Abstract
Suppressor of cytokine signalling 3 (SOCS3) negatively regulates STAT3 activation in response to several cytokines such as those in the gp130-containing IL-6 receptor family. Thus, SOCS3 may play a major role in immune responses to pathogens. In the present study, the role of SOCS3 in M. tuberculosis infection was examined. All Socs3fl/fl LysM cre, Socs3fl/fl lck cre (with SOCS3-deficient myeloid and lymphoid cells, respectively) and gp130F/F mice, with a mutation in gp130 that impedes binding to SOCS3, showed increased susceptibility to infection with M. tuberculosis. SOCS3 binding to gp130 in myeloid cells conveyed resistance to M. tuberculosis infection via the regulation of IL-6/STAT3 signalling. SOCS3 was redundant for mycobacterial control by macrophages in vitro. Instead, SOCS3 expression in infected macrophages and DCs prevented the IL-6-mediated inhibition of TNF and IL-12 secretion and contributed to a timely CD4+ cell-dependent IFN-γ expression in vivo. In T cells, SOCS3 expression was essential for a gp130-independent control of infection with M. tuberculosis, but was neither required for the control of infection with attenuated M. bovis BCG nor for M. tuberculosis in BCG-vaccinated mice. Socs3fl/fl lck cre mice showed an increased frequency of γδ+ T cells in different organs and an enhanced secretion of IL-17 by γδ+ T cells in response to infection. Socs3fl/fl lck cre γδ+ T cells impaired the control of infection with M. tuberculosis. Thus, SOCS3 expression in either lymphoid or myeloid cells is essential for resistance against M. tuberculosis via discrete mechanisms.
Author Summary
Tuberculosis is a severe disease caused by infection with the intracellular bacteria Mycobacterium tuberculosis. The protein “suppressor of cytokine signalling 3” (SOCS3) inhibits the responses of cells to several cytokines and growth factors that signal via the STAT3 transcription factor. Since STAT3 is a major controller of immune and inflammatory responses, we studied the role of SOCS3 in the control of infection with M. tuberculosis. Mice deficient in the expression of SOCS3 either in myeloid or lymphoid cells were extremely susceptible to infection with M. tuberculosis as measured by elevated bacterial levels, worsened pathology and reduced survival. In myeloid cells, SOCS3 hindered a detrimental role of IL-6. In absence of SOCS3, IL-6 hampered the release of IL-12 by antigen-presenting cells. In T cells, SOCS3-mediated protection was independent of IL-6 signals, and of adequate IFN-γ secretion by antigen-specific T cells. Instead, SOCS3 inhibited the in vivo accumulation of, and the IL-17 secretion by γδ+ T cells. γδ+ T cells accounted in part for the susceptibility to M. tuberculosis infection of mice with SOCS3-deficient T cells. Thus, SOCS3 controls diverse immune mechanisms of myeloid and lymphoid cells that are required for containment of M. tuberculosis.
Introduction
Tuberculosis (TB), an infectious disease caused by Mycobacterium tuberculosis, remains a leading public health problem worldwide. The global incidence of TB is rising with 8.8 million new cases and 2 million deaths each year [1]. However, while immune responses to TB clearly show their importance in host defence, it is clear that there are still gaps in our knowledge of the host factors determining the outcome of infection.
Host responses of mycobacterial infections are primarily Th1 immune responses involving cellular effector mechanisms such as macrophage activation. IFN-γ is known to be an important mediator of mycobacterial control during clinical and experimental infections [2]. IL-12 is crucial for optimal differentiation and maintenance of IFN-γ-secreting antigen-specific Th1 cells [3], [4], and in controlling mycobacterial infections in mice and man [5], [6].
The “suppressor of cytokine signalling” (SOCS) proteins are a family of eight members that inhibit STAT activation by different receptors. SOCS proteins bind either the Janus-activated kinases (JAKs) directly inhibiting their kinase activity, or the intracellular domain of cytokine receptors thereby targeting the receptor complex for ubiquitination and subsequent proteasome-mediated degradation [7]. SOCS3 inhibits STAT3-mediated signalling by binding to the IL-6 receptor family subunit gp130, G-CSF, leptin and the IL-12 receptor [8]. Since SOCS3-deficient mice die during embryogenesis [9], [10], the role of SOCS3 in vivo has been studied using conditional knockdown mice. Conditional knockdown of SOCS3 in macrophages protects mice from LPS shock by reducing the secretion of IL-12 and TNF due to the enhanced anti-inflammatory effect of STAT3 [11]. However, mice with SOCS3-deficient macrophages and neutrophils succumb to toxoplasmosis, probably due to reduced IL-12 and IFN-γ responses [12]. Furthermore, SOCS3 can also inhibit STAT1 activation thereby preventing IFN-γ-like responses in cells stimulated with IL-6 [13], [14]. SOCS3 also may have several roles in T cell function. SOCS3 expression in T cells can both obstruct the differentiation of inflammatory IL-17-producing Th17 cells [15], [16] and inhibit the secretion of anti-inflammatory IL-10 and TGF-β by T cells [17] and mice with SOCS3-deficient T cells are more susceptible to infection with Leishmania major [17]. On the other hand, SOCS3 has also been shown to impair T-cell memory development, T cell-mediated IFN-γ secretion and LCMV virus clearance in mice [18].
In the present study, the role of SOCS3 in the outcome of infection with M. tuberculosis was investigated. We report that the expression of SOCS3, in either myeloid or T cells, is independently required for the control of M. tuberculosis infection in mice. SOCS3 expression in myeloid cells allows a proper IL-12 secretion by hampering an IL-6-mediated inhibition of IL-12 expression. SOCS3 expression in T cells reduces the frequency of γδ+ T cells in different organs and the secretion of IL-17 by + T cells in response to infection in a gp130-independent manner.
