Abstract
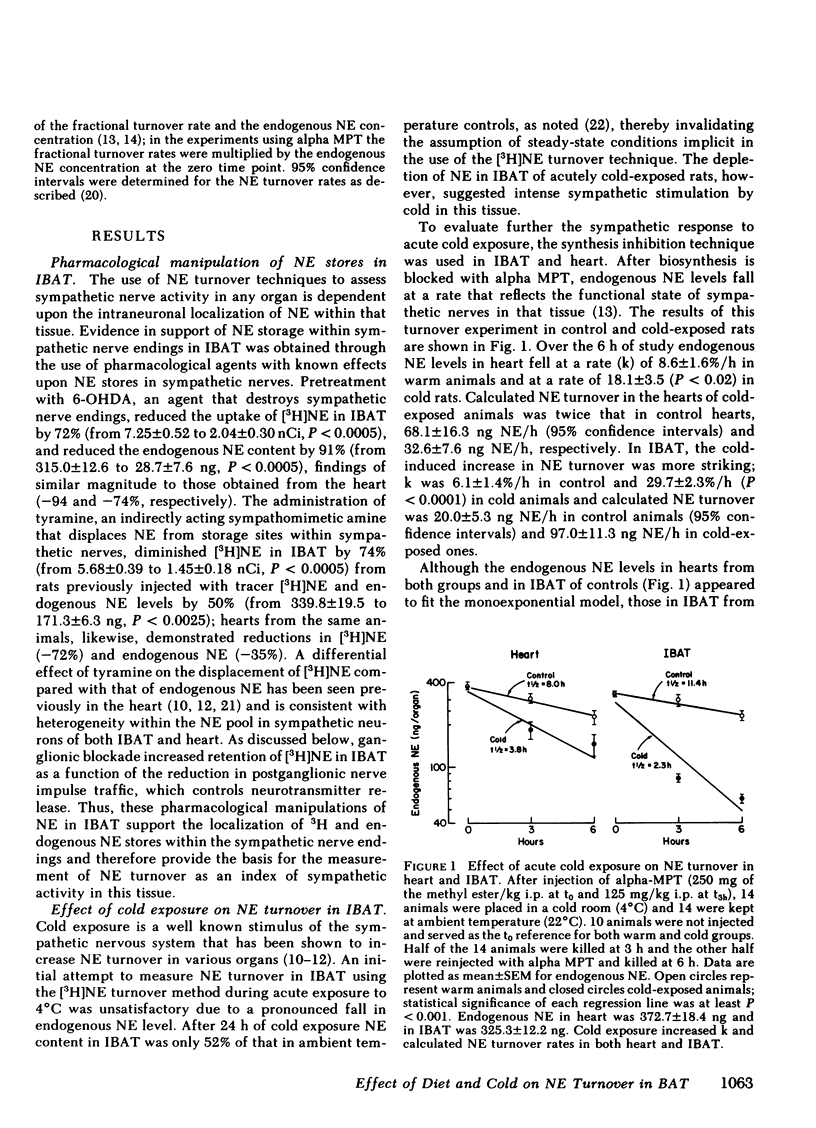
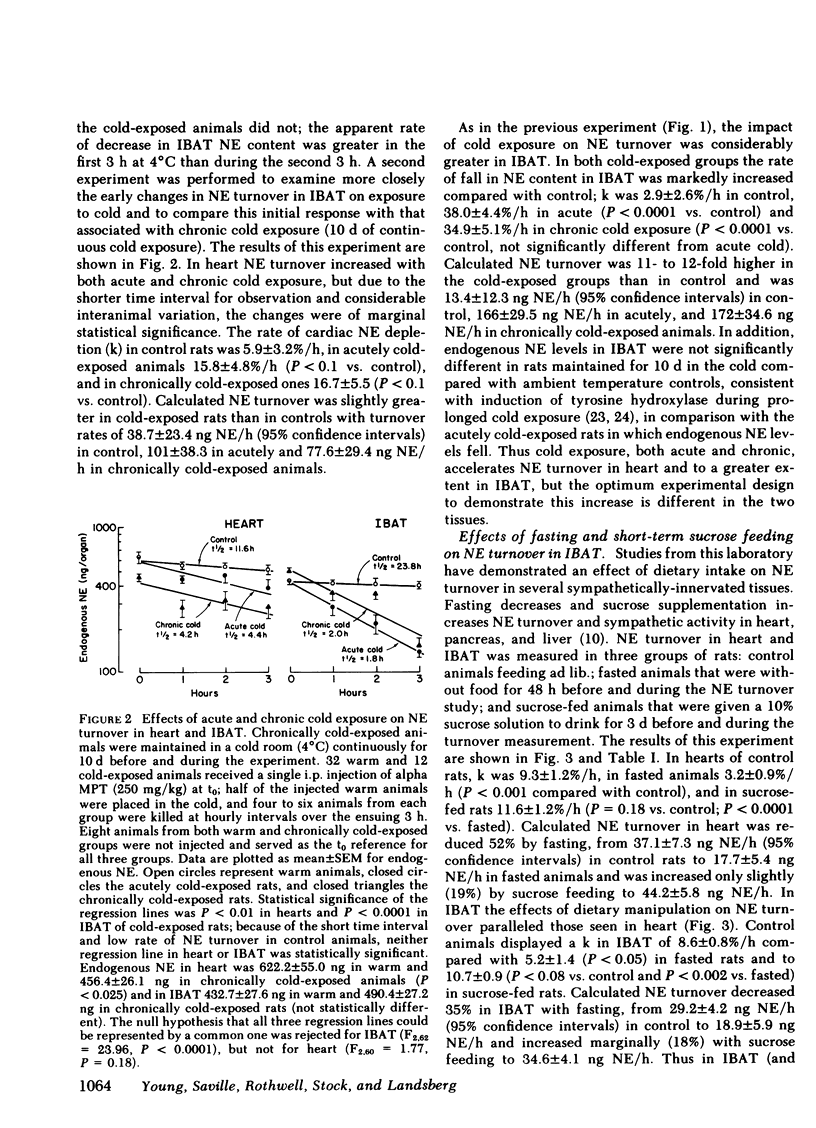
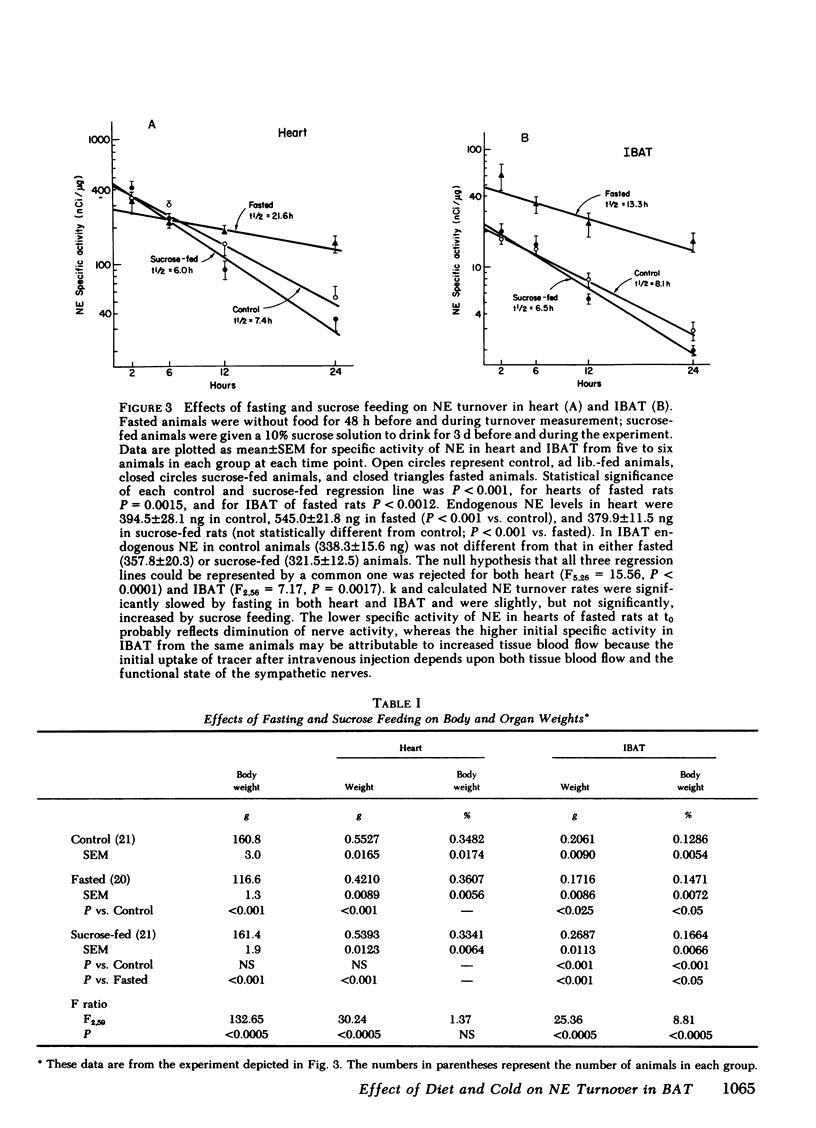
Brown adipose tissue (BAT) is an important site of adaptive changes in thermogenesis in the rat. The sympathetic nervous system, which richly supplies BAT, is thought to play an important role in the regulation of BAT thermogenesis because catecholamines stimulate and beta adrenergic blocking agents inhibit oxygen consumption in this tissue. The present studies were carried out to assess directly sympathetic activity in BAT in response to cold exposure and to changes in dietary intake, both of which alter heat production in the rat. Sympathetic