9. Different letters indicated significant differences (p<0.05). Bars represent the means ± standard deviation (S.D.). Each was replicated four times.
To test the UV stability of P. orientale extracts, we investigated the antibacterial activities of different treatments. There were no statistically significant (p<0.05) differences between samples that were exposed to UV light for different times (Figure 5 B), implying that P. orientale extracts were not impacted by exposure to UV light.
Protective Effects of P. orientale Leaf Extracts
To determine whether P. orientale leaf extracts exerted in vivo inhibition against C. michiganense subsp. sepedonicum, an inoculation experiment was performed. As shown in Figure 6 A, water solution of P. orientale leaf extracts had 35.29% protective effect at a lower concentration of 12 mg/mL, 50.49% protective effect at 25 mg/mL. At 50 mg/mL, 75 mg/mL and 100 mg/mL concentrations, the protective effect reached 73.55%, 76.47% and 79.44%. However, there were no statistically significant (p<0.05) differences between 50 mg/mL, 75 mg/mL and 100 mg/mL concentrations, and there were statistically significant (p<0.05) differences between 12 mg/mL, 25 mg/mL and 50 mg/mL concentrations, based on ANOVA. These results clearly demonstrated strong in vivo inhibition against C. michiganense subsp. sepedonicum by P. orientale leaf extracts. Figure 6 B showed tuber lesions caused by bacteria.
Figure 6. In vivo inhibition analysis of P. orientale leaf extracts against C. michiganense subsp. sepedonicum.
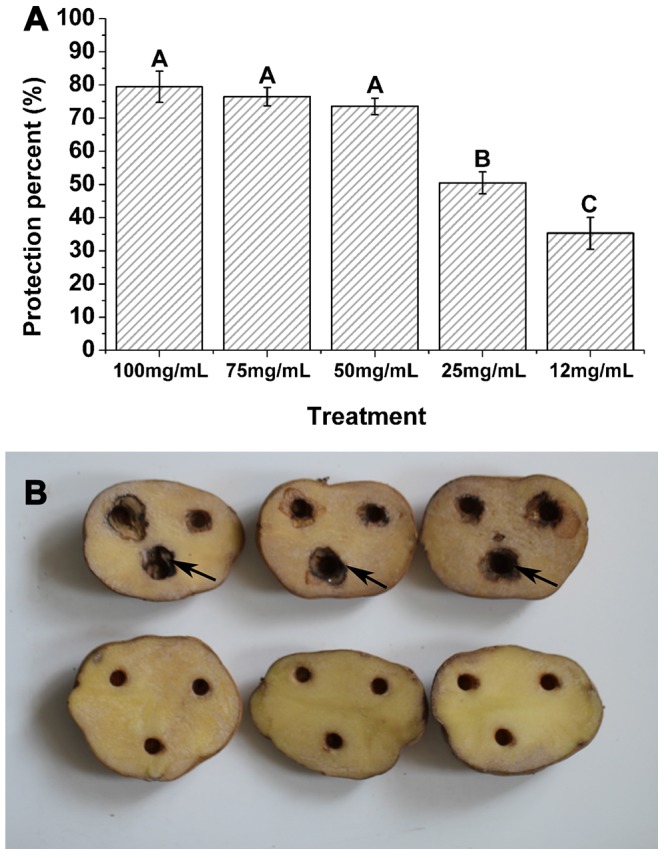
Protection percent treated with 100 mg/mL, 75 mg/mL, 50 mg/mL, 25 mg/mL, 12 mg/mL of P. orientale leaf extracts (A). Different letters indicated significant differences (p<0.05, ANOVA and Duncan’s multiple range test). Bars represent the means ± standard deviation (S.D.). Each was replicated four times. Control, the number of potatoes was 51, which treated with distilled water showing disease symptom. Tuber lesions caused by bacteria (B). Prior to incubation, water solution of P. orientale leaf extracts at concentration of 50 mg/mL was put into the holes of the treatment group (bottom), whereas sterilized water was put into the control (top). Then, all tubers were inoculated with C. michiganense subsp. sepedonicum, and incubated for three days. Tubers treated with water solution of P. orientale leaf extracts at concentration of 50 mg/mL were uninfected (bottom). By contrast, the tubers treated with sterilized water in the control group were severely infected by C. michiganense subsp. sepedonicum and manifested aggressive lesions (top). The resulting lesions were highlighted with arrowheads.
Plant Growth Parameters under Field Conditions
In the present study, growth parameters were recorded after 8 weeks from date of planting during the 2010 and 2011 two growing seasons. Data in Table 4 showed that P. orientale leaf extracts at different concentrations were able to increase the whole plant length and shoot length significantly (p<0.05), compared to negative control, during both growing seasons. 50 mg/mL of extracts increased root length, plant fresh weight, shoot fresh weight and dry weight significantly (p<0.05) in both seasons, compared to negative control. Thus, extracts at 25 mg/mL and 12 mg/mL concentrations were not effective in increasing these parameters. The root fresh weight showed no significantly differences between the P. orientale leaf extract treatments and the negative control in 2010 season. Thus, 50 mg/mL of extracts increased root fresh weight significantly (p<0.05) compared to 25 mg/mL and 12 mg/mL of extracts and negative control, in 2011 season. Extracts at 50 mg/mL and 25 mg/mL concentrations increased the final number of leaves significantly (p<0.05), in 2010 season. In contrast, 50 mg/mL, 25 mg/mL and 12 mg/mL of extracts were all effective in increasing the final number of leaves, in 2011 season.