Results
Socs3fl/fl LysM cre mice are highly susceptible to infection with M. tuberculosis
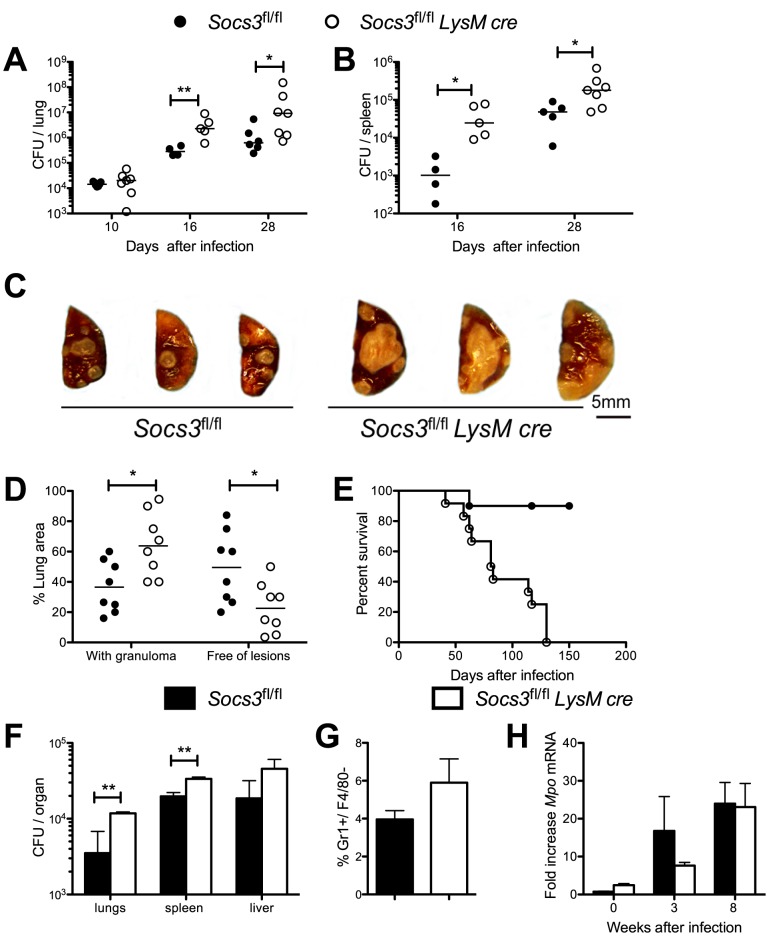
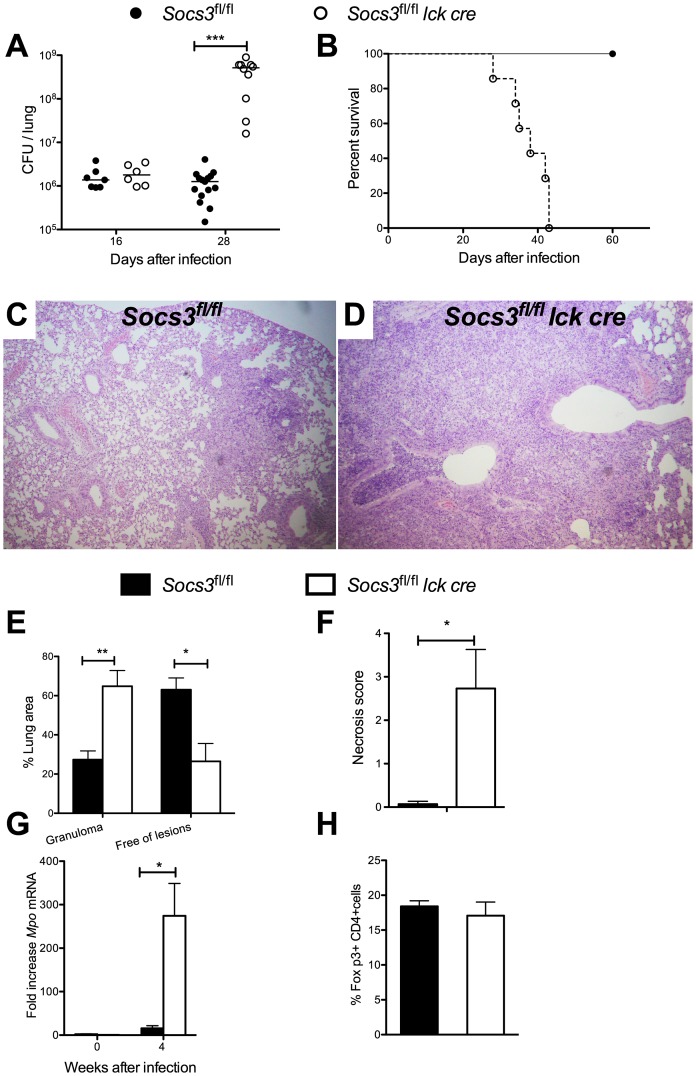
First, the role of Socs3 expression in myeloid cells in the control of infection with M. tuberculosis was examined by using Socs3fl/fl LysM cre mice [19]. Lungs and spleens from Socs3fl/fl LysM cre mice showed significantly higher M. tuberculosis levels than Socs3fl/fl littermates at 16 and 28 days of infection (Figure 1A, B). A larger area of the lung parenchyma of Socs3fl/fl LysM cre mice was occupied by granulomas as compared to controls 4 weeks after infection (Figure 1C, D). Furthermore, M. tuberculosis-infected Socs3fl/fl LysM cre mice also showed a higher cumulative mortality (Figure 1E). Socs3fl/fl LysM cre mice infected with the attenuated M. bovis BCG displayed higher bacterial levels in the lungs and spleen (but not the liver), although the differences in BCG levels with infected Socs3fl/fl littermates were not as striking as those observed after infection with M. tuberculosis (Figure 1F).
Figure 1. Socs3fl/fl LysM cre mice show higher susceptibility to infection with M. tuberculosis.
Socs3fl/fl LysM cre and Socs3fl/fl littermate controls were sacrificed at indicated time points after aerosol infection with M. tuberculosis and colony forming units (CFU) per lung (A) and spleen (B) were assessed. The CFU per lung of individual mice and the median per group (n≥4) at the indicated time points after infection are depicted. Differences in CFU are significant (*p<0.05 and **p<0.01 Mann Whitney U test).Gross-pathology photograph of the lungs from Socs3fl/fl and Socs3fl/fl LysM cre mice 8 weeks after infection with M. tuberculosis (C). Histopathological scoring of hematoxylin-eosin stained paraffin lung sections from Socs3fl/fl LysM cre and Socs3fl/fl mice measured 4 weeks after infection with M. tuberculosis (D). The mean % lung area with granulomas or free of lesions ± SEM is displayed. Differences with controls are significant (n = 8 per group, *p<0.05 Student t test). The cumulative mortality of Socs3fl/fl and Socs3fl/fl LysM cre mice (n = 10) after aerosol infection with M. tuberculosis is depicted (E). Survival curves are different (Log-rank test p<0.005). CFU per lung, spleen and liver in Socs3fl/fl and Socs3fl/fl LysM cre mice (n≥5 per group) were assessed 6 weeks after infection with 106 BCG i.v. (F). The median CFU and interquartile range per group are depicted. Differences in CFU are significant (**p<0.01 Mann Whitney U test). The mean percentage of Gr1+F4/80- neutrophils in the lung of Socs3fl/fl and Socs3fl/fl LysM cre mice (n = 5 per group) 3 weeks after infection with M. tuberculosis ± SEM was determined by FACS analysis (G). The accumulation of myeloid peroxidase (Mpo) transcripts in lungs from mice at 0, 3 or 8 weeks after M. tuberculosis infection (n≥5 per group) was determined by real time PCR. The mean fold Mpo mRNA increase ± SEM in is depicted (H).
Since the LysM promoter is active in neutrophils and SOCS3 has been shown to be a negative regulator of granulopoiesis [20], [21], we studied whether the increased susceptibility to M. tuberculosis of Socs3fl/fl LysM cre mice was associated to increased numbers of neutrophils at the site of infection. Comparable numbers of Gr1+/F4/80- neutrophils and similar mRNA levels of the neutrophil enzyme myeloperoxidase were detected in lungs from M. tuberculosis-infected Socs3fl/fl LysM cre and control mice (Figure 1G, H).
SOCS3-deficient macrophages are not impaired in bacterial control and can respond to IFN-γ
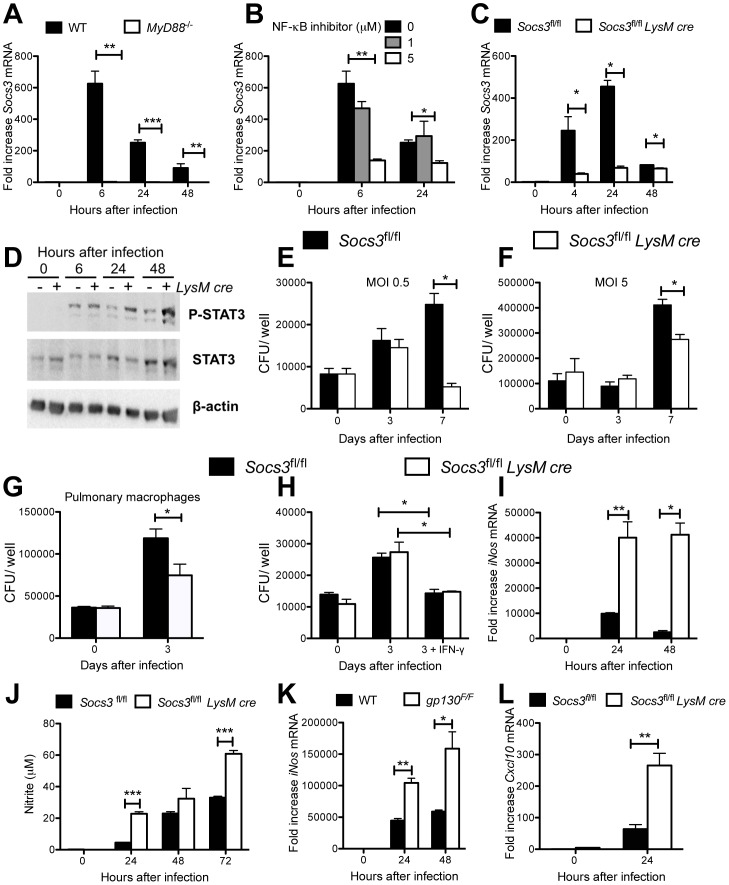
Next, we studied the expression and role of SOCS3 in mycobacteria-infected macrophages. Bone marrow-derived macrophages (BMM) from wild type (WT) mice showed increased accumulation of Socs3 mRNA after infection with either M. tuberculosis or BCG (Figure 2A–C, S1A, B). Recognition by innate immune receptors was required for SOCS3 expression, since Socs3 mRNA levels after infection were reduced in the Toll-like receptor adaptor molecule MyD88 −/− BMM and BMM incubated with a NF-κB inhibitor but not in Irf3 −/− BMM (Figure 2A, B and S1A, B). IRF3 has been shown to detrimentally affect M. tuberculosis infection [22]. As expected, Socs3 mRNA levels were reduced in M. tuberculosis-infected Socs3fl/fl LysM cre BMM when compared to controls (Figure 2C), and in vitro infection of BMM with M. tuberculosis stimulated STAT3 phosphorylation that was prolonged in Socs3fl/fl LysM cre BMM (Figure 2D).