activity was determined from the rate of norepinephrine (NE) turnover in interscapular brown adipose tissue (IBAT) after preliminary experiments validated the use of NE turnover techniques in IBAT. Acute exposure to 4°C increased NE turnover in IBAT 4- to 12-fold compared with ambient temperature controls, depending upon the interval over which the turnover measurement was made, while in the heart NE turnover doubled in response to the same cold stimulus. In animals exposed to cold continuously for 10 d before study, NE turnover measurements in IBAT and in the heart were elevated comparably to those obtained during acute exposure. Alterations in feeding were also associated with changes in NE turnover in IBAT. Fasting for 2 d decreased NE turnover in IBAT (-35% from 29.2±4.2 ng NE/h to 18.9±5.9) and in heart (-52%). In animals fed a “cafeteria” diet, a model of voluntary overfeeding in the rat, NE turnover was increased in both IBAT (+108% from 24.8±4.5 ng NE/h to 51.7±6.8) and heart (+66%). Because ganglionic blockade exerted a greater effect on NE turnover in IBAT in cafeteria-fed rats than in controls, the increase in NE turnover in IBAT with this overfeeding regimen reflects enhanced central sympathetic outflow. Thus NE turnover techniques can be satisfactorily applied to the assessment of sympathetic nervous system activity in IBAT.