Table 4. Effect of different concentrations (50 mg/mL, 25 mg/mL and 12 mg/mL) of P. orientale leaf extracts on different growth parameters of potato under field conditions during the 2010 and 2011 growing seasons.
| Treatments | Whole plantlength (cm) | ShootLength (cm) | Rootlength (cm) | Plant freshweight (g) | Shoot freshweight (g) | Root freshweight (g) | Dry weight (g) | Number of leaves |
| Season 2010 | ||||||||
| 50 mg/mL ofextracts | 45.40±5.46A | 24.60±1.14A | 20.80±3.41A | 38.58±4.38AB | 32.08±3.92AB | 6.50±0.82A | 6.00±1.58AB | 50.33±2.52A |
| 25 mg/mL ofextracts | 39.20±4.49A | 23.40±4.72A | 15.80±2.49AB | 31.28±9.37BC | 25.90±9.73BC | 5.38±0.87A | 4.48±0.90BC | 49.00±1.73A |
| 12 mg/mL ofextracts | 38.60±1.14A | 22.10±3.44A | 16.50±3.81AB | 23.60±4.10C | 18.84±2.47BC | 4.76±2.11A | 3.92±0.87C | 38.33±8.02B |
| 50 mg/L of copper sulfate(positive control) | 45.60±2.00A | 24.00±1.48A | 21.60±1.79A | 46.58±7.35A | 40.68±10.45A | 5.90±1.65A | 6.72±1.31A | 56.67±9.02A |
| Untreated(negativecontrol) | 29.00±3.15B | 15.20±4.63B | 13.80±3.29B | 19.70±3.00C | 14.68±4.31C | 5.02±0.34A | 3.54±0.43C | 28.33±1.53B |
| Season 2011 | ||||||||
| 50 mg/mL ofextracts | 49.60±2.16A | 27.57±0.38A | 22.03±1.82A | 45.13±3.00A | 37.87±4.00A | 7.26±0.98A | 7.26±0.70A | 55.33±4.16AB |
| 25 mg/mL ofextracts | 46.00±4.00A | 27.03±1.10A | 18.97±3.10AB | 31.82±2.21B | 25.91±1.61B | 5.91±0.60B | 4.91±0.69B | 53.67±5.51B |
| 12 mg/mL ofextracts | 45.00±2.00A | 26.47±1.03A | 18.53±3.01AB | 31.05±2.63B | 25.05±2.75B | 6.00±0.25B | 4.62±0.46B | 53.33±3.06B |
| 50 mg/L of copper sulfate(positive control) | 48.00±2.00A | 25.73±1.60A | 22.27±0.42A | 45.96±3.30A | 38.45±3.92A | 7.51±0.78A | 6.77±0.33A | 61.33±3.06A |
| Untreated (negativecontrol) | 30.00±1.55B | 14.80±1.71B | 15.20±0.30B | 27.13±1.59B | 22.03±1.83B | 5.10±0.36B | 4.01±0.54B | 30.67±2.52C |
All data are average of three replications. Each value was the mean with standard deviation (S.D.). Different letters indicated significant differences (p<0.05, ANOVA and Duncan’s multiple range test). Untreated potato tubers were used as the negative control, and 50 mg/L of copper sulfate was used as the positive control.
Partition of the Ethanol Extracts
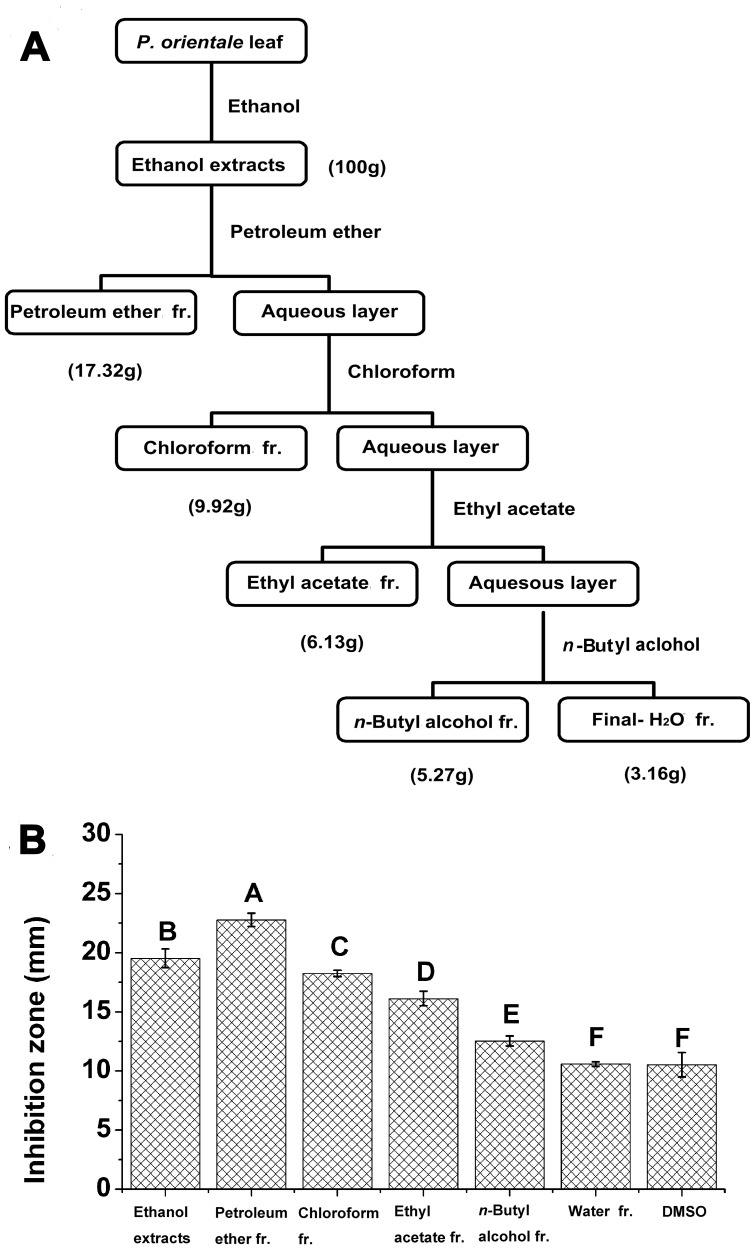
The ethanol extracts were partitioned with four solvents and the antibacterial activity was measured in five partitions (Figure7). The amount of petroleum ether fractionation (17.32 g) was the largest among the five fractionations, compared with 9.92 g in chloroform fractionation, 6.13 g in ethyl acetate fractionation, 5.27 g in n-butyl alcohol fractionation and 3.16 g in water fractionation (Figure 7 A). At a concentration of 1 mg/mL, the petroleum ether fractionation showed the highest (p<0.05) antibacterial activity, followed by ethanol extracts (positive control), chloroform fractionation, ethyl acetate fractionation, n-butyl alcohol fractionation, water fractionation and DMSO (negative control, Figure7 B). These results suggested that the petroleum ether fractionation was the most efficient in inhibiting the C. michiganense subsp. sepedonicum.