Figure 2. SOCS3-deficient macrophages do not display increased M. tuberculosis growth.
Mouse BMM were infected with M. tuberculosis at a MOI of 5∶1 (A–C). BMM were treated with the indicated concentrations of BAY-117082 1 h before infection (B). Total RNA was isolated from MyD88−/− (A) WT (C57Bl/6) (A, B), Socs3fl/fl (C) or Socs3fl/fl LysM cre (C) BMM at the indicated time points after infection. The mean fold Socs3 mRNA induction ± SEM measured by real time PCR is depicted. A representative of 3 experiments is shown (C). Differences with WT (A, C) or untreated (B) BMM are significant (*p<0.05, **p<0.01, ***p<0.001 Student t test). Phosphorylated STAT3, total STAT3 and actin in lysates Socs3fl/fl LysM cre and Socs3fl/fl BMM after infection with M. tuberculosis was detected by Western Blot (D). Bacterial levels were determined in Socs3fl/fl LysM cre and Socs3fl/fl BMM after infection M. tuberculosis H37Rv at a MOI of 0.5∶1 (E) or 5∶1 (F). The mean CFU ± SEM from triplicate cell cultures is shown. Two independent experiments for each panel were performed. (*p<0.05 Student t test) Socs3fl/fl LysM cre and Socs3fl/fl pulmonary macrophages were infected with M. tuberculosis at a MOI of 1. The CFU were determined at the indicated time points in triplicate cell cultures (*p<0.05 Student t test)(G). Socs3fl/fl LysM cre and Socs3fl/fl BMM were infected with M. tuberculosis at a MOI of 5. One hundred U/ml recombinant IFN-γ were added 24 h after infection. The CFU were determined in triplicate cell cultures (H). One out of two independent experiments is shown. Differences with Socs3fl/fl BMM are significant (*p<0.05 Student t test). Total RNA was extracted from Socs3fl/fl LysM cre, Socs3fl/fl (I, L), WT and gp130F/F (K) BMM at the indicated times after infection with M. tuberculosis at a MOI of 5. The relative accumulation of iNos, Cxcl10 and Hprt mRNA was measured by real time PCR. The mean fold increase of iNos (I, K), or Cxcl10 (L) mRNA ± SEM in triplicate samples for each genotype and time point in one out of two independent experiments is depicted. Differences with control BMM are significant (*p<0.05 and **p<0.01Student t test). Nitrite concentrations in supernatants of Socs3fl/fl LysM cre and Socs3fl/fl BMM at different times after infection with M. tuberculosis. The mean NO2 − concentration ± SEM in triplicate cultures per condition from one of two independent experiments is depicted (***p<0.001 Student t test)(J).
Whether a defect of macrophages to control intracellular mycobacterial growth could account for the enhanced susceptibility of Socs3fl/fl LysM cre mice to mycobacteria was then studied. Socs3fl/fl LysM cre BMM, pulmonary and peritoneal macrophages showed diminished intracellular levels of M. tuberculosis (Figure 2E–G and data not shown). The IFN-γ-mediated control of mycobacteria by macrophages is essential for the intracellular control of M. tuberculosis. Incubation of BMM with IFN-γ decreased the number of intracellular M. tuberculosis. Similar bacterial levels were measured in Socs3fl/fl LysM cre and control BMM after incubation with IFN-γ (Figure 2H). Macrophages have been shown to kill mycobacteria through the generation of nitric oxide (NO) by the IFN-γ-regulated inducible NO synthase (iNOS) [23]. M. tuberculosis-infected Socs3fl/fl LysM cre BMM contained higher iNos mRNA and nitrite levels than Socs3fl/fl BMM (Figure 2I, J). Similarly, infection of Socs3fl/fl LysM cre BMM with BCG or stimulation with Pam3CSK4, an agonist for TLR2, a receptor that plays a prominent role in the initiation of responses against M. tuberculosis [24], led to higher NO and iNos mRNA levels compared to controls (Figure S1C, D).
Cells derived from gp130F/F mice, harbouring a gp130 Y757F mutation to ablate SOCS3 binding to gp130, show an exaggerated gp130-mediated STAT3 signalling as a consequence of an impaired negative feedback loop by SOCS3 to down-modulate gp130/STAT3 signalling [25]. Similar to Socs3fl/fl LysM cre BMM, gp130F/F BMM showed increased iNos mRNA and nitrite levels after infection with either M. tuberculosis or BCG, or stimulation with Pam3CSK4 (Figure 2K and S1E, F). Thus, the increased iNOS response of SOCS3-deficient macrophages was dependent on signalling via gp130. Similarly, the mRNA expression levels of the IFN-γ-induced chemokine CXCL10 was also increased in either M. tuberculosis- or BCG-infected Socs3fl/fl LysM cre BMM (Figure 2L and S1G).
Altogether, these data demonstrated that the higher susceptibility to M. tuberculosis of Socs3fl/fl LysM cre mice was not associated with a defect of BMM or pulmonary macrophages in controlling intracellular bacterial growth in vitro.
SOCS3-deficient macrophages show decreased TNF and IL-12 responses during mycobacterial infection
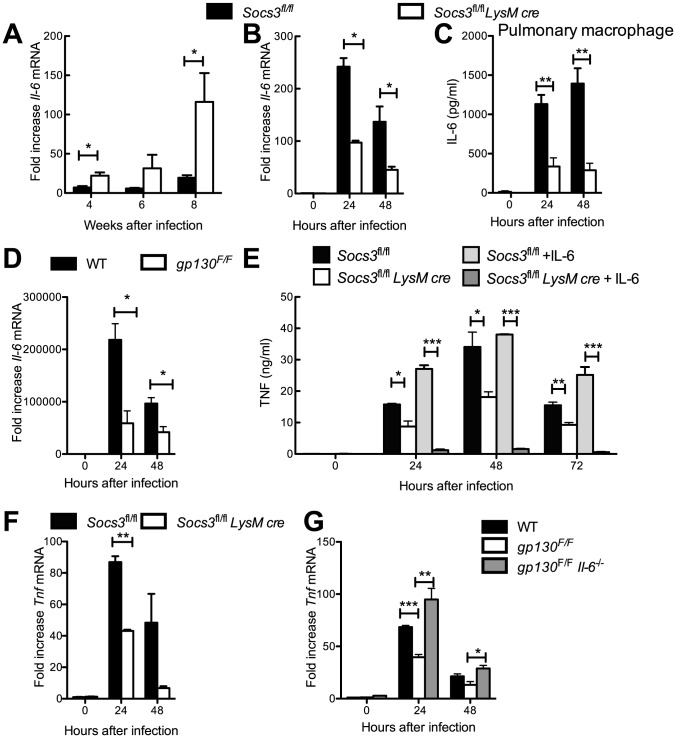
After infection with M. tuberculosis, Il-6 mRNA levels were strikingly increased in lungs from Socs3fl/fl LysM cre mice when compared to littermates (Figure 3A). Therefore, we evaluated whether IL-6 secretion was also elevated in mycobacteria-infected Socs3fl/fl LysM cre BMM. Unexpectedly, we found diminished Il-6 mRNA and protein levels in M. tuberculosis- or BCG-infected or in Pam3CSK4-stimulated Socs3fl/fl LysM cre BMM, peritoneal and pulmonary macrophages as compared to controls (Figure 3B, C and S2A–C). Similarly, gp130F/F BMM expressed lower IL-6 protein and mRNA levels after infection with either M. tuberculosis or BCG, or stimulation with Pam3CSK4 (Figure 3D and S2D). In conclusion, although IL-6 levels are increased in lungs of infected Socs3fl/fl LysM cre mice, SOCS3 does not impair IL-6 secretion by mycobacteria-infected BMM.
