Abstract
Fibrodysplasia ossificans progressiva (FOP) is a rare heritable disease characterized by progressive heterotopic ossification of connective tissues, for which there is presently no definite treatment. A recurrent activating mutation (c.617G→A; R206H) of activin receptor-like kinase 2 (ACVR1/ALK2), a BMP type I receptor, has been shown as the main cause of FOP. This mutation constitutively activates the BMP signaling pathway and initiates the formation of heterotopic bone. In this study, we have designed antisense oligonucleotides (AONs) to knockdown mouse ALK2 expression by means of exon skipping. The ALK2 AON could induce exon skipping in cells, which was accompanied by decreased ALK2 mRNA levels and impaired BMP signaling. In addition, the ALK2 AON potentiated muscle differentiation and repressed BMP6-induced osteoblast differentiation. Our results therefore provide a potential therapeutic approach for the treatment of FOP disease by reducing the excessive ALK2 activity in FOP patients.
Introduction
BMPs are multifunctional growth factors that play key roles in bone formation, and heart and liver development [1], [2], [3]. The activity of the BMP pathway is precisely regulated to elicit its function in different cellular contexts. Perturbation of BMP pathways can lead to multiple diseases, including fibrodysplasia ossificans progressiva (FOP), a genetic disease caused by constitutively activated BMP signaling [4], [5], [6], [7].
FOP is a rare disease in which acute inflammation results in progressively ossified fibrous tissue. Minor traumas such as intramuscular immunization, muscle fatigue or muscle trauma from bumps or bruises can initiate the formation of heterotopic bones in the soft tissue [6]. Since surgical trauma also induces ectopic bone formation, surgery to remove ectopic bone is not an option for FOP patients. In the past decade, a variety of gene mutations in the activin receptor type IA/activin-like kinase 2 (ACVR1/ALK2) gene, encoding one of the type I BMP receptors, were found in most FOP patients [4]. The most common ALK2 FOP mutation is a change of guanine (G) into adenine (A) causing an arginine to histidine substitution (R206H) in the ALK2 GS domain [4]. Due to this mutation, the FOP ALK2 shows a lower binding affinity for its negative regulator FKBP12, which results in elevated BMP signaling in cells, both in the presence and absence of exogenous BMP ligands [5], [8], [9].
The recurrent ALK2 mutation in FOP patients provides a specific target for drug development. Plausible therapeutic approaches for inhibiting the excessive BMP signaling in FOP include ALK2 inhibitory RNA technology, anti-ALK2 monoclonal antibodies, and ALK2 small molecule inhibitors [10], [11]. Several small molecules already have been developed that efficiently inhibit ALK2 activity, such as dorsomorphin and LDN-193189 (LDN) [12], [13]. However, these compounds in addition also inhibit the activity of BMPR1 (ALK3), another type I BMP receptor [12], [13]. Other studies have suggested that dorsomorphin and LDN are not specific for BMP signaling as the inhibitors could block TGF-β-induced activity at higher concentrations [14]. The ideal BMP inhibitor for FOP patients would be an agent that normalizes the (excessive) ALK2 activity without affecting the functions of other kinases. Using the allele specific siRNA technique, two separate research groups have successfully obtained siRNAs that target the disease-causing ALK2, without affecting normal ALK2 expression [15], [16]. The siRNAs were used in cells from FOP patients to restore BMP activity and osteogenic differentiation [15], [16].
In addition to siRNAs, antisense oligonucleotides (AONs) mediated exon skipping might be a potential tool to modulate ALK2 activity. AONs are short synthetic, chemically modified single-stranded oligonucleotides between 20–30 base pairs in length. AONs can be used to modify splicing by specifically binding pre-mRNA sequences to block the access of spliceosome and other splicing factors, thereby excluding the target exon from the mature mRNA [17]. AON-mediated exon skipping has enabled the successful reframing of the mutated dystrophin mRNA and the restoration of dystrophin protein synthesis in skeletal muscle of Duchenne muscular dystrophy (DMD) patients [18], [19]. Systemic delivery of AONs is less challenging than for siRNAs, since AONs are single stranded, which is pharmacokinetically advantageous, allowing uptake by many tissues at significant levels after subcutaneous and intravenous administration without the need for specific formulations [20]. Therefore, adjustment of aberrant gene expression via exon skipping or RNaseH knockdown might be an attractive therapeutic option for genetic diseases.
In this study, ALK2 AON was designed to selectively modulate pre-mRNA splicing of mouse ALK2 to inhibit Alk2 expression. The effects of ALK2 knockdown on ALK2-mediated BMP functions were assessed by analyzing myogenic differentiation and osteoblast differentiation. In line with the fact that BMP represses myogenic differentiation and potentiates osteoblast differentiation, we found ALK2 AON to potentiate myogenic differentiation of C2C12 myoblasts and inhibit osteoblast differentiation in mouse endothelial cells, suggesting that the endogenous BMP signaling in C2C12 cells and mouse endothelial cells were repressed by the ALK2 AON.
Materials and Methods
Antisense Oligonucleotides
ALK2 AON was specifically designed to target exon 8 of wild type mouse Alk2; AONs with full length phosphorothioate backbones and 2′-O-methyl-modified ribose molecules were obtained from Prosensa Therapeutics B.V. (Leiden, the Netherlands). AONs sequences are listed in Table 1.