Figure 7. Fractionation charts of P. orientale extracts (A), and antibacterial activities of fractionations (B).
Fr. means fractionation. Different letters indicated significant differences (p<0.05, ANOVA and Duncan’s multiple range test). Bars represent the means ± standard deviation (S.D.). Each was replicated four times.
Observation of Interior Damage
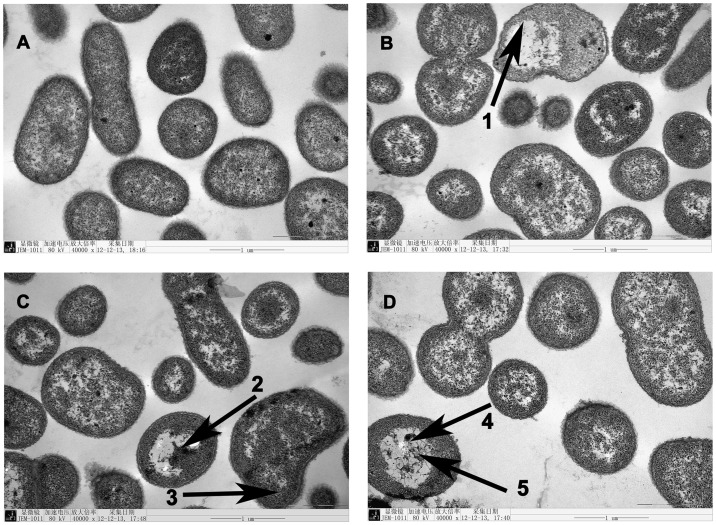
The influence of petroleum ether fractionation from P. oriental extracts on the cell morphology of C. michiganense subsp. sepedonicum was investigated by TEM. Untreated cells showed no changes in cell morphology after eight hours. Cell showed a typical cell wall, cytoplasmic membrane, periplasmic space, and cytoplasmic content (Figure 8 A). In contrast, C. michiganense subsp. sepedonicum treated with petroleum ether fractionation (0.05 mg/mL) exhibited a wide range of abnormalities (Figure 8 B–D). Compared with undamaged cells, it was easy to find small vacuoles inside the cells (Figure 8 B–D). Some cells showed formation of big vacuoles (pure-white regions of bacteria) and lack of cytoplasmic material (Figure 8 (1, 2, 5)), others showed bacteria misshapen (Figure 8 (1, 3)). In addition, electron-dense particles were also observed in damaged bacterial cell (Figure 8 (4)).
Figure 8. Transmission electron micrographs of C. michiganense subsp. sepedonicum cells.
Untreated (A, 40.000x). Treated with the petroleum ether fractionation of P. orientale extracts (B,C,D, 40.000x). Malformation of cells (1, 3). Formation of vacuoles and loss of cytosol structure and contents inside the cells (1, 2, 5). The cytoplasm was coagulated (4).
Discussion
The exploitation of plant products for the management of plant diseases has made significant progress due to its readily available nature, easy biodegradability, non-phytotoxicity [28]. Recently, several studies have been reported to use plant extracts in controlling plant diseases [29]–[30]. In the present study, we evaluated the antibacterial activity in P. orientale extracts against C. michiganense subsp. sepedonicum. Among root, stem, leaf, flower and whole plant extracts of P. orientale, the leaf extracts showed significantly (p<0.05) highest antibacterial activity (Figure 1). This demonstrated that P. orientale played an important role in biological control of C. michiganense subsp. sepedonicum, the causal agent of bacterial ring rot of potato. In addition, this is the first report of P. orientale as a potential agent against C. michiganense subsp. sepedonicum.
Solvent played a key role in extraction of antibacterial substances from P. orientale. In this study, we used ethanol. Ethanol has high polarity index, dielectric constant and cohesive energy, as compared with other solvents, which provides strong bounding between solvent molecules and compounds from the solutes, causing their dissolution [31]. In addition, ethanol has several advantages such as low toxicity, economical, and lower boiling point [31]. There are many factors affecting the extraction, among them, extraction time (A), extraction temperature (B), and solid to liquid ratio (C) are key factors. Single factor experiment was performed by one factor varied with different levels while other factors being fixed. Shorter extraction times would result in incomplete extraction. Longer extraction times would lead to waste of time and energy, and antibacterial components might be decomposed (Figure 2 A) [32]. Increasing temperature enhanced diffusivity and thus the yield of antibacterial activity in extracts was increased with higher temperature [33]. When temperature was too high, ethanol volatilization was accelerated and the solid to liquid ratio was lowed, and thus the yield of antibacterial activity was decreased (Figure 2 B). When the solid to liquid ratio was too low, the contact between antibacterial substance and solvent was not sufficient enough, and it was not conducive to extract maximal amount of antibacterial substances. When the solid to liquid ratio was too high, concentration time would be long and antibacterial components might be decomposed (Figure 2 C) [34].
The orthogonal experimental design was used to study optimization of parameters for efficient extraction of antibacterial substances from P. orientale. The advantage of orthogonal experimental design is that its economical for characterizing a complicated process in fewer experiments. However, it requires a specialized experimental design to properly set up the test and specialized statistics to analyze data[35]–[37]. The results (Table 2, Table3) revealed that factor C (Solid to liquid ratio) had significant effect on the antibacterial activity, while the other factors and interactions were not identified as significant factors and interactions under the selected conditions based on ANOVA. We concluded that solid to liquid ratio was the major factor affecting P. orientale extraction. Thus, we should pay more attention to the factor in extraction. The optimum extraction conditions for P. orientale were defined as below: extraction time: 6 h, temperature: 80°C, solid to liquid ratio: 1∶10 (g:mL). Compared with conventional extraction conditions, our optimum extraction conditions in this study are economic, convenient and efficient [38]. Further, this extraction method meets the actual needs and is also compliant with environmental regulations.