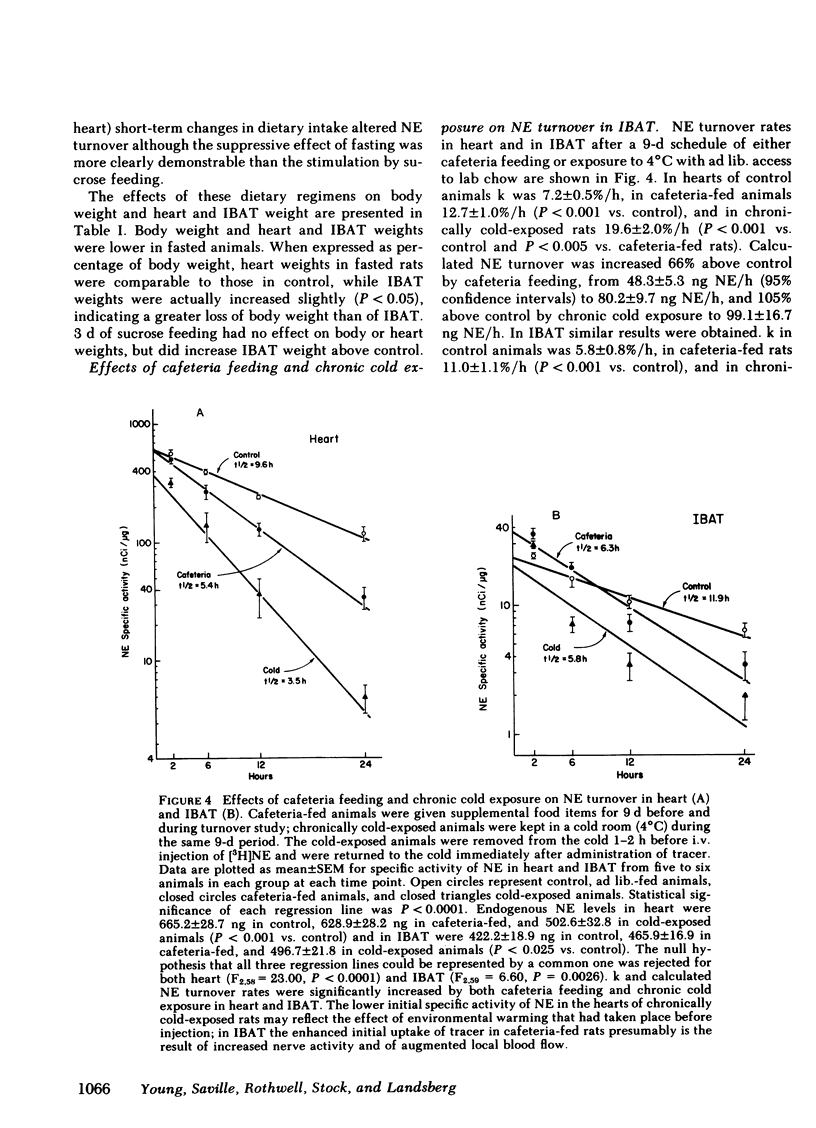
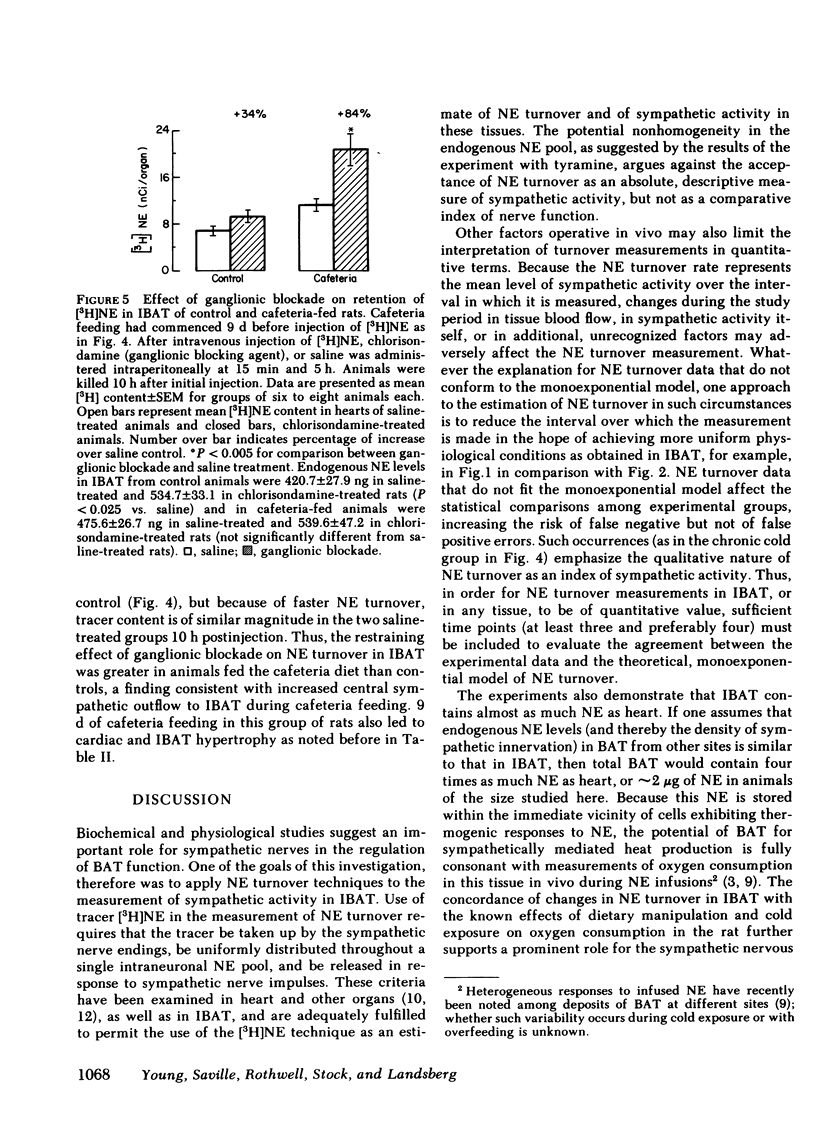
The experiments reported here demonstrate changes in sympathetic activity in IBAT that parallel known adaptive changes in heat production in the rat. These studies, therefore, support the concept that the increased thermogenesis of chronic cold exposure and of cafeteria feeding occur by similar mechanisms and imply an important role for the sympathetic nervous system, mediated in part through BAT, in the regulation of energy balance in the rat.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Bralet J., Beley A., Lallemant A. M. Modifications du taux de renouvellement de la noradrénaline dans différents organes périphériques du rat au cours de l'exposition et de l'acclimatation au froid. Pflugers Arch. 1972;335(3):186–197. doi: 10.1007/BF00592156. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966 Dec;154(3):493–498. [PubMed] [Google Scholar]
- Cottle W. H., Nash C. W., Veress A. T., Ferguson B. A. Release of noradrenaline from fat of cold-acclimated rats. Life Sci. 1967 Nov 1;6(21):2267–2271. doi: 10.1016/0024-3205(67)90034-3. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Depocas F., Frydman M. L. Noradrenaline-induced calorigenesis in warm- and cold-acclimated rats: relations between concentration of noradrenaline in arterial plasma, blood flow to differently located masses of brown adipose tissue, and calorigenic response. Can J Physiol Pharmacol. 1980 Aug;58(8):915–924. doi: 10.1139/y80-140. [DOI] [PubMed] [Google Scholar]
- Foster D. O., Frydman M. L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978 Feb;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- Gale C. C. Neuroendocrine aspects of thermoregulation. Annu Rev Physiol. 1973;35:391–430. doi: 10.1146/annurev.ph.35.030173.002135. [DOI] [PubMed] [Google Scholar]