Table 1. Antisense oligonucleotides used in this study.
| AON | Sequences (5′-3′) | Targeting |
| ALK2 AON | GGGUUAUCUGGCGAGCCACCGUUCU | Mouse ALK2 exon 8 |
| Control AON | UCAAGGAAGAUGGCAUUUCU | Control |
Cell Culture
Mouse C2C12 myoblast cells were maintained in DMEM medium (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), and penicillin/streptomycin (Invitrogen). For myogenic differentiation, C2C12 cells were cultured in DMEM (Gibco) supplemented with 2% FBS (Invitrogen). Mouse endothelial cells (MEECs [21] and 2H11 [22]) and mouse osteoprogenitor cells KS483 [23] were cultured in α-MEM (Gibco) and 10% fetal bovine serum (FBS) (Invitrogen), and penicillin/streptomycin (Invitrogen), the plates were pretreated with 0.1% gelatin (Sigma, St Louis, MO, USA). All of the cells were grown at 37°C in a humidified incubator with 5% CO2.
AON Transfection
ExGen 500, the linear 22 kDa form of polyethyleneimine (PEI, MBI Fermentas, St.Leon-Rot, Germany), was used as a transfection reagent for KS483, MEECs and 2H11; DharmaFECT Duo (Thermo Scientific, Pittsburgh, PA, USA) was used as transfection reagent for C2C12 cells. Transfection was performed according to the manufacturer’s instructions.
RNA Isolation and Quantitative Real-time PCR Analysis
Total RNA was isolated using the RNAII isolation kit (Machery Nagel, Düren, Germany) according to the manufacturer’s instructions. The RNA quantity and integrity were measured using RNA 6000 Nanochip in the Agilent 2100 bioanalyzer (Agilent Technologies, Amstelveen, the Netherlands). Reverse transcriptase-polymerase chain reaction was performed using the RevertAid H Minus First strand cDNA synthesis kit (Fermentas, St.Leon-Rot, Germany) according to manufactures instructions. Quantitative real-time PCR (qPCR) analysis was performed using the Roche LightCycler 480 and the relative expression levels of the genes of interest were determined in triplicate for each sample using the 2−ΔΔCT method. Values were normalized to Gapdh expression, qPCR primers are listed in Table 2.
Table 2. Primers used in this study.
| Primers | Sequences(5′-3′) | Used for |
| mouse ALK2 E7 FW | AAGTTGGCCTTATCATCC | ALK2 exon 8 skip PCR |
| mouse ALK2 E9 RV | GTACAATTCCGTCTCCCT | |
| mouse ALK2 FW | TGGCTCCGGTCTTCCTTT | ALK2 qPCR |
| mouse ALK2 RV | AGCGACATTTTCGCCTTG | |
| mouse GAPDH FW | AACTTTGGCATTGTGGAAGG | GAPDH qPCR |
| mouse GAPDH RV | ACACATTGGGGGTAGGAACA | |
| mouse BSP FW | AGGGAACTGACCAGTGTTGG | BSP qPCR |
| mouse BSP RV | ACACATTGGGGGTAGGAACA | |
| mouse OSC FW | AGACTCCGGCGCTACCT | OSC qPCR |
| mouse OSC RV | CTCGTCACAAGCAGGGTTAA | |
| mouse RunX2 FW | GAATGCTTCATTCGCCTCAC | Runx2 qPCR |
| mouse RunX2 RV | GTGACCTGCAGAGATTAACC |
Immunofluorescence
Antibodies used for immunofluorescence were Desmin (Santa Cruz, Santa Cruz, CA, USA) and Myosin heavy chain (MF20; Developmental Studies hybridoma Bank, USA). The immunofluorescence procedure was performed as described previously [24].
Alkaline Phosphatase (ALP) Assay
2×104 MEECs cells were seeded in a 48-well plate. One day after AON transfection, cells were stimulated with TGF-β3 (kindly provided by Dr. K Iwata, OSI Pharmaceuticals, Melville, NY, USA) for 2 days. The ALP activity assay was performed after treatment with BMP6 in proliferation medium for another 2 days. KS483 cells were seeded in a 96-well plate with 5×103 cells per well. Two days after AON transfection, cells were stimulated with 100 ng/ml BMP6 (R&D, Minneapolis, MN, USA) for further 2 days. Histochemical examination of ALP activity was performed using naphtol AS-MX phosphate (Sigma) and fast blue RR salt (Sigma), as described previously [5]. To quantify the data, the histochemically stained cell material was solubilized in 50 mM NaOH in ethanol, and absorbance was measured at 550 nm.
Mineralization Assay
AON-transfected MEECs were stimulated with 5 ng/ml of TGF-β3 for 2 days in growth medium. Mineralization assay was performed after the cells were cultured in osteogenic medium, which is comprised of α-MEM supplemented with 5% FBS, 0.2 mM of ascorbic acid (Sigma), dexamethasone (Sigma) and 10 mM of β-glycerolphosphate (Sigma), containing 100 ng/ml of BMP6 for another 4 days. Confluent KS483 cells were transfected for 2 days, then stimulated with 100 ng/ml BMP6 for 4 days in growth medium. The mineralization assay was performed after subsequent 14 days of culturing in osteogenic medium, medium refreshed every 3–4 days. To visualize mineralization, cells were stained with 2% alizarin red S solution (Sigma).
Transcription Reporter Assays
2H11 cells were seeded in 24-well plates, and then transfected with the BMP Responsive Element (BRE)-Luc reporter construct using PEI (Fermentas) as described previously [24]. After overnight serum starvation, the cells were stimulated with BMP6 (50ng/ml) for 8 hours. Harvested cells were assayed for luciferase activity with a Perkin Elmer luminometer. Each experiment was performed in triplicate and data represent the mean ± SD of three independent experiments with normalization to β-galactosidase activity.