Environmental factors often influence the efficacy of bactericides [39]. In this study, we tested whether pH and UV could influence the efficacy of P. orientale leaf extracts. Data (Figure 5 A) showed that the highest (p<0.05) antibacterial activity was observed when pH was 5, excluding the effect of control. This indicated that either organic acids or other pH-dependent antibacterial compounds were responsible for the antibacterial effect [40]. These results indicated that P. orientale leaf extracts would be best used when pH was 5. As exposure time changed, no statistically significant (p<0.05) differences were observed between different UV treatments (Figure 5 B). The results showed that extracts were stable following exposure to UV.
Although in vitro test of plant extracts is an important first step in selecting plants with potential antibacterial activity against plant pathogens, in vivo test is reproducible [41]. Results (Figure 6) obtained from the in vivo study indicated that P. orientale leaf extracts contained strong antibacterial activity against C. michiganense subsp. sepedonicum in vivo. Our finding of the protective effect of P. orientale leaf extracts provides evidence that the antibacterial potentiality of P. orientale leaf extracts can be used as an alternative to bactericides.
P. orientale leaf extracts were tested at different concentrations for increasing the plant growth under field condition in the 2010 and 2011 two growing seasons. All the treatments were significant (p<0.05) for increases in whole plant length and shoot length promotion, compared to negative control, during both growing seasons (Table 4). A clear correlation was observed between plant growth parameters and concentrations of extraction. Among different treatments, 50 mg/mL had the best performance for most of the parameters assessed, in both 2010 and 2011 growing seasons (Table 4). Our results indicated that application of P. orientale leaf extracts was effective in increasing the plant growth under field condition.
The results of partition showed that the antibacterial material exists largely in the petroleum ether phase. Su et al, (2012) [42] had identified chemical composition of petroleum ether fractionation from P. orientale ethanol extracts by using GC-MS method, for anti-tumor research. Therefore, we did not repeat this experiment. As the results, forty eight components were identified. The major components were separated into six classes, including nexplanon (18.31%), alkane (1.62%), ester (10.95%), alcohol (9.65%), organic acids (10.55%) and ketone (7.36%). The mechanism of action of these compounds is not fully understood. But among these compounds, ethyl laurate, hexadecanoic acid, ethyl oleate, hexadecane, clionasterol, eicosanoic acid and stigmasterol are known to exhibit antibacterial activity [43]–[54]. On the other hand, it is speculated that cell membrane disruption by lipophilic compounds (nexplanon, alkane, ester and ketone) may be involved [55]. Because the highly lipophilic compounds easily pass through cell membranes to induce biological responses [56]. Third, it is possible that the active components might be involved in some type of synergism in antibacterial activity with lipophilic compounds [57].
To investigate possible changes in cell morphology, TEM was applied. C. michiganense subsp. sepedonicum, treated with petroleum ether fractionation of P. oriental extracts, showed electron-dense particles, bacteria misshapen, formation of vacuoles and lack of cytoplasmic materials (Figure 8). These results indicated that compounds from the petroleum ether fractionation had penetrated the cell walls and cell membranes, and interacted with cellular contents. Considering the constituents of petroleum ether fractionation from P. oriental extracts, it was most likely that antibacterial activity was not attributable to one specific mechanism, since there were several targets in the cell. The components of petroleum ether fractionation included nexplanon, alkane, ester, alcohol, organic acids and ketone. Lipophilic compounds had the ability to interact with hydrophobic structures, like bacterial membranes [58]. It was speculated that lipophilic compounds disrupted the cytoplasmic membrane of C. michiganense subsp. sepedonicum, thereby causing leakage of the bacterial cell content. Furthermore, the dysfunction and disruption of the membrane, interference with the energy generation system in cell, and enzyme inhibition preventing substrate utilization for energy production might also lead to the death of bacterial cells [59]–[61]. In TEM, the appearance of electron-dense particles might be result from several possible events. The petroleum ether fractionation contained antibacterial substances, such as ethyl laurate, hexadecanoic acid, ethyl oleate, hexadecane, clionasterol, eicosanoic acid and stigmasterol. These substances might be due to the precipitation of abnormal proteins, and we could see protein aggregation as electron-dense particles in the TEM. Based on the present research, schematic model for proposed mechanism was described as follows. Lipophilic materials in petroleum ether fractionation made a break through the outer membrane firstly, causing the leakage of cellular contents. Secondly, antibacterial materials in petroleum ether fractionation entered the inner membrane, thus inhibiting respiration and growth of cells. Simultaneously, antibacterial materials could affect some proteins, resulting in cell decomposition and death eventually.
Conclusion
In conclusion, P. oriental extracts consistently showed significant antibacterial activity against C. michiganense subsp. sepedonicum in in vitro, in vivo and in field experiments, respectively. The optimum extraction conditions were investigated using single factor experimental design and L273(13) orthogonal experimental design. The maximum efficiency of antibacterial activity was observed when pH was 5. The extracts were relatively stable when exposed to UV radiation. From partition study, it has become clear that petroleum ether fractionation of P. orientale extracts showed the greatest potential to inhibit the growth of C. michiganense subsp. sepedonicum. TEM investigated the possible mechanism of petroleum ether fractionation against C. michiganense subsp. sepedonicum. Results of TEM revealed that petroleum ether fractionation of P. orientale extracts caused cytoplasm coagulated in cell, bacterial misshapen, formation of vacuoles and lack of cytoplasmic material. These findings indicate that P. oriental extracts have a great potential for biological control of C. michiganense subsp. sepedonicum. The antibacterial activity of the agent against C. michiganense subsp. sepedonicum should be applied in the field for potato protection. The agent offers a safe alternative to synthetic bactericide.
Materials and Methods
Plant Material and Pathogen
Polygonum orientale L. was collected from wetland of Fenhe River in Taiyuan section, Shanxi Province, China, in July 2008. The collection of plant is not need specific permissions, and the field studies did not involve endangered or protected species. Taxonomic identification was performed in our lab. Clavibater michiganense subsp. sepedonicum (Spieckermann & Kotthoff) Davis et al. (ATCC 33113) was provided by Chinese Academy of Agricultural Science. Potato cultivar ‘Jinhan-1′ was obtained from a local seed agency.