- HALE H. B., MEFFERD R. B., Jr, VAWTER G., FOERSTER G. E., CRISCUOLO D. Influence of long-term exposure to adverse environments on organ weights and histology. Am J Physiol. 1959 Mar;196(3):520–524. doi: 10.1152/ajplegacy.1959.196.3.520. [DOI] [PubMed] [Google Scholar]
- HEROUX O., GRIDGEMAN N. T. The effect of cold acclimation on the size of organs and tissues of the rat, with special reference to modes of expression of results. Can J Biochem Physiol. 1958 Feb;36(2):209–216. [PubMed] [Google Scholar]
- Himms-Hagen J. Cellular thermogenesis. Annu Rev Physiol. 1976;38:315–351. doi: 10.1146/annurev.ph.38.030176.001531. [DOI] [PubMed] [Google Scholar]
- Ikemoto H., Hiroshige T., Ito S. Oxygen consumption of brown adipose tissue in normal and hypothyroid mice. Jpn J Physiol. 1967 Oct 15;17(5):516–522. doi: 10.2170/jjphysiol.17.516. [DOI] [PubMed] [Google Scholar]
- Johnson T. S., Young J. B., Landsberg L. Norepinephrine turnover in lung: effect of cold exposure and chronic hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1981 Sep;51(3):614–620. doi: 10.1152/jappl.1981.51.3.614. [DOI] [PubMed] [Google Scholar]
- Kennedy D. R., Hammond R. P., Hamolsky M. W. Thyroid cold acclimation influences on norepinephrine metabolism in brown fat. Am J Physiol. 1977 Jun;232(6):E565–E569. doi: 10.1152/ajpendo.1977.232.6.E565. [DOI] [PubMed] [Google Scholar]
- Landsberg L., Berardino M. B., Silva P. Metabolism of 3-H-L-dopa by the rat gut in vivo-evidence for glucuronide conjugation. Biochem Pharmacol. 1975 Jun 15;24(11-12):1167–1174. doi: 10.1016/0006-2952(75)90057-x. [DOI] [PubMed] [Google Scholar]
- Neff N. H., Tozer T. N., Hammer W., Costa E., Brodie B. B. Application of steady-state kinetics to the uptake and decline of H3-NE in the rat heart. J Pharmacol Exp Ther. 1968 Mar;160(1):48–52. [PubMed] [Google Scholar]
- POTTER L. T., AXELROD J., KOPIN I. J. Differential binding and release of norepinephrine and tachyphylaxis. Biochem Pharmacol. 1962 Mar;11:254–256. doi: 10.1016/0006-2952(62)90082-5. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., FAWCETT D. W. The effect of peripheral nerve section on some metabolic responses of brown adipose tissue in mice. Anat Rec. 1954 Mar;118(3):487–507. doi: 10.1002/ar.1091180303. [DOI] [PubMed] [Google Scholar]