Western Blotting
Western blotting was performed as previously described using standard techniques [14]. The antibodies used for immunoblotting were phosphorylated Smad1/5/8 antibody (1∶1000, Cell signaling Technology, Danvers, MA, USA) and GAPDH antibody (1∶40,000, Sigma). GAPDH was used as the loading control.
Results
Design of AON for ALK2 Exon Skipping in Mouse Cells
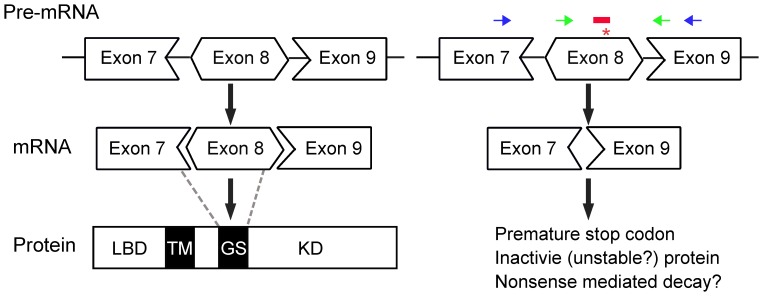
The classic FOP mutation, a G-A substitution in exon 7 of the human ALK2 gene, leads to a R206H substitution in the GS domain of ALK2 protein, causing elevated BMP signaling in FOP patients [4], [5]. The mutated ALK2 exon sequence is highly conserved between mouse and human, and the exon 7 in human corresponds to exon 8 in mouse [25]. In an attempt to modulate ALK2-mediated BMP signaling, an ALK2 AON was designed to specifically target exon 8 encoding the GS domain of mouse ALK2 and the sequence of ALK2 AON overlapped with the location of hot spot mutation in FOP (Figure 1). Upon transfection and entry into the cell nucleus, the AON was anticipated to modulate mouse ALK2 pre-mRNA splicing by masking and subsequent skipping of exon 8, which disrupts the reading frame (exon 8 is 100 base pairs, not dividable by three) (Figure 1). We have chosen AON against exon internal site as we demonstrated previously that they outperform AONs targeting splice sites [26]. The truncated ALK2 mRNA without exon 8 may be degraded via nonsense-mediated decay due to the introduction of a premature stop codon. The locations of primers to detect the skipped exon are indicated as black arrows in Figure 1.
Figure 1. Schematic overview of ALK2 exon skipping.
Left panel: The ALK2 protein structural domains include ligand binding domain (LBD), transmembrane domain (TM), GS domain (GS), and kinase domain (KD). The position of each exon relative to each domain is denoted by broken lines. Right panel: The FOP mutation hot spot (c.617G→A; R206H) is in exon 8 (*). ALK2 AON (red bars, the sequence covered the FOP mutation hot spot) hybridizes and hides exon 8 from the splicing machinery, resulting in skipping exon 8 upon mRNA splicing. This out-of-frame mutation will lead to non-sense mediated decay of Alk2 mRNA. qPCR primers to detect Alk2 expression are indicated by green arrows; The primers for detecting of skipped band are indicated by blue arrows. While we targeted exon 8 for exon skipping, we do not exclude that other AONs targeting other exons can be designed that also inhibit ALK2 expression.
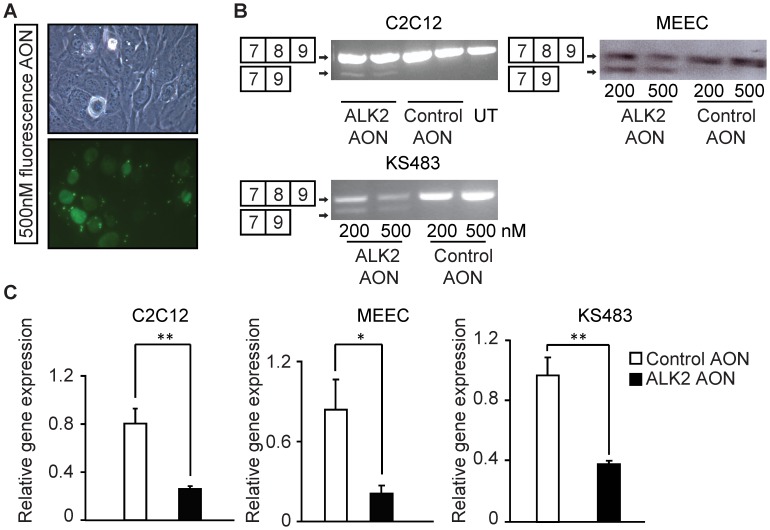
To test whether the designed ALK2 AON could induce exon skipping and decrease full-length ALK2 expression and/or ALK2 activity in cultured cells we applied ALK2 AON in various cell types. These cells were shown to be transfected with AONs with high efficiency (>70%) as visualized by the 5′-fluorescein (FAM)-labeled control AON (Figure 2A). RT-PCR on RNA harvested 2 days after transfection showed a skipped band representing the transcript without exon 8 upon transfection of the ALK2 and not the control AON (Figure 2B). qPCR analysis showed that Alk2 expression was decreased about 70–80% in the cells treated with ALK2 AON (Figure 2C).
Figure 2. AON-induced ALK2 exon 8 skipping in various cell types.