Preparation of Plant Extracts
The roots, stems, leaves and flowers of P. orientale were cut into small pieces (2–4 cm) respectively. Each was washed several times with running tap water, then with sterile water, and dried at room temperature for 15 days [62]. Dry materials were ground to fine powders in a grinder. Then 100 g of each powder was blended in 1 L of ethanol at room for 24 h [63]. The extracts were concentrated to dryness using a rotary evaporator after filtrating.
In vitro Assays
Determination of antibacterial activity was accomplished by agar diffusion method (ADM) [64]. Each residue was dissolved in DMSO (dimethyl sulphoxide) to give a final concentration of 1 mg/mL. Fresh strain (18–24 h old) grown in nutrient broth was used for the studies. The medium contained 1 L distilled water, 5 g beef extract, 10 g pepton, 5 g sodium chloride, 20 g agar. The agar surface was perforated with 10 mm diameter holes, aseptically cut and filled with 200 µL of each sample. The DMSO was used as control since it does not inhibit microorganism growth [65]. After the diffusion of the solution in each hole, the plates were inverted and incubated at 28°C for 24 h. Antibacterial activity was determined by measuring the radius of the inhibition zone around the hole. Each treatment was replicated nine times.
Optimization of Extraction Condition
Single factor experiments
The three factors including extraction time (h), extraction temperature (°C), solid to liquid ratio (g:mL) could affect extraction efficiency. Single factor experiments were applied to decide appropriate levels. For each single factor, five different levels were designed, with other factors being kept constant. For each experiment, 100 g of P. orientale leaf sample was added to corresponding volume of ethanol and extracted as described in Table 5. Then, antibacterial activity of extracts (1 mg/mL) from each sample was analyzed by ADM to choose three reasonable levels of three factors for orthogonal experimental design. Each treatment was replicated nine times.
Table 5. Single factor experiment design.
| Factors | Conditions | Levels | ||||
| 1 | 2 | 3 | 4 | 5 | ||
| Extraction time (h) | Temperature 80°C | 2 | 4 | 6 | 8 | 10 |
| Solid to liquid ratio 1∶5 | ||||||
| Temperature (°C ) | Extraction time 8h | 40 | 50 | 60 | 70 | 80 |
| Solid to liquid ratio 1∶5 | ||||||
| Solid to liquid ratio (g:mL) | Extraction time 8h | 1∶5 | 1∶10 | 1∶15 | 1∶20 | 1∶25 |
| Temperature 80°C | ||||||
Orthogonal experimental design
On the basis of single factor experiments, three levels of three factors were selected as described in Table 1. Then orthogonal array L27(313) matrix was used to determine the optimum extraction conditions of antibacterial substances from P. orientale, with the consideration of the interactions between the parameters [66]–[67]. For each experiment, 100 g of P. orientale leaf sample was added to corresponding volume of ethanol and extracted as described in (Table 2). Four replicates were used for each extraction. Then, antibacterial activity of extracts (1 mg/mL) from each sample was analyzed by ADM. Each treatment was replicated four times. ANOVA were performed by using SPSS software package (version 17.0) to identify significant extraction factor and interaction between samples.
pH and UV Stability Assays
The effect of pH on antibacterial activity in P. orientale leaf extracts was examined by pH stability assays [68]–[69]. Tests were conducted in two sets: test sets of P. orientale leaf extracts were adjusted with 5 M NaOH or 5 M HCl to different pH values ranging from 2 to 12. The control sets were prepared using the same method with DMSO except that no P. orientale leaf extract was added. To test the impact of UV, P. orientale leaf extracts were incubated under UV light (256rim, 6W, 5 cm) for a period ranging from 1 h to 5 h. Then, antibacterial activity of extracts (1 mg/mL) was analyzed by ADM. Each treatment was replicated four times.
In vivo Assays
The dried powder of P. orientale leaf extracts was dissolved in distilled water to produce a series of concentration solutions, including 100 mg/mL, 75 mg/mL, 50 mg/mL, 25 mg/mL and 12 mg/mL [70]–[73]. To examine in vivo effect of P. orientale leaf extracts, healthy potatoes without physical injuries or infections were scraped (1 mm deep and 8 mm wide) with a sterile nail. Then, 50 µL of 100 mg/mL, 75 mg/mL, 50 mg/mL, 25 mg/mL and 12 mg/mL solution or 50 µL of sterile distilled water (control) was put into each hole (one potato contained three holes). After 24 h, 10 µL of C. michiganense subsp. sepedonicum at 106 CFU/mL was put into each hole. The treated potatoes were put in trays covered with plastic bags to maintain a relative humidity of approximately 95%, then incubated at 28°C. Protection percentage was calculated on the fourth day after inoculation using the following formula:
Control : The number of potatoes showing disease symptoms, treated with distilled water.
Treatment: The number of potatoes showing disease symptoms, treated with P. orientale leaf extracts.
Each experiment was repeated four times, with 60 potatoes per experiment.
Field Experiments
Based on the results of in vivo assays, the dried powder of P. orientale leaf extracts was dissolved in distilled water to produce 50 mg/mL, 25 mg/mL and 12 mg/mL solutions. Overnight culture of C. michiganense subsp. sepedonicum was adjusted to 106 CFU/mL, and was then incubated 20 µL in the tuber. When the first symptoms of bacterial ring rot of potato occurred naturally, tubers were soaked in water solutions of P. orientale extracts at 50 mg/mL, 25 mg/mL and 12 mg/mL concentration, respectively, for 15 mins. Untreated potato tubers and potato tubers treated with 50 mg/L of copper sulfate (chemical bactericides) were used as controls. Each treatment consisted of three replicates with 50 tubers each. A total of 150 tubers were treated in each variant. All field experiments were conducted on the farm of Shanxi Academy of Agricultural Science, Shanxi Province, China, during the 2010 and 2011 two growing seasons. The land accessed is not privately owned or protected. The treatments were arranged in a complete randomized block design with three plots as replicates. Ten tubers per line (distances between plants: 30 cm, and distances between lines: 40 cm) were sown and five lines per plot (4 m×3 m) were maintained. The observation of growth parameters (whole plant length, shoot length, root length, plant fresh weight, shoot fresh weight, root fresh weight, dry weight, and number of leaves) were recorded and analyzed after 8 weeks from date of planting, in both seasons.