Figure 3. SOCS3-deficient BMM show diminished TNF secretion after infection with M. tuberculosis.
Socs3fl/fl LysM cre and Socs3fl/fl mice were infected with M. tuberculosis Harlingen via the aerosol route. Animals were sacrificed at the indicated time points after infection and the total RNA was extracted from lungs. The accumulation of Il-6 and Hprt transcripts was measured by real time PCR. The mean fold Il-6 mRNA increase ± SEM in lungs from infected mice (n≥5 per group) was calculated (*p<0.05 Student t test)(A). The levels of Il-6 mRNA in Socs3fl/fl and Socs3fl/fl LysM cre (B) or gp130F/F and WT (D) BMM infected with M. tuberculosis were determined by real time PCR. The mean fold increase of mRNA level ± SEM in triplicate independent cultures per condition compared to non-infected cultures in one out of two independent experiments is depicted (*p<0.05 Student t test). The mean IL-6 concentration ± SEM in supernatants of M. tuberculosis-infected Socs3fl/fl and Socs3fl/fl LysM cre pulmonary macrophages as determined by ELISA is depicted. IL-6 secretion by BCG-infected Socs3fl/fl and Socs3fl/fl LysM cre peritoneal macrophages is shown (C). The concentration of TNF was measured in supernatants of M. tuberculosis-infected Socs3fl/fl and Socs3fl/fl LysM cre BMM co-incubated with or without 50 ng/ml of recombinant IL-6 (E). The levels of Tnf mRNA in Socs3fl/fl and Socs3fl/fl LysM cre (F) or gp130F/F, gp130F/F Il-6−/− and WT (G) BMM infected with M. tuberculosis were determined by real time PCR. The mean fold increase of mRNA level ± SEM in triplicate independent cultures per condition compared to non-infected cultures of one of two independent experiments is depicted (*p<0.05, **p<0.01, ***p<0.001 Student t test).
The LPS-induced production of TNF and IL-12 is reduced in SOCS3-deficient macrophages if IL-6 is added [11]. Consistent with this observation, the Tnf mRNA or protein accumulation was reduced in Socs3fl/fl LysM cre BMM incubated with either M. tuberculosis, BCG- or Pam3CSK4 as compared to controls (Figure 3E and S2E).
Next, we studied whether IL-6 signalling accounted for SOCS3/gp130-mediated regulation of TNF levels. Tnf mRNA and protein levels were reduced in M. tuberculosis- or BCG-infected gp130F/F BMM and were partially restored in gp130F/F Il-6 −/− BMM (Figure 3G and S2F, G), indicating that SOCS3 allows proper infection-induced TNF secretion in macrophages by hampering gp130/IL-6 receptor-mediated signalling. Moreover, the co-incubation with recombinant IL-6 (rIL-6) further diminished TNF levels in M. tuberculosis-infected Socs3fl/fl LysM cre but not in control BMM (Figure 3E).
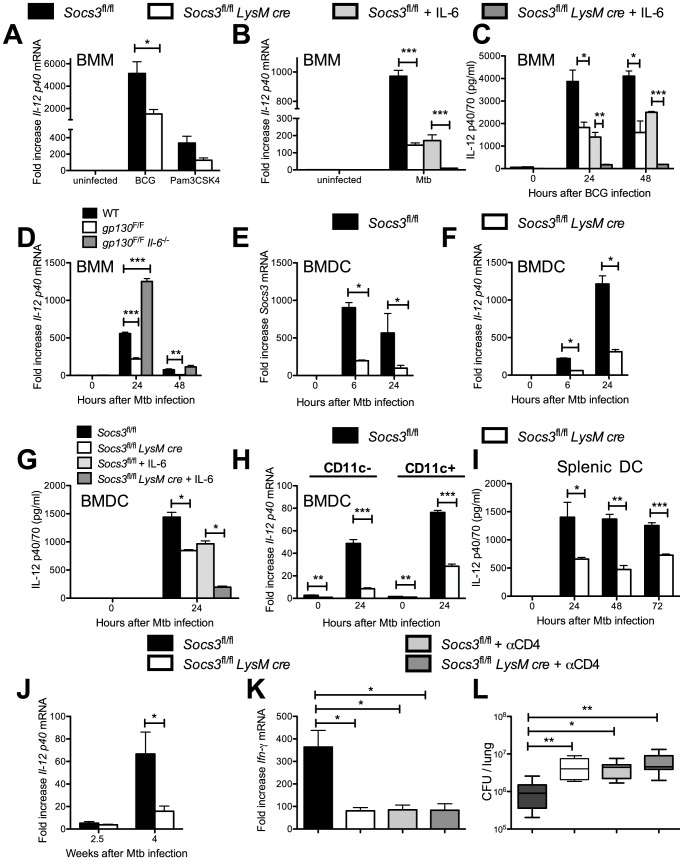
Similar to the results observed for TNF, the Il-12 p40 mRNA and protein levels were reduced in Socs3fl/fl LysM cre BMM after stimulation with Pam3CSK4, BCG or M. tuberculosis when compared to controls (Figure 4A–C). IL-12 levels were further decreased in cultures of infected mutant macrophages incubated with rIL-6 (Figure 4B, C). Accordingly, IL-12 p40 mRNA and protein levels were reduced in M. tuberculosis- or BCG-infected gp130F/F BMM, and such defect was restored in infected gp130F/F Il-6−/−BMM (Figure 4D and S3A).
Figure 4. Reduced IL-12 secretion in Socs3fl/fl LysM cre macrophages and DCs after mycobacterial infection.