(A) C2C12 cells were transfected with 500 nM non-targeting, fluorescently-labeled AONs fluorescent AON in differentiation medium and fluorescent images were taken 2 days after transfection. (B) Various of cells were transfected with indicated control AON or ALK2 AON. RNA was isolated 2 days after transfection, and RT-PCR was performed to visualize the full length Alk2 (composed of exon 7, 8 and 9); and skipped Alk2 (composed of exon 7 and exon 9). (C) MEECs cells were transfected with 100 nM control AON or 100 nM ALK2 AON, RNA was isolated 1 day after transfection. C2C12 cells and KS483 cells were transfected with AONs in proliferation medium; RNA was isolated 2 days post transfection. cDNA was synthesized using random hexamer primers and used for the quantification of Alk2 expression. The relative full length ALK2 mRNA expression was analyzed. Alk2 RNA level was normalized to Gapdh; the level of expression for untreated sample was defined as 1. Values and error bars represent the means ± SD of triplicates. Statistical analysis was performed using Student’s t-test. *P<0.05, **P<0.005.
Exon-skipping in ALK2 Reduced Alk2 Expression and Potentiated Muscle Differentiation
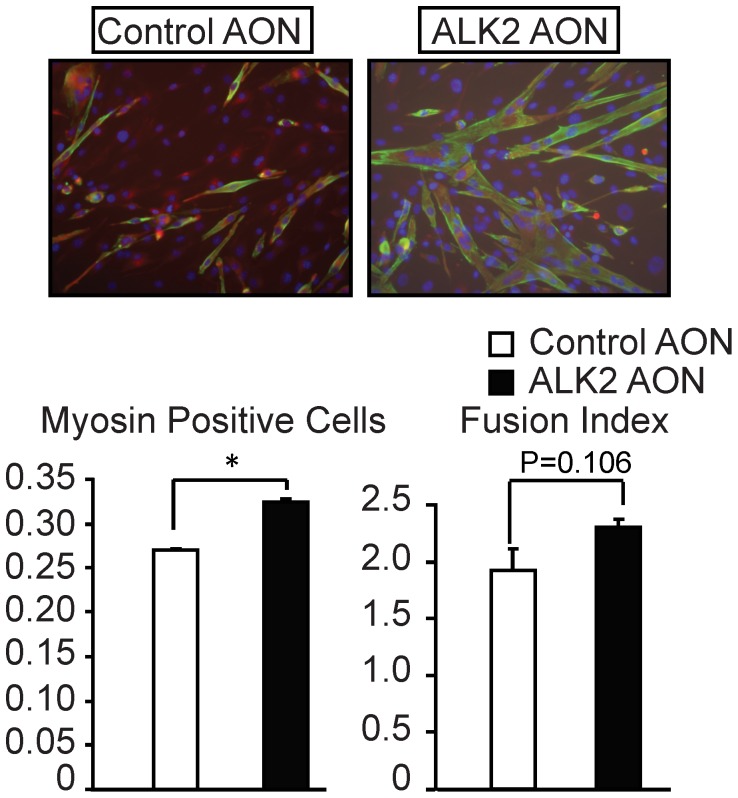
BMP signaling is known to repress myogenic differentiation, and BMP inhibitors have been shown to potentiate the differentiation of myoblasts into myotubes [24], [27]. We therefore examined whether the ALK2 AON can decrease BMP signaling and function as a BMP inhibitor to potentiate myogenic differentiation of C2C12 myoblasts. Seven days after transfection, cells were fixed and immunostained for myosin to visualize the differentiated myotubes. The degree of differentiation was measured by determining the differentiation and the fusion indexes. The differentiation index was calculated as the percentage of myosin-positive cells out of all myogenic (desmin-positive) cells. The fusion index was calculated as the average number of nuclei in the differentiated myotubes. The ALK2 AON was found to enhance both the differentiation index and the fusion index in C2C12 cells (Figure 3), suggesting that AON-mediated ALK2 knockdown can potentiate myoblast differentiation via repression of endogenous BMP signaling.
Figure 3. ALK2 AON enhanced myogenic differentiation in C2C12 cells.
C2C12 cells were transfected with 200 nM control AON or 200 nM ALK2 AON in differentiation medium for 7 days. Then cells were immunostained with myosin and desmin. Myosin (green) was used to label the differentiated cells while desmin (red) was used to label myogenic cells. The values for the percentage of myosin positive cells or for the fusion index represent the average levels of two independent samples. Values and error bars represent the means ± SD. Statistical analysis was performed using Student’s t-test, using the untransfected samples as reference. *P<0.05.