Solvent Partition of P. orientale Extracts
100 g of dried powder of P. orientale extracts was taken and mixed with 500 mL of sterile water. Then the sample was sequentially extracted using petroleum ether, chloroform, ethyl acetate, and n-butyl alcohol as extraction solvents (1∶1, v/v). Each partitioned extract was then concentrated to dryness. The process was repeated four times for each of the four solvents [74]. Then each dried powder from all four partitioned extracts and water partition was dissolved in DMSO to give a final concentration of 1 mg/mL for antibacterial activity assays (ADM).
Transmission Electron Microscopy (TEM)
TEM technique was used to observe the structural changes in C. michiganense subsp. sepedonicum (ATCC 33113) induced by petroleum ether fractionation of P. oriental extracts. Logarithmic phase cells of C. michiganense subsp. sepedonicum (each approximately 109 CFU/L) were treated with petroleum ether fractionation of P. oriental extracts at 0.05 mg/mL for 8 h. No treatment with petroleum ether fractionation of P. oriental extracts was as control. Cells were then collected by centrifugation and washed with 0.05 mol/L phosphate buffer saline (PBS), pH7.0. The samples were transferred to fresh 0.5% glutaraldehyde, and kept for 30 min at 4°C, centrifuged at 13,000 rpm, and fixed in 3% glutaraldehyde. Cells were further fixed in 1% OSO4, dehydrated in gradually increased acetone solutions, and embedded in Epon812. Ultrathin sections were cut and stained with uranyl acetate and lead citrate. Electron micrographs were taken with a JEM–1011(Tokio, Japan) transmission electron microscope at 80 kV.
Statistical Analysis
ANOVA and t-test were performed on the data, using the SPSS package software (Version 17.0).
Acknowledgments
We thank Edanz Group China for the critical reviews of the manuscript and editorial assistance with the English.
Funding Statement
The authors have no support or funding to report.
References
- 1. Fousek J, Mráz I (2003) Determination of genetic differences between fluid and nonfluid variants of Clavibacter michiganensis subsp. sepedonicus using rep-PCR technique. Folia Microbiol 48: 682–686. [DOI] [PubMed] [Google Scholar]
- 2. van der Wolf JM, van Beckhoven JRCM, Hukkanen A, Karjalainen R, Müller R (2005) Fate of Clavibacter michiganensis ssp. sepedonicus, the causal organism of bacterial ring rot of potato, in weeds and field crops. J Phytopathology 153: 358–365. [Google Scholar]
- 3. Fu WJ, Dou LX, Chen H (2005) The control of bacterial ring rot of potato. Forest By-Product Specialityin Chin (77): 40. [Google Scholar]
- 4. Wang L, Zhang J, Zhao R, Li Y, Li C, et al. (2010) Adsorption of Pb (II) on activated carbon prepared from Polygonum orientale Linn.: kinetics, isotherms, pH, and ionic strength studies. Bioresource Technol 101: 5808–5814. [DOI] [PubMed] [Google Scholar]
- 5. Secor GA, De Buhr L, Gudmestad NC (1987) Chemical sanitation for bacterial ring rot control. Am Potato J 64: 699–700. [Google Scholar]
- 6. Qiu YE (2004) The occurrence and control of ring-rot in potato. Xinjiang Agric Sci (41): 88–89. [Google Scholar]
- 7. Chen Y, Yue XL, Wang YC (2010) Features and integrated management of ring-rot in potato. J Shanxi Agric Sci 38 (7): 140–141. [Google Scholar]
- 8. Knight SC, Anthony VM, Brady AM, Greenland AJ, Heaney SP, et al. (1997) Rationale and perspectives on the development of fungicides. Annu Rev Phytopathol 35: 349–372. [DOI] [PubMed] [Google Scholar]
- 9. Demoz BT, Korsten L (2006) Bacillus subtilis attachment, colonization, and survival on avocado flowers and its mode of action on stemend rot pathogens. Biol Control 37: 68–74. [Google Scholar]
- 10.Brady NC (1984) The nature and properties of soils. Mac Millan Publishing Company, New York, p. 528.
- 11. Ippolito A, Nigro F (2000) Impact of preharvest application of biological control agents on postharvest diseases of fresh fruits and vegetables. Crop Prot 19: 715–723. [Google Scholar]
- 12. Cardoso RA, Pires LTA, Zucchi TD, Zucchi FD, Zucchi TMAD (2010) Mitotic crossing-over induced by two commercial herbicides in diploid strains of the fungus Aspergillus nidulans . Gen Mol Res 9: 231–238. [DOI] [PubMed] [Google Scholar]
- 13. Carson CF, Riley TV (2003) Non-antibiotic therapies for infectious diseases. Commun Dis Intell 27: S143–S146. [PubMed] [Google Scholar]
- 14. Chen Y, Dai G (2012) Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber. J Sci Food Agric 92: 1937–1943. [DOI] [PubMed] [Google Scholar]
- 15. Wilson CL, Solar JM, Ghaouth AEL, Wisniewski ME (1997) Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea . Plant Dis 82: 204–210. [DOI] [PubMed] [Google Scholar]
- 16. Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19: 603–608. [Google Scholar]
- 17. Liu YJ, Zhang KQ (2004) Antimicrobial activity of selected Cyathus species. Mycopathologia 157: 185–189. [DOI] [PubMed] [Google Scholar]
- 18. Mares D, Romagnoli C, Tosi B, Andreotti E, Chillemi G, et al. (2005) Chicory extracts from Cichorium intybus L. as potential antifungals. Mycopathologia 160: 85–92. [DOI] [PubMed] [Google Scholar]
- 19. Soylu EM, Soylu S, Kurt S (2006) Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthora infestans . Mycopathologia 161: 119–128. [DOI] [PubMed] [Google Scholar]
- 20. Akhtar Y, Yeoung R, Isman MB (2008) Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusiani and Pseudaletia unipuncta . Phytochem Rev 7: 77–88. [Google Scholar]