The levels of Il-12 p40 mRNA were measured in triplicate cultures of Socs3fl/fl LysM cre and Socs3fl/fl BMM infected with BCG (A) or M. tuberculosis (B) or treated with Pam3CSK4 for 24 h (A). Additionally, 50 ng/ml recombinant IL-6 was added to M. tuberculosis-infected samples (B). One out of two independent experiments is shown (*p<0.05 and ***p<0.001 Student t test). The concentration of IL-12 in supernatants from BCG-infected Socs3fl/fl and Socs3fl/fl LysM cre BMM co-incubated with 50 ng/ml IL-6 was determined by ELISA (C). The mean IL-12 concentration ± SEM from triplicate cultures per condition in one of two independent experiments is depicted, (*p<0.05, **p<0.01, ***p<0.001 Student t test). The levels of Il-12 p40 mRNA were measured in triplicate cultures of gp130F/F, gp130F/F Il-6−/− or WT BMM infected with M. tuberculosis (D), (**p<0.05 and ***p<0.001 Student t test). The levels of Socs3 (E) and Il-12 p40 mRNA (F) in triplicate cultures of M. tuberculosis-infected Socs3fl/fl LysM cre and Socs3fl/fl BMDC were determined by real time PCR. One of two independent experiments is shown, (*p<0.05 Student t test). The concentration of IL-12 was determined by ELISA in supernatants from triplicate cultures of M. tuberculosis-infected Socs3fl/fl LysM cre and Socs3fl/fl BMDC co-incubated or not with 50 ng/ml IL-6 (G), (*p<0.05, Student t test). Total RNA was extracted from M. tuberculosis-infected CD11c+ and CD11c- Socs3fl/fl and Socs3fl/fl LysM cre BMDC cultures 24 h after M. tuberculosis infection. The mean Il-12p40 mRNA levels ± SEM levels measured by real time PCR are depicted (H). The concentration of IL-12p40 in supernatants from M. tuberculosis-infected Socs3fl/fl and Socs3fl/fl LysM cre splenic DCs was determined by ELISA (I). The mean IL-12p40 ± SEM pg/ml from triplicate cultures is depicted (*p<0.05, **p<0.01, ***p<0.001 Student t test). The fold increase of Il-12 p40 mRNA in the lungs of M. tuberculosis-infected Socs3fl/fl LysM cre and Socs3fl/fl mice relative to uninfected mice is displayed (J). The data is pooled from 2 independent experiments with n≥5 animals per group in each one (*p<0.05, Student t test). Total RNA was isolated from the lungs of Socs3fl/fl LysM cre and Socs3fl/fl mice (n≥7 per group) treated or not with CD4 cell-depleting antibodies 2.5 weeks after infection with M. tuberculosis (K). The mean Ifn-γ mRNA ± SEM is depicted, (*p<0.05, Student t test). Bacterial loads in lungs from Socs3fl/fl and CD4+ cell-depleted Socs3fl/fl and Socs3fl/fl LysM cre mice (n≥5) 2.5 weeks after M. tuberculosis infection are shown (L). A box and whisker diagram showing the median CFU, quartiles and the 99th percentiles is depicted, (*p<0.05, **p<0.01 Mann Whitney U test).
Since IL-12 secretion by dendritic cells (DCs) is required for Th1 differentiation, we investigated whether DCs from Socs3fl/fl LysM cre mice also showed an impaired secretion of IL-12. Socs3 mRNA levels were reduced in Socs3fl/fl LysM cre bone marrow-derived dendritic cells (BMDC) indicating the expression of the LysM cre recombinase (Figure 4E). The Il-12 p40 mRNA and IL-12 protein expression by Socs3fl/fl LysM cre BMDC after infection with M. tuberculosis was reduced as compared to controls (Figure 4F, G). As shown for BMM, incubation with exogenous IL-6 further diminished IL-12 secretion by M. tuberculosis-infected Socs3fl/fl LysM cre BMDC (Figure 4G). In order to exclude that the diminished IL-12 secretion in BMDC cultures was due to the response of contaminant macrophages in the culture, the expression of Il-12 p40 mRNA was tested in CD11c+ sorted cells. Both, CD11c+ and CD11c- cells showed diminished IL-12 p40 mRNA accumulation after infection with M. tuberculosis compared with controls (Figure 4H). Moreover, Socs3fl/fl LysM cre splenic DCs displayed a diminished secretion of IL-12 after M. tuberculosis (Figure 4I). Thus, SOCS3 expression promotes IL-12 secretion in M. tuberculosis-stimulated DCs.
Since IL-12 is required for IFN-γ secretion by NK cells, we tested the effect of SOCS3 expression by M. tuberculosis-infected DCs in the regulation of IFN-γ secretion by NK cells. Co-incubation with M. tuberculosis-infected splenic CD11c+ DCs induced IFN-γ secretion by NK cells. IFN-γ expression by NK cells was reduced when these cells were incubated with Socs3fl/fl LysM cre DCs (Figure S3B, C).
Lower Il-12 p40 mRNA levels were also found in lungs of M. tuberculosis-infected Socs3fl/fl LysM cre mice compared to controls (Figure 4J) and similarly, lower Ifn-γ mRNA accumulation in lungs from Socs3fl/fl LysM cre mice was detected 2.5 weeks after M. tuberculosis infection (Figure 4K). In order to examine whether an effect of SOCS3-deficient myeloid cells on the cytokine production by CD4+ T cells accounted for the elevated numbers of bacteria in Socs3fl/fl LysM cre mice, we depleted CD4+ cells during M. tuberculosis infection by administration of anti-CD4 neutralizing antibodies (Figure S4A). CD4+ cell depletion decreased the Ifn-γ mRNA accumulation in lungs from Socs3fl/fl mice. The Ifn-γ mRNA levels in infected Socs3fl/fl LysM cre mice were similar to those measured in CD4+ cell-depleted mice (Figure 4K). Moreover, lungs from Socs3fl/fl LysM cre and control mice depleted of CD4+ cells showed similar bacterial levels (Figure 4L). In contrast to the decreased Ifn-γ mRNA expression, the frequency of CD44+ and CD62L+ CD4+ activated T cells in the lungs of M. tuberculosis-infected Socs3fl/fl LysM cre and Socs3fl/fl mice was similar, and higher than in uninfected animals (Figure S4B, C). When we compared cytokine levels in Socs3fl/fl LysM cre and Socs3fl/fl littermates at later time points after M. tuberculosis infection. Higher Ifn-γ and iNos, but similar Tnf-mRNA levels were measured in lungs from Socs3fl/fl LysM cre compared to control mice 8 weeks after infection (Figure S4D–F).
Regulatory FoxP3+ T-cells have been shown to expand in mice with SOCS3-deficient DCs [26]. However, comparable levels of FoxP3+ CD4+ T cells were found in lungs and pulmonary lymph nodes of infected mutant and control mice (Figure S4G), suggesting that the susceptibility of Socs3fl/fl LysM cre mice to M. tuberculosis is not due to higher frequencies of regulatory T cells.
Altogether, the enhanced susceptibility of Socs3fl/fl LysM cre mice to M. tuberculosis could be associated to a reduced IL-12 secretion resulting in a delayed CD4+ -cell dependent IFN-γ-expression.
Mice with SOCS3-deficient T cells are dramatically susceptible to infection with M. tuberculosis
Next, the role of SOCS3 expression by T cells in the control of infection with M. tuberculosis was studied. Lungs and spleens from Socs3fl/fl lck cre mice showed higher numbers of M. tuberculosis bacteria 4 weeks after aerosol infection (Figure 5A and data not shown), with 500-fold higher bacterial levels in lungs compared to Socs3fl/fl littermate controls. In contrast, no differences in bacterial load were registered 2 weeks after infection (Figure 5A). Infected Socs3fl/fl lck cre mice had a median survival of 38 days after infection while controls survived more than 200 days (Figure 5B). Four weeks after infection, Socs3fl/fl lck cre mice displayed an increased severity of pulmonary pathology (Figure 5C–E) with granulomas containing large necrotic areas (Figure 5F) and elevated levels of neutrophil myeloperoxidase transcripts (Figure 5G). The frequency of Foxp3+ CD4+ regulatory T cells in pulmonary lymph nodes was similar (Figure 5H).
Figure 5. Mice with SOCS3-deficient T cells are susceptible to infection with M. tuberculosis.
Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed at the indicated time points after aerosol infection with M. tuberculosis, and CFU in lungs assessed (A). The CFU per lung of individual mice and the median per group (n≥5) are depicted. Results are pooled from three independent experiments. Differences in CFU are significant (***p<0.001 Mann Whitney U test). The cumulative mortality of Socs3fl/fl lck cre and Socs3fl/fl mice (n = 10 mice per group) after aerosol infection with M. tuberculosis is depicted (B). Differences in survival curves are significant (Log-rank test p<0.0005) with a median survival of 38 days for Socs3fl/fl lck cre mice. Hematoxylin-eosin stained paraffin lung sections from Socs3fl/fl (C) and Socs3fl/fl lck cre mice (D) and their histopathological scoring (E, F) determined 4 weeks after infection with M. tuberculosis. The mean % lung area with granulomas or free of lesions ± SEM and the mean score of lymphocytes within the granuloma or in perivascular spaces ± SEM is shown (F). Differences with controls are significant (n = 5 per group, *p<0.05 Student t test). Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed 4 weeks after M. tuberculosis infection and the total RNA was extracted from lungs. The accumulation of Mpo and Hprt transcripts was measured by real time PCR (G). The mean fold increase of Mpo mRNA ± SEM in lungs from infected mice (n≥5 per group) was calculated. Differences with infected Socs3fl/fl mice are significant (*p<0.05 Student t test). The mean frequency of FoxP3+ within CD4+ pulmonary lymph node CD3+ T cells from Socs3fl/fl lck cre and Socs3fl/fl mice (n = 5 per group) was determined by FACS analysis 4 weeks after infection with M. tuberculosis (H).
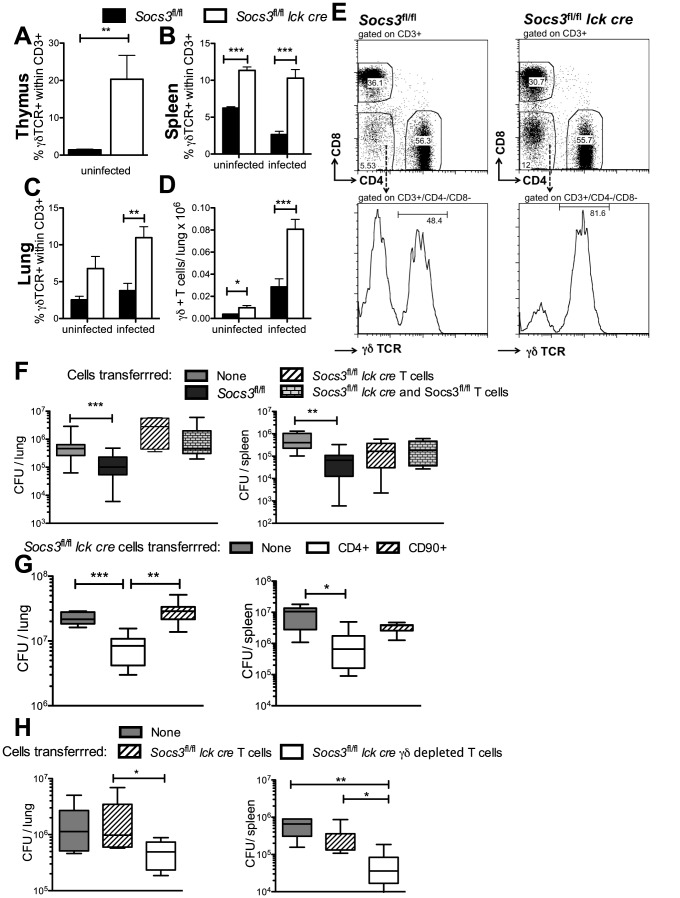
Whether the susceptibility of Socs3fl/fl lck cre mice was associated to an altered frequency of T cell populations was then evaluated. While the percentage of CD4+ and CD8+ T cells in lungs and spleens from Socs3fl/fl lck cre and Socs3fl/fl mice before or after M. tuberculosis infection was similar (Figure S5A–D), the percentage of γδ+ T cells in the thymus, spleen and lungs of uninfected Socs3fl/fl lck cre mice was strikingly elevated and remained high after M. tuberculosis infection when compared to Socs3fl/fl controls (Figure 6A–C, E). T cells accumulated in the lungs after M. tuberculosis infection and higher numbers of γδ+ T cells were observed in lungs from infected Socs3fl/fl lck cre compared to Socs3fl/fl mice (Figure 6D).
Figure 6. γδ+ T cell numbers are increased in organs of Socs3fl/fl lck cre mice.
The frequency of γδ+ T cells within CD3+ cells in the thymus (A), spleen (B) and lung (C) of Socs3fl/fl lck cre and Socs3fl/fl mice obtained before or 2.5 weeks after M. tuberculosis infection were determined by FACS. Mean percentage (A–C) and the total numbers (D) of γδ+ within CD3+ T cells in the lungs ± SEM are depicted. Differences with Socs3fl/fl mice (n = 4 per group) are significant (*p<0.05, ***p<0.001 Student t test). The gating strategy and representative dot-plots for spleens of infected Socs3fl/fl lck cre and Socs3fl/fl mice are shown (E). Two million Socs3fl/fl lck cre or Socs3fl/fl CD90+ T cells positively selected from spleens using magnetic beads were inoculated i.v. into Rag1−/− mice. A group of animals was also inoculated with both Socs3fl/fl lck cre and Socs3fl/fl T cells (F). Rag1−/− mice were alternatively transferred with 1.2×106 CD4+ or 2×106 CD90+ Socs3fl/fl lck cre spleen cells (G) or in a different experiment with 2×106 FACS-sorted CD3+ depleted of γδ+ T cells or total CD3+ Socs3fl/fl lck cre spleen cells (H). Two weeks after transfer, mice were infected with M. tuberculosis via the aerosol route. Mice (n≥6 per group) were sacrificed 4 weeks after infection. Box and whisker diagrams showing the median CFU, quartiles and the 99th percentiles in lungs and spleens are depicted (F–H). Differences in CFU are significant (*p<0.05, **p<0.001, ***p<0.001 Mann Whitney U test).
The outcome of infection with M. tuberculosis of Rag1 −/− mice reconstituted with control or Socs3fl/fl lck cre T cells was then compared. Lungs and spleens from Rag1 −/− mice transferred with Socs3fl/fl total T cells (CD90+) showed lower bacterial levels than non-transferred mice while Socs3fl/fl lck cre T cells failed to transfer protection (Figure 6F). Moreover, the transfer of a 1∶1 mixture of Socs3fl/fl lck cre and control T cells conferred no protection to Rag1−/− mice (Figure 6F), suggesting that SOCS3-deficient T cells can hamper M. tuberculosis control by wild type T cells. Rag1−/− mice transferred with CD4+ Socs3fl/fl lck cre cells unlike those transferred with total T cells from the same mice, showed reduced M. tuberculosis levels compared to non-transferred controls indicating that in Socs3fl/fl lck cre mice CD3+CD4− T cells hamper the protective ability of CD4+ cells (Figure 6G). Therefore, we examined whether γδ+ T cells could account for the suppressive activity of CD3+CD4− cells. Indeed, Rag1−/− mice transferred with γδ+ cell-depleted CD90+ Socs3fl/fl lck cre T cells showed lower bacterial levels than those transferred with total Socs3fl/fl lck cre T cells (Figure 6 H).