ALK2 AON-mediated Exon Skipping Decreased BMP Signaling and Mineralization in Endothelial Cells
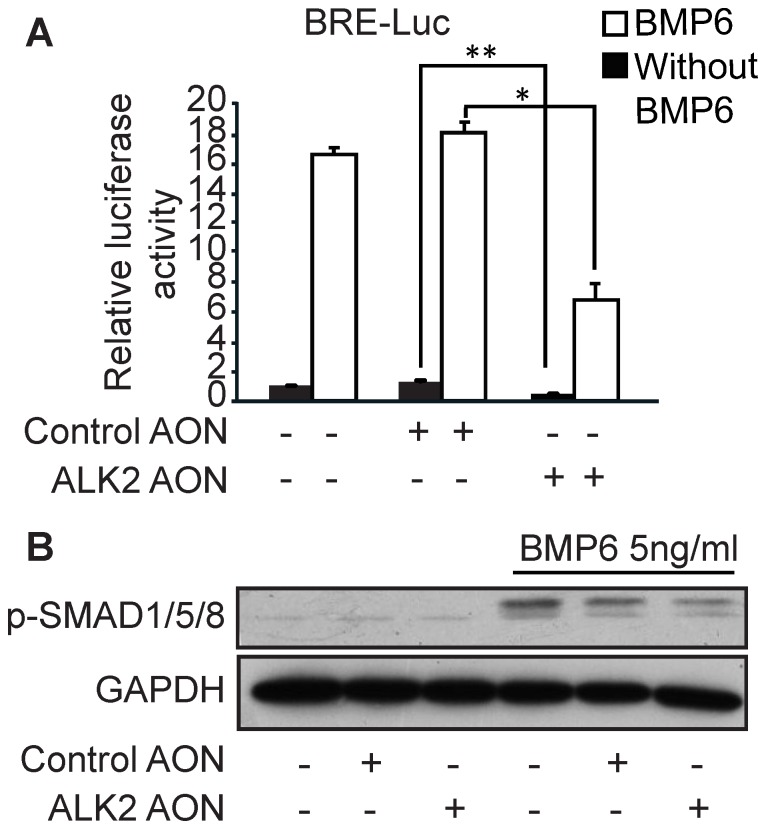
Recently, endothelial cells were reported as potential osteoprecursor cells for ectopic bone formation in FOP patients [28], [29]. Therefore, our next step was to test whether the ALK2 AON can repress BMP signaling and osteoblast differentiation in endothelial cells. The ALK2 AON (and not a control AON) decreased BMP-induced BMP-responsive element (BRE)-driven luciferase reporter activity (Figure 4A). BMP6-induced Smad1/5 phosphorylation was also inhibited by the ALK2 AON, albeit weakly (Figure 4B).
Figure 4. ALK2 AON-induced exon skipping decreased BMP signaling in 2H11 endothelial cells.
(A) 2H11 cells were cotransfected with 100 nM of the indicated AON and the BMP reporter construct BRE-Luc for 16 hours. Subsequently, cells were starved for 8 hours, and then stimulated with 50 ng/ml BMP6 overnight. Luciferase reporter activity was measured after stimulation and normalized with β-gal activity. (B) 2H11 cells were transfected with 100 nM control AON or 100 nM ALK2 AON in proliferation medium. One day after transfection, the cells were serum starved for overnight and stimulated with 5 ng/ml BMP6 for 1 hour. Protein was isolated and western blotting was performed to check the Smad1/5/8 phosphorylation. GAPDH was used as loading control. Data are means ± SD from three independent experiments. Statistical analysis was performed using Student’s t-test, using the untransfected samples as reference. *P<0.05, **P<0.005.
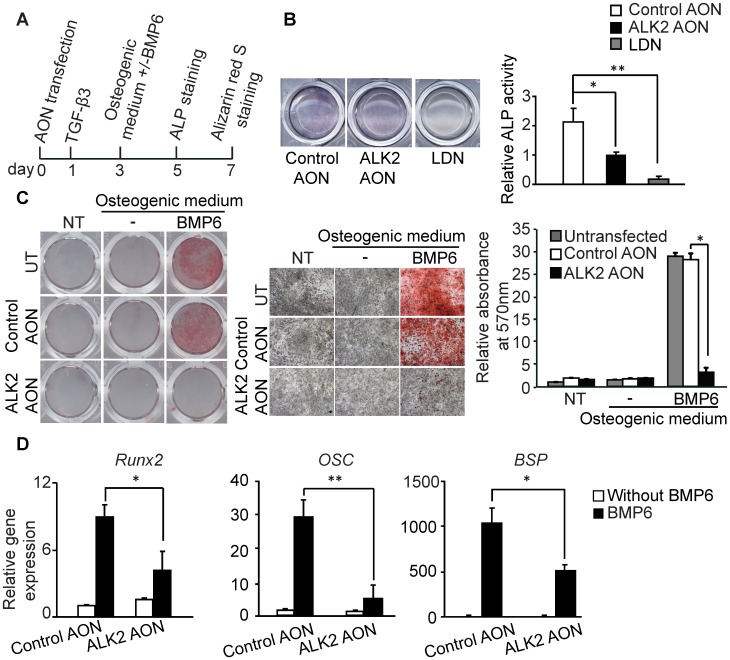
We next evaluated the therapeutic potential of the ALK2 AON in the treatment of excessive bone formation such as occurring in FOP patients. For this purpose we investigated the effect of the ALK2 AON on endothelial to osteoblast transdifferentiation by using MEECs cultured under osteogenic conditions. MEECs were chose due to the short transdifferentiation period. Transfected MEECs were treated with TGF-β3 for 2 days and then refreshed with osteogenic medium with or without BMP6 for several days (Figure 5A). The osteoblast differentiation can be measured by determining alkaline phosphatase (ALP) activity, an early marker for osteoblast differentiation. Histochemical staining revealed that ALP activity in ALK2 AON treated cells was significantly decreased (Figure 5B). Compared to LDN-193189 treated sample in which most of the ALP activity was blocked, ALP activity in ALK2 AON treated cells was only partly blocked (Figure 5B). Furthermore, we analyzed the effect of ALK2 AON on osteoblast differentiation by alizarin red S staining, a staining to detect calcium mineralization. BMP6 induced mineralization was significantly decreased by exon skipping of ALK2 (Figure 5C). Consistent with the ALP activity and alizarin red S staining results, qPCR analysis confirmed that exon skipping in ALK2 can decrease the expression of Runx2, bone sialoprotein (BSP) and osteocalcin (OSC) (Figure 5D). Together these data indicate that ALK2 AON can decrease BMP-induced osteoblast differentiation.