- 21. Yazaki K, Sugiyama A, Morita M, Shitan N (2008) Secondary transport as an efficient membrane transport mechanism for plant secondary metabolites. Phytochem Rev 7: 513–524. [Google Scholar]
- 22. Wang L, Zhang J, Zhao R, Li C, Li Y, et al. (2010) Adsorption of basic dyes on activated carbon prepared from Polygonum orientale Linn: equilibrium, kinetic and thermodynamic studies. Desalination 254: 68–74. [Google Scholar]
- 23. Wei Y, Chen X, Jiang X, Ma Z, Xiao J (2009) Determination of taxifolin in Polygonum orientale and study on its antioxidant activity. J Food Compos Anal 22: 154–157. [Google Scholar]
- 24. Xiong JH, Liu ZH, Wang KQ (2007) Extraction and purification of polyphenols in Ilex paraguarensis and its antibacteriostatic activities. Food Mach 23(5): 78–80. [Google Scholar]
- 25. Wang RX, He YC, Zhao TC, Tian HX, Li YF, et al. (2010) Identification, disease-preventing role and growth-promoting effect of biocontrol strain P1 to potato bacteria ring rot. Acta Phytopathologica Sinica 40(1): 66–73. [Google Scholar]
- 26. Antony J (2006) Taguchi or classical design of experiments: a perspective from a practitioner. Sens Rev 26: 227–230. [Google Scholar]
- 27. Kilickap K (2010) Modeling and optimization of burr height in drilling of Al-7075 using Taguchi method and response surface methodology. Int J Adv Manuf Technol 49: 911–923. [Google Scholar]
- 28. Harish S, Saravanakumar D, Radjacommare R, Ebenezar EG, Seetharaman K (2008) Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. BioControl 53: 555–567. [Google Scholar]
- 29. Kagale S, Marimuthu T, Thayumanavan B, Nandakumar R, Samiyappan R (2004) Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae . Physiol Mol Plant Pathol 65: 91–100. [Google Scholar]
- 30. Elbadri GA, Lee DW, Park JC, Yu HB, Choo HY (2008) Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita . J Asia-Pac Entomol 11: 99–102. [Google Scholar]
- 31.Xie BX, Lu ZK 2004. Effect of extract condition on extract ratio and restraining bacteria of Sinocalamus latiflorus leave. Nonwood Forest Res 22(3), 5–8.
- 32. Yang DM, Zhu XY, Feng LD, Bi Y, Ying TJ (2010) Study on the extraction technology of the antibacterial components from Potentilla ansterina L. J Chin Inst Food Sci Technol. 10: 47–51. [Google Scholar]
- 33. Liu RM, Zhang K, Cui QX (2002) Study on extraction of pumpkin seed oil by supercritical CO2 . Food Ferm Indus 29: 61–65. [Google Scholar]
- 34. Bernardo-Gil MG, Casquiho M, Esquível MM, Ribeiro AM (2009) Supercritical fluid extraction of fig leaf gourd seeds oil: fatty acids composition and extraction kinetics. J Supercrit Fluids 49: 32–36. [Google Scholar]
- 35. Sun L, Lee HK (2003) Optimization of microwave–assisted extraction and supercritical fluid extraction of carbamate pesticides in soil by experimental design methodology. J Chromatogr A 1014: 165–177. [DOI] [PubMed] [Google Scholar]
- 36. Nishida M, Yashiki M, Namera A, Kimura K (2006) Single hair analysis of methamphetamine and amphetamine by solid phase microextraction coupled with in matrix derivatization. J Chromatogr B 842: 106–110. [DOI] [PubMed] [Google Scholar]
- 37. Díez C, Barrado E, Marinero P, Sanz M (2008) Orthogonal array optimization of a multiresidue method for cereal herbicides in soils. J Chromatogr A 1180: 10–23. [DOI] [PubMed] [Google Scholar]
- 38. Li Z, Pan Q, Cui X, Duan C (2010) Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci Biotechnol 19(4): 1047–1053. [Google Scholar]
- 39. Qasem JR, Abu-Blan HA (1995) Antifungal activity of aqueous extracts from some common weed species. Ann Appl Biol 127: 215–219. [Google Scholar]
- 40. Li H, Liu L, Zhang S, Cui W, Lv J (2012) Identification of antifungal compounds produced by Lactobacillus casei AST18. Curr Microbiol 65: 156–161. [DOI] [PubMed] [Google Scholar]
- 41. Askarne L, Talibi I, Boubaker H, Boudyach EH, Msanda F, et al. (2012) In vitro and in vivo antifungal activity of several Moroccan plants against Penicillium italicum, the causal agent of citrus blue mold. Crop Pro 40: 53–58. [Google Scholar]
- 42. Su FP, Lou YC, Zhang DQ, Hu JX, Wang C, et al. (2012) Antitumor effect and chemical constitutes of the petroleum ether fraction from P. orientale extracts. Chin Tradit Pat Med 34(5): 938–940. [Google Scholar]
- 43. Viswanathan MB, Jeya Ananthi JD, Sathish Kumar P (2012) Antimicrobial activity of bioactive compounds and leaf extracts in Jatropha tanjorensis. . Fitoterapia 83: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 44. Tao R, Wang CZ, Kong ZW (2013) Antibacterial/antifungal activity and synergistic interactions between polyprenols and other lipids isolated from Ginkgo Biloba L. leaves. Molecules 18: 2166–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aziz AN, Ibrahim H, Rosmy Syamsir D, Mohtar M, Vejayan J, et al. (2013) Antimicrobial compounds from Alpinia conchigera . J Ethnopharmacol 145: 798–802. [DOI] [PubMed] [Google Scholar]
- 46. Tanaka A, Shimizu K, Kondo R (2013) Antibacterial compounds from shoot skins of moso bamboo (Phyllostachys pubescens). J Wood Sci 59: 155–159. [Google Scholar]
- 47. Joshi S, Bharucha C, Desai AJ (2008) Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresource Technol 99: 4603–4608. [DOI] [PubMed] [Google Scholar]
- 48. Mercan N, Kivrak I, Duru ME, Katircioglu H, Gulcan S, et al. (2006) Chemical composition effects onto antimicrobial and antioxidant activities of propolis collected from different regions of Turkey. Ann Microbiol 56(4): 373–378. [Google Scholar]