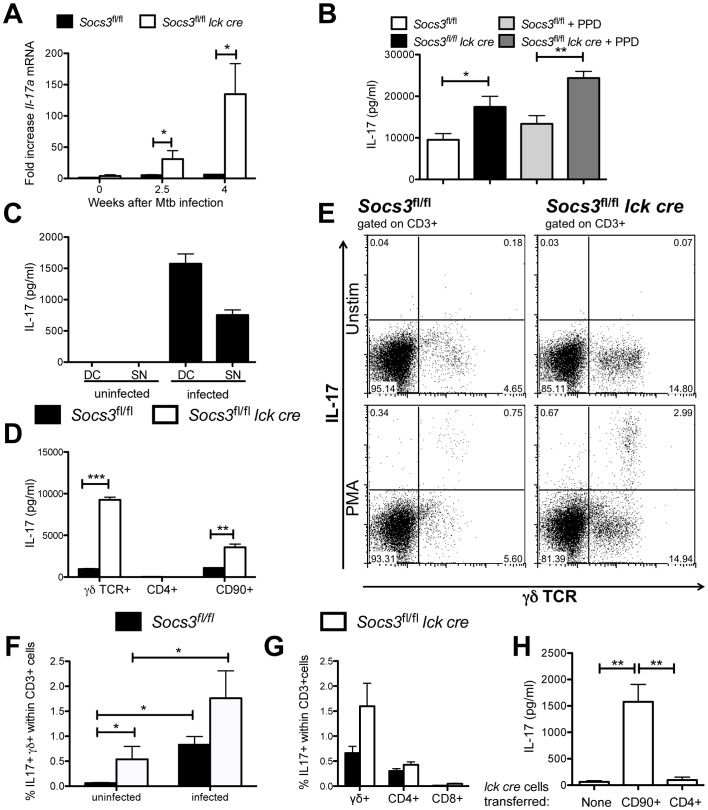
Since SOCS3 expression in T cells has been shown to impair IL-17 production [27], we speculated that a differential release of IL-17 could be related with the susceptibility of Socs3fl/fl lck cre mice to M. tuberculosis. Lungs from M. tuberculosis-infected Socs3fl/fl lck cre mice showed higher levels of Il-17 mRNA than controls (Figure 7A). The levels of IL-17 in supernatants from lung cells of Socs3fl/fl lck cre mice stimulated or not with mycobacterial Purified protein derivate (PPD) were higher than controls 2.5 weeks after M. tuberculosis infection, when no differences in bacterial load in lungs were detected (Figure 7B). Thus, the impaired control of M. tuberculosis-infection in Socs3fl/fl lck cre mice was associated with increased IL-17 levels. γδ+ T cells have been shown to dominate IL-17 secretion during infection with M. tuberculosis [28]. In line with this observation, the incubation of naïve spleen T cells with mycobacteria-infected BMDCs or their supernatants resulted in the secretion of IL-17 (Figure 7C). Furthermore, IL-17 was secreted by γδ+ and total (CD90+) but not by CD4+ T cells after incubation supernatants from mycobacteria-infected BMDCs. The levels of IL-17 secreted by γδ+ T cells were higher than those by similar numbers of total T cells. The IL-17 content in supernatants from γδ+ and total Socs3fl/fl lck cre T cells was higher compared to Socs3fl/fl controls (Figure 7D).
Figure 7. SOCS3-deficient γδ+ T cells secrete IL-17 during M. tuberculosis infection.
Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed before and at 2.5 and 4 weeks after M. tuberculosis infection and the total RNA was extracted from lungs. The accumulation of Il-17a and Hprt transcripts was measured by real time PCR (A). The mean fold increase of IL-17a mRNA ± SEM in lungs from infected mice (n≥5 per group) was calculated. One out of two independent experiments is depicted. Differences with infected Socs3fl/fl mice are significant (*p<0.05 Student t test). Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed 2.5 weeks after aerosol infection with M. tuberculosis. Lung cell suspensions were stimulated or not with 20 µg/ml PPD for 48 h. The IL-17 level in supernatants was determined by a cytokine bead assay (CBA) (B). The mean IL-17 concentration ± SEM (n≥6 animals per group) is depicted. Differences in cytokine concentrations are significant (*p<0.05, **p<0.01 ANOVA with Bonferroni correction). CD90+ naïve spleen T cells were co-cultured either with uninfected, BCG-infected BMDCs (DC) or with their 48 h supernatants (SN). After 72 h, the IL-17 levels in culture supernatants were measured by ELISA. A representative out of three independent experiments is shown (C). 105 γδ+, CD4+ or CD90+ FACS sorted T cells from Socs3fl/fl lck cre and Socs3fl/fl mice were co-cultured with supernatants from BCG-infected BMDCs for 72 h. The mean IL-17 concentration in supernatants from triplicate cultures ± SEM is depicted (D). Differences in cytokine concentrations are significant (**p<0.01, ***p<0.001 Student t test). The presence of IL-17-secreting cells in PMA/ionomycin-stimulated lung cell suspensions from Socs3fl/fl lck cre or Socs3fl/fl mice before or 16 days after infection with M. tuberculosis was measured by FACS as described in materials and methods. Representative FACS dot plots from CD3+ gated infected lung cells before or after PMA/ionomycin stimulation are shown (E). The frequency of IL-17-secreting γδ+ within CD3+ cells in uninfected or infected mice is displayed (n = 6, *p<0.05 Mann Whitney U test) (F). The mean frequency of IL-17-secreting CD4+, CD8+ and γδ+ within CD3+ cells in lungs of infected mice (5 mice per group) ± SEM is depicted (G). Rag1 −/− mice were infected with M. tuberculosis 2 weeks after inoculation with either 1.2×106 CD4+ or 2×106 CD90+ Socs3fl/fl lck cre spleen cells. Mice were sacrificed 4 weeks after infection and lung cell suspensions incubated for 48 h. The mean concentration of IL-17 in supernatants ± SEM (n = 6) is depicted (H). Differences in cytokine concentrations are significant (**p<0.01 Student t test).
IL-17-secreting cells were enumerated by intracellular cytokine staining in PMA/ionomycin-stimulated lung cell suspensions from Socs3fl/fl lck cre and Socs3fl/fl mice 16 days after M. tuberculosis infection. The majority of IL-17-secreting lung T cells in infected mice were γδ+ rather than CD4+ or CD8+ cells (Figure 7E, G). Furthermore, M. tuberculosis infection stimulated the IL-17 secretion capability of γδ+ T cells (Figure 7F). However, the frequency of IL-17-secreting cells among γδ+ T cells from infected Socs3fl/fl lck cre and Socs3fl/fl was similar, suggesting that the lack of SOCS3 does not alter the differentiation of γδ+ T cells into IL-17-secreting cells.
IL-17 was measured in supernatants from lung cell suspensions from CD4+ or CD90+ Socs3fl/fl lck cre T cell-transferred Rag1−/− mice 4 weeks after M. tuberculosis infection. While IL-17 levels were strikingly higher in supernatants from mice transferred with total T cells compared with non-transferred controls, the IL-17 concentration in cultures from mice inoculated with CD4+ cells was not increased (Figure 7H). Thus, the inhibition of CD4+ cell-mediated protection in Rag1 −/− mice transferred with CD90+ cells (Figure 6G) was associated to an increased IL-17 secretion.
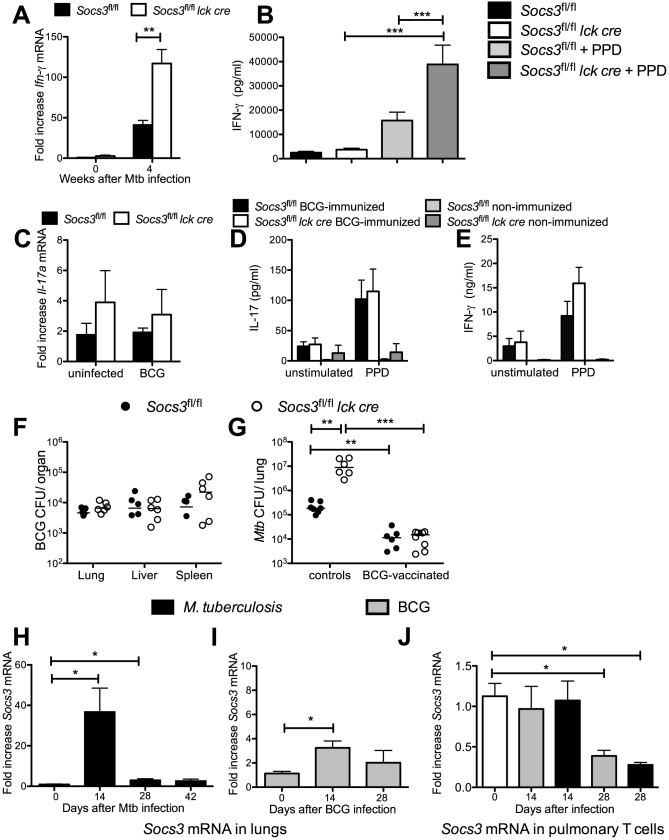
Lung cells from M. tuberculosis-infected Socs3fl/fl lck cre mice contained higher levels of Ifn-γ mRNA (Figure 8A) and secreted higher levels of IFN-γ when stimulated with PPD than Socs3fl/fl controls (Figure 8B). Thus, the susceptibility of Socs3fl/fl lck cre mice was not associated to an impaired secretion of IFN-γ.