Figure 5. BMP-induced osteoblast differentiation was impaired by ALK2 AON-induced exon skipping in MEECs.
(A)As a mineralization assay, MEECs were seeded into a 24-wells or 48-wells plate. One day after transfection, cells were stimulated with 5 ng/ml TGF-β3 for 2 days and switched to osteogenic medium with or without 100 ng/ml BMP6 for several days (with medium refreshment after 4 days). ALP staining was performed 2 days after maintaining in osteogenic medium. After 4 days in osteogenic medium, the cells were fixed and stained with 2% alizarin red S solution for mineralization staining. (B) MEECs were transfected with 200 nM control AON or 200 nM ALK2 AON, and stimulated with osteogenic medium indicated in figure 4A. The LDN sample indicates LDN-193189 was present during the whole experiment. Representative microscopic pictures of staining (upper panel) are shown; ALP activity was represented as the average of three independent samples, and was normalized by protein concentration. (C) MEECs were transfected with 100 nM control AON or 100 nM ALK2 AON in grow medium and treated as in panel 4A. The mineralization was visualized by alizarin red S staining. The plate was scanned (4C, left panel), and scanned under a microscope (4x, 4C, middle panel). Alizarin red S staining was quantified by stain extraction and absorbance reading at 570 nm (4C, right panel). (D) RNA was collected in experiment 4C. mRNA expression of Runx2, OSC and BSP was measured by qPCR. All the experiments were performed for three times and were normalized to Gapdh. Each value is the means ± SD. Statistical analysis was performed using Student’s t-test, using the untransfected samples as reference. *P<0.05, **P<0.005.
The ALK2 AON Decreased BMP6-induced Osteoblast Differentiation in KS483 Osteoprogenitor Cells
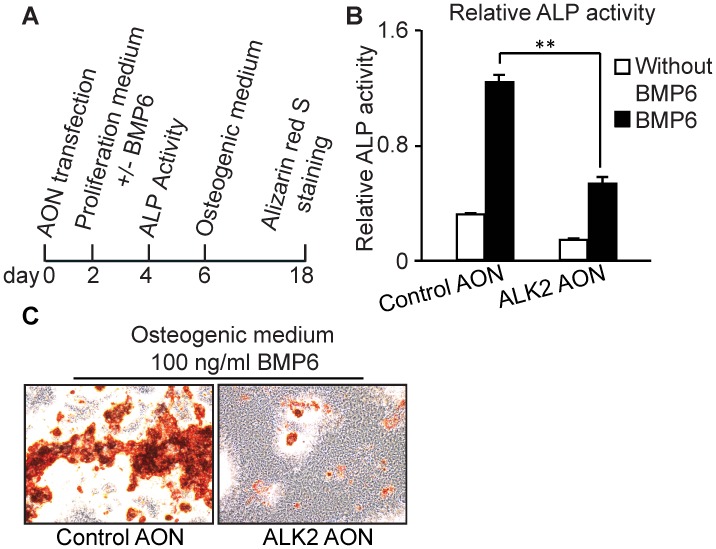
In addition to endothelial cells, mesenchymal stem cells are considered as another source of osteoprecursors responsible for BMP induced ectopic bone formation in mice [6], [30]. In FOP patients, the mesenchymal stem cell-like cells derived from endothelial cells are considered to be in part responsible for heterotopic ossification [29]. Pluripotent mesenchymal stem cells KS483 cells can be differentiated into osteoblasts, chondrocytes and adipocytes in vitro [31], [32]. Two days after transfection, KS483 cells were maintained in proliferation medium with or without BMP6 for 2 days before measuring ALP activity. For alizarin red S staining, transfected cells were cultured in proliferation medium for 4 days and then refreshed with osteogenic medium with or without BMP6 for 12 days (Figure 6A). The ALK2 AON also efficiently repressed BMP6-induced osteoblast differentiation in KS483 cells, as visualized by the ALP activity (Figure 6B) and the mineralization assay (Figure 6C). qPCR analysis confirmed that exon skipping in ALK2 can decrease the expression of BMP6-induced osteogenic gene expression (data not shown).
Figure 6. ALK2 AON reduced BMP-induced osteogenic differentiation in KS483.
(A) Confluent KS483 cells were transfected for 2 days in a 96-wells or 24-wells plate. Two days after AON transfection, cells were stimulated with 100 ng/ml BMP6 (R&D, MN, USA) for 2 additional days and ALP assay was performed. For mineralization assay, after stimulation with 100 ng/ml BMP6 (R&D, MN, USA) for 4 days, cells then switched to osteogenic medium for subsequent 14 days. Medium was refreshed every 3–4 days. (B) Confluent KS483 cells were transfected with 200 nM control AON, or 200 nM ALK2 AON for 2 days in proliferation medium. Then cells were stimulated with 100 ng/ml BMP6 for another 2 days in proliferation medium. Cells lysates were harvested and ALP activity was measured. Data are presented as means ±SD. (C) Confluent KS483 cells were transfected with 200 nM control AON, or 200 nM mouse ALK2 AON for 2 days in proliferation medium. The cells were then stimulated with 100 ng/ml BMP6 in proliferation medium for 4 days. Then cells were maintained in osteogenic medium for another 12 days. Medium was refreshed every 3–4 days. The cells were finally stained with alizarin red S solution to visualize the mineralized area in KS483 cells. Statistical analysis was performed using Student’s t-test, using the untransfected samples as reference. **P<0.005.