- 49. Teixeira PC, Leite GM, Domingues RJ, Silva J, Gibbs PA, et al. (2007) Antimicrobial effects of a microemulsion and a nanoemulsion on enteric and other pathogens and biofilms. Int J Food Microbiol 118: 15–19. [DOI] [PubMed] [Google Scholar]
- 50. Ismail S, Jalilian FA, Talebpour AH, Zargar M, Shameli K, et al. (2013) Chemical composition and antibacterial and cytotoxic activities of Allium hirtifolium boiss. BioMed Res Int 2013: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Choi GJ, Jang KS, Choi YH, Yu JH, Kim JC (2010) Antifungal activity of lower alkyl fatty acid esters against powdery mildews. Plant Pathol J 26 (4): 360–366. [Google Scholar]
- 52. Zhao HY, Wei F, Zhou CQ (2013) Effect of extraction methods on the chemical composition and antibacterial activity in vitro of essential oil from whole plants of Origanum vulgare L. Food Sci. 34: 236–240. [Google Scholar]
- 53. Zheng XD, Hu HB, Hu HS (2009) Study on the constituent and fungistasis of the four kinds of complex preparation from herb medicine. Gansu Agr Sci Techn 1: 11–14. [Google Scholar]
- 54. Wang B, Mei WL, Zuo WJ, Zeng YB, Liu GD, et al. (2012) Analysis of liposoluble components from Elephantopus scaber and Elephantopus tomentosus by GC-MS and studies on antibacterial function. Nat Prod Res Dev 24: 23–27. [Google Scholar]
- 55. Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chao LK, Hua KF, Hsu HY, Cheng SS, Liu JY, et al. (2005) Study on the antiinflammatory activity of essential oil from leaves of Cinnamomum osmophloeum . J Agri Food Chem 53: 7274–7278. [DOI] [PubMed] [Google Scholar]
- 57. Marino M, Bersani C, Comi G (2001) Impedance measurements to study the antimicrobial activity of essential oils from Lamiaceae and Compositae . Int J Food Microbiol 67: 187–195. [DOI] [PubMed] [Google Scholar]
- 58. Sikkema J, de Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59: 201–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ibrahim HR, Higashiguchi S, Koketsu M, Juneja LR, Kim M, et al. (1996) Partially unfolded lysozyme at neutral pH agglutinates and kills Gram-negative and Gram-positive bacteria through membrane damage mechanism. J Agri Food Chem 44: 3799–3806. [Google Scholar]
- 60. Holley RA, Patel D (2005) Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol 22: 273–292. [Google Scholar]
- 61. Khan MS, Ahmad I (2011) In vitro antifungal, anti-elastase and anti-keratinase activity of essential oils of Cinnamomum-, Syzygium- and Cymbopogon-species against Aspergillus fumigatus and Trichophyton rubrum . Phytomedicine 19: 48–55. [DOI] [PubMed] [Google Scholar]
- 62. Abdel-Monaim MF, Abo-Elyousr KAM, Morsy KM (2011) Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Prot 30: 185–191. [Google Scholar]
- 63. Zhang ZY, Dai GH, Zhuge YY, LI YB (2008) Protrctive effect of Robinia pseudoacacia Linn1 extracts against cucumber powdery mildew fungus, Sphaerotheca fuliginea . Crop Prot 27: 920–925. [Google Scholar]
- 64. Michielin EMZ, Salvador AA, Riehl CAS, Smânia Jr A, Smânia EFA, et al. (2009) Chemical composition and antibacterial activity of Cordia verbenacea extracts obtained by different methods. Bioresource Technol 100: 6615–6623. [DOI] [PubMed] [Google Scholar]
- 65. Smânia Jr A, Delie Monache F, Smânia EFA, Cuneo RS (1999) Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int J Med Mushrooms 1: 325–330. [Google Scholar]
- 66. Jia XY, Li NB, Luo HQ (2010) Determination of ursolic acid in force loquat capsule by ultrasonic extraction and ionic liquid based reverse dispersive LLME. Chromatographia 71: 839–843. [Google Scholar]
- 67. Zhou W, Zhang X, Xie M, Chen Y, Li Y, et al. (2010) Infrared-assisted extraction of adenosine from radix isatidis using orthogonal experimental design and LC. Chromatographia 72: 719–724. [Google Scholar]
- 68. Cheikhyoussef A, Pogori N, Chen H, Tian F, Chen W, et al. (2009) Antimicrobial activity and partial characterization of bacteriocin-like inhibitory substances (BLIS) produced by Bifidobacterium infantis BCRC 14602. Food Control 20: 553–559. [Google Scholar]
- 69. Wu G, Ding J, Li H, Li L, Zhao R, et al. (2008) Effects of cations and pH on antimicrobial activity of thanatin and s-thanatin against Escherichia coli ATCC 25922 and B. subtilis ATCC 21332. Curr Microbiol 57: 552–557. [DOI] [PubMed] [Google Scholar]
- 70. Tegegne G, Pretorius JC, Swart WJ (2008) Antifungal properties of Agpanthus africanus L. extracts against plant pathogens. Crop Prot 27: 1052–1060. [Google Scholar]
- 71. Zhang ZY, Dai GH, Zhuge YY, Li YB (2008) Protective effect of Robinia pseudoacacia Linn1 extracts against cucumber powdery mildew fungus, Sphaerotheca fuliginea . Crop Prot 27: 920–925. [Google Scholar]
- 72. Corato UD, Maccioni O, Trupo M, Sanzo GD (2010) Use of essential oil of Laurus nobilis obtained by means of a supercritical carbon dioxide technique against post harvest spoilage fungi. Crop Prot 29: 142–147. [Google Scholar]
- 73. Chen Y, Dai G (2012) Antifungal activity of plant extracts against Colletotrichum lagenarium, the causal agent of anthracnose in cucumber. J Sci Food Agric 92: 1937–1943. [DOI] [PubMed] [Google Scholar]
- 74. Wang HK, Yan YH, Wang JM, Zhang HP, Qi W (2012) Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. Plos One 7(1): e29452. [DOI] [PMC free article] [PubMed] [Google Scholar]