- Sclafani A., Springer D. Dietary obesity in adult rats: similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976 Sep;17(3):461–471. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Sellers E. A., Flattery K. V., Steiner G. Cold acclimation of hypothyroid rats. Am J Physiol. 1974 Feb;226(2):290–294. doi: 10.1152/ajplegacy.1974.226.2.290. [DOI] [PubMed] [Google Scholar]
- Seydoux J., Girardier L. Control of brown fat thermogenesis by the sympathetic nervous system. Experientia. 1977 Sep 15;33(9):1128–1130. doi: 10.1007/BF01922280. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Taubin H. L., Djahanguiri B., Landsberg L. Noradrenaline concentration and turnover in different regions of the gastrointestinal tract of the rat: an approach to the evaluation of sympathetic activity in the gut. Gut. 1972 Oct;13(10):790–795. doi: 10.1136/gut.13.10.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco J. L., Flattery K. V., Sellers E. A. Effects of thyroid hormones and cold exposure on turnover of norepinephrine in cardiac and skeletal muscle. Can J Physiol Pharmacol. 1977 Jun;55(3):515–522. doi: 10.1139/y77-073. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Wimpfheimer C., Saville E., Voirol M. J., Danforth E., Jr, Burger A. G. Starvation-induced decreased sensitivity of resting metabolic rate to triiodothyronine. Science. 1979 Sep 21;205(4412):1272–1273. doi: 10.1126/science.224460. [DOI] [PubMed] [Google Scholar]
- Wirsén C., Hamberger B. Catecholamines in brown fat. Nature. 1967 May 6;214(5088):625–626. doi: 10.1038/214625a0. [DOI] [PubMed] [Google Scholar]
- Young J. B., Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in pancreas and liver. Am J Physiol. 1979 May;236(5):E524–E533. doi: 10.1152/ajpendo.1979.236.5.E524. [DOI] [PubMed] [Google Scholar]