Discussion
In this study, an ALK2 exon-skipping AON was designed based on previous published guidelines [33]. The mouse ALK2 AON we designed can specifically induce skipping of exon 8 in different cell types, including C2C12 myoblasts, KS483 osteoprogenitor cells, and two types of endothelial cells (2H11 and MEECs). The removal of exon 8 might cause the transcripts to be degraded via nonsense-mediated decay. We observed a weak skip product in the RT-PCR analysis, which suggested that the transcript with premature stop codon might not be stable. Potentially, the truncated protein, if stably produced, could have dominant negative receptor activity. However, high amounts of kinase inactive ALK2 are required to achieve dominant negative effects in cultured cells, while the accumulated mutant ALK2 in AON transfected cells may be readily degraded. From studies examining the effect of mismatches on efficiency, we know that AONs with 1 mismatch at the 5′ of 3′ end of the AON can still work, but these AONs may show a reduced efficiency. More mismatches leads to poor or inefficient AONs [34]. As there is no complete overlap of ALK2 AON with another region in the genome as determined by blasting the ALK2 AON sequence with the full genome, and ALK2 AON did not inhibit the expression of other related type I receptors, such as ALK3 and ALK1 (or housing keeping genes) (data not shown), we conferred that ALK2 AON specifically targets ALK2 in vitro.
Importantly, the ALK2 AON also can downregulate ALK2 and BMP-induced osteoblast differentiation in endothelial cells, which has recently been reported to be the major bone progenitor cell population in FOP patients [29]. Endothelial cells were first found to dedifferentiate into a mesenchymal stem cell-like phenotype by endothelial-to-mesenchymal transition, and subsequently to differentiate into cartilage and bone [29]. The ALK2 AON downregulated the levels of ALK2 mRNA and significantly reduced BMP-induced signaling responses and osteogenic differentiation in MEECs and more mature 2H11 cells. The effect of the ALK2 AON on BMP-induced Smad1/5 phosphorylation was relatively weak compared to other responses. This could be because of the different thresholds required for BMP-induced responses; BMP6-induced Smad1/5 phosphorylation as measured in a total cell lysate at the time point examined may need more efficient knockdown to observe a strong effect.
BMPs transmit signals through induction of heterotetrameric complexes consisting with type I receptors and type II receptors [7]. Utilization of type I receptors differs depending on BMP ligands; BMP-6 binds principally to ALK2, but also ALK3, ALK6 [35]. The role of ALK2 played in BMP6 induced osteoblast differentiation may be different depending on the cell types [9], [36]. While LDN can strongly block the BMP signal pathway and osteoblast differentiation (Figure 5B), ALK2 AON has a slightly lower inhibitory effect, which may explained by the minor role ALK2 played in BMP signal pathway in endothelial cells. Alternatively, as AON-mediated depletion of ALK2 does not affect the expression ALK3, the cells with ALK2 knockdown may still partly respond to BMP6 by signaling via ALK3 or other BMP type I receptors.
Our research provides a new approach for the therapy of FOP. The next step is to test whether the ALK2 AON can efficiently decrease ALK2 expression and ALK2-mediated BMP signaling in vivo. In this respect, it will be of great interest to test whether the ALK2 AON can inhibit the heterotopic ossification in ALK2 R206H knock-in mice that have recently been developed [37]. The specific chemistry of the type of AONs used in this study (2′-O-methyl phosphorothioate RNA) enhances nuclease resistance and stability of the AON-target RNA duplex [38]. The phosphorothioate backbones of AONs also prevent renal clearance [38], resulting in a long serum half-life and uptake by most of tissues. Phase 3 clinical trials are currently ongoing for DMD [39]. The research of applying AON to DMD holds a promise for the application of AON in FOP. The final goal would be to examine whether ALK2 AON can counteract the excessive BMP activity in tissues from FOP patients. We have attempted to specifically target the ALK2 R206H allele with AON-mediated strategies by human ALK2 AON, but so far this has not been successful. Taken together, the results presented here underscore that AONs have potential for the treatment of FOP, although more studies should be performed in this sense.
Acknowledgments
We would like to acknowledge Midory Thorikay for expert technical assistance, Prosensa B.V. for their generous supply of antisense oligonucleotides, and thank Hans van Dam for critical reading of the manuscript.
Funding Statement
This study was supported by Dutch Ministry for Economic Affairs (IOP Genomics grant IGE7001) “Towards broad clinical and technological application of gene expression engineering by exon skipping”, Netherlands Research Council (NWO-MW), Cancer Genomics Centre Netherlands and Centre for Biomedical Genetics; and by funds from LeDucq foundation and AO Start-up Grant S-12-27S: Targeting Endothelial-to-Mesenchymal transition in Fibrodysplasia Ossificans Progressiva. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schlange T, Andree B, Arnold HH, Brand T (2000) BMP2 is required for early heart development during a distinct time period. Mech Dev 91: 259–270. [DOI] [PubMed] [Google Scholar]
- 2. Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, et al. (2009) Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 41: 478–481. [DOI] [PubMed] [Google Scholar]
- 3. Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, et al. (2006) BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 4. Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, et al. (2006) A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38: 525–527. [DOI] [PubMed] [Google Scholar]
- 5. van Dinther M, Visser N, de Gorter DJ, Doorn J, Goumans MJ, et al. (2010) ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res 25: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 6.Shi S, de Gorter DJ, Hoogaars WM, t Hoen PA, Ten Dijke P (2012) Overactive bone morphogenetic protein signaling in heterotopic ossification and Duchenne muscular dystrophy. Cell Mol Life Sci. [DOI] [PMC free article] [PubMed]
- 7. Cai J, Pardali E, Sanchez-Duffhues G, ten Dijke P (2012) BMP signaling in vascular diseases. FEBS Lett 586: 1993–2002. [DOI] [PubMed] [Google Scholar]
- 8. Groppe JC, Wu J, Shore EM, Kaplan FS (2011) In vitro analyses of the dysregulated R206H ALK2 kinase-FKBP12 interaction associated with heterotopic ossification in FOP. Cells Tissues Organs 194: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song GA, Kim HJ, Woo KM, Baek JH, Kim GS, et al. (2010) Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem 285: 22542–22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, et al. (2008) Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol 22: 191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Gorter DJ, Jankipersadsing V, Ten Dijke P (2012) Deregulated bone morphogenetic protein receptor signaling underlies fibrodysplasia ossificans progressiva. Curr Pharm Des 18: 4087–4092. [DOI] [PubMed] [Google Scholar]
- 12. Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, et al. (2008) BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 14: 1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, et al. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol 4: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu PB, Deng DY, Beppu H, Hong CC, Lai C, et al. (2008) Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 283: 3877–3888. [DOI] [PubMed] [Google Scholar]
- 15. Kaplan J, Kaplan FS, Shore EM (2012) Restoration of normal BMP signaling levels and osteogenic differentiation in FOP mesenchymal progenitor cells by mutant allele-specific targeting. Gene Ther 19: 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi M, Katagiri T, Furuya H, Hohjoh H (2012) Disease-causing allele-specific silencing against the ALK2 mutants, R206H and G356D, in fibrodysplasia ossificans progressiva. Gene Ther 19: 781–785. [DOI] [PubMed] [Google Scholar]
- 17. Aartsma-Rus A, van Ommen GJ (2007) Antisense-mediated exon skipping: a versatile tool with therapeutic and research applications. RNA 13: 1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, et al. (2007) Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 357: 2677–2686. [DOI] [PubMed] [Google Scholar]
- 19. Goemans NM, Tulinius M, van den Akker JT, Burm BE, Ekhart PF, et al. (2011) Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med 364: 1513–1522. [DOI] [PubMed] [Google Scholar]
- 20. Spitali P, Aartsma-Rus A (2012) Splice modulating therapies for human disease. Cell 148: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 21. Larsson J, Goumans MJ, Sjostrand LJ, van Rooijen MA, Ward D, et al. (2001) Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. EMBO J 20: 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walter-Yohrling J, Morgenbesser S, Rouleau C, Bagley R, Callahan M, et al. (2004) Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res 10: 2179–2189. [DOI] [PubMed] [Google Scholar]
- 23. Yamashita T, Ishii H, Shimoda K, Sampath TK, Katagiri T, et al. (1996) Subcloning of three osteoblastic cell lines with distinct differentiation phenotypes from the mouse osteoblastic cell line KS-4. Bone 19: 429–436. [DOI] [PubMed] [Google Scholar]
- 24. Shi S, Hoogaars WM, de Gorter DJ, van Heiningen SH, Lin HY, et al. (2011) BMP antagonists enhance myogenic differentiation and ameliorate the dystrophic phenotype in a DMD mouse model. Neurobiol Dis 41: 353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, et al. (2009) Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aartsma-Rus A, Houlleberghs H, van Deutekom JC, van Ommen GJ, t Hoen PA (2010) Exonic sequences provide better targets for antisense oligonucleotides than splice site sequences in the modulation of Duchenne muscular dystrophy splicing. Oligonucleotides 20: 69–77. [DOI] [PubMed] [Google Scholar]
- 27. Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, et al. (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127: 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, et al. (2009) Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am 91: 652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, et al. (2010) Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 16: 1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, et al. (2011) BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res 26: 1166–1177. [DOI] [PubMed] [Google Scholar]
- 31. Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW (2002) Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17: 394–405. [DOI] [PubMed] [Google Scholar]
- 32. de Gorter DJ, van Dinther M, Korchynskyi O, ten Dijke P (2011) Biphasic effects of transforming growth factor beta on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res 26: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 33. Aartsma-Rus A, van Vliet L, Hirschi M, Janson AA, Heemskerk H, et al. (2009) Guidelines for antisense oligonucleotide design and insight into splice-modulating mechanisms. Mol Ther 17: 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aartsma-Rus A, Kaman WE, Bremmer-Bout M, Janson AA, den Dunnen JT, et al. (2004) Comparative analysis of antisense oligonucleotide analogs for targeted DMD exon 46 skipping in muscle cells. Gene Ther 11: 1391–1398. [DOI] [PubMed] [Google Scholar]
- 35. Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, et al. (1999) Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci 112 (Pt 20): 3519–3527. [DOI] [PubMed] [Google Scholar]
- 36. Lavery K, Swain P, Falb D, Alaoui-Ismaili MH (2008) BMP-2/4 and BMP-6/7 differentially utilize cell surface receptors to induce osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. J Biol Chem 283: 20948–20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, et al. (2012) An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res 27: 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oberbauer R, Schreiner GF, Meyer TW (1995) Renal uptake of an 18-mer phosphorothioate oligonucleotide. Kidney Int 48: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 39. Aartsma-Rus A, Bremmer-Bout M, Janson AA, den Dunnen JT, van Ommen GJ, et al. (2002) Targeted exon skipping as a potential gene correction therapy for Duchenne muscular dystrophy. Neuromuscul Disord 12 Suppl 1S71–77. [DOI] [PubMed] [Google Scholar]