Figure 8. BCG-immunization protects Socs3fl/fl and Socs3fl/fl lck cre mice equally well against M. tuberculosis challenge.
M. tuberculosis-infected Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed 4 weeks after infection and the total RNA was extracted from lungs. The accumulation of Ifn-γ (A) or Hprt transcripts was measured by real time PCR. The mean fold cytokine mRNA increase ± SEM in lungs from infected mice (n≥5 per group) was calculated. One out of two independent experiments is depicted. Differences with controls are significant (*p<0.05, **p<0.01 Student t test). Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed 2.5 weeks after infection with M. tuberculosis. Lung cell suspensions were stimulated with 20 µg/ml PPD and concentrations of IFN-γ (B) in supernatants after 48 h were measured by cytokine bead assay (CBA). The mean cytokine concentration ± SEM (n≥6 animals per group) is depicted. Differences in cytokine concentrations are significant (***p<0.001 ANOVA with Bonferroni correction). Total RNA was extracted from lungs of Socs3fl/fl lck cre and Socs3fl/fl mice 6 weeks after BCG infection. Levels of Il-17a (C) mRNA expression was determined by real time PCR (n = 6 mice per group). Socs3fl/fl lck cre and Socs3fl/fl mice were immunized s.c. with 5×106 heat-killed BCG and boosted after 2 weeks with 2.5×106 heat-killed BCG. Mice were sacrificed four weeks after the priming, and spleen cell suspensions from immunized or non-immunized control mice were co-incubated with 15 µg/ml PPD for 72 h. The concentration of IL-17 (D) and IFN-γ (E) in the culture supernatants was determined by ELISA. The mean cytokine concentration ± SEM (n = 6 animals per group) is depicted. Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed 6 weeks after i. v. infection with 106 BCG, and the CFU per lung, spleen and liver were quantified. The individual and median CFU per group (n≥6) are depicted (F). Socs3fl/fl lck cre and Socs3fl/fl mice were infected with 106 BCG i.v. and were challenged with M. tuberculosis 10 weeks post BCG infection. Four weeks after M. tuberculosis infection, mice were sacrificed and the CFU in the lungs were quantified. The individual and median CFU per group (n≥6) are shown (G). Differences in CFU are significant (**p<0.05, ***p<0.001 Mann Whitney U test). Wild type mice were either infected with 106 BCG i.v. (I, J) or via aerosol with M. tuberculosis Harlingen (H, J). The total RNA was isolated from lungs or pulmonary CD90+ T cells at the indicated time points. The mean fold Socs3 mRNA increase ± SEM (n = 5 per group) was determined by real-time PCR (H–J). Differences with between groups are significant (*p<0.05 Student t test).
The accumulation of Il-6 mRNA was increased in lungs of Socs3fl/fl lck cre M. tuberculosis-infected mice at 4 but not at 2.5 weeks after infection compared to controls (Figure S6A). The IL-6 levels in supernatants from Socs3fl/fl lck cre and Socs3fl/fl lung cells obtained 2.5 weeks after infection, stimulated or not with PPD, were similar (Figure S6B). SOCS3 has been shown to regulate IL-10 secretion by T cells [17]. However, Socs3fl/fl lck cre and Socs3fl/fl lung cells from M. tuberculosis-infected mice, stimulated or not with PPD, secreted similar levels of IL-10 (Figure S6C).
In contrast to results from M. tuberculosis-infected mice, Il-6 and Il-17a mRNA levels in lungs from BCG-infected Socs3fl/fl lck cre and Socs3fl/fl mice were similar (Figure 8C and S6D). Spleen cells from BCG-immunized Socs3fl/fl lck cre and control mice showed comparable IL-17, IFN-γ or IL-6 secretion in response to PPD stimulation when compared with controls (Figure 8D, E and S6E). The organs from Socs3fl/fl lck cre and Socs3fl/fl mice infected with M. bovis BCG contained similar bacterial levels (Figure 8F). Moreover, bacterial levels in Socs3fl/fl lck cre and Socs3fl/fl mice challenged with M. tuberculosis after BCG immunization were comparable (Figure 8G).
Next, we studied whether a differing stimulation of SOCS3 expression could explain the divergent susceptibility of Socs3fl/fl lck cre mice to M. tuberculosis and BCG infection. Lungs from mice infected with either M. tuberculosis or BCG showed higher Socs3 mRNA levels compared to uninfected mice. Socs3 transcript levels were higher in M. tuberculosis- than in BCG-infected animals (Figure 8H, I). However, Socs3 mRNA levels in pulmonary T cells before or after infection with BCG or M. tuberculosis were similar (Figure 8J). Thus, different expression levels of SOCS3 in T cells do not explain the distinct susceptibility of Socs3fl/fl lck cre mice to M. tuberculosis and BCG infection.
Gp130F/F mice display dramatic susceptibility to infection with M. tuberculosis
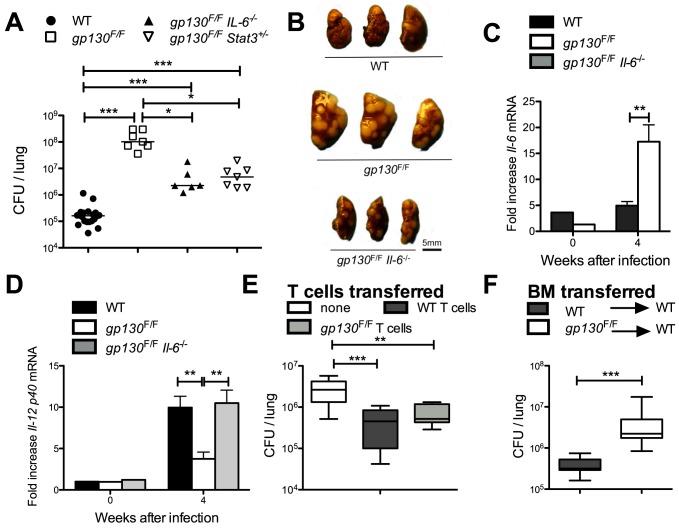
To further characterize the function of SOCS3 in myeloid and T cells in the control of infection with M. tuberculosis, gp130F/F knock-in mice were used. gp130F/F mice displayed a dramatically enhanced susceptibility to M. tuberculosis as measured by their increased bacterial load, severity of pathology in lungs (Figure 9A, B), and increased cumulative mortality (gp130F/F mice died before 48 days after infection whereas all WT controls survived for more than 100 days). Since IL-6 mediated, at least in part, the inhibition of TNF and IL-12 secretion by SOCS3-deficient BMM and BMDC, the role of IL-6 in the susceptibility of gp130F/F mice to infection with M. tuberculosis was studied. We found that gp130F/FIl-6 −/− as well as gp130F/FStat3+/− mutant mice displayed lower levels of M. tuberculosis bacteria in lungs and diminished severity of pulmonary pathology when compared to gp130F/F mice, indicating that the increased susceptibility of gp130F/F mice is in part mediated by IL-6 and STAT3 activation (Figure 9A, B). Lungs from gp130F/F mice displayed higher Il-6 and lower Il-12 p40 mRNA accumulation than infected control mice, while Il-12 p40 mRNA accumulation in lungs from gp130F/FIl-6−/− was comparable to WT mice (Figure 9C, D).
Figure 9. Gp130F/F mice display dramatic susceptibility to infection with M. tuberculosis.
Gp130F/F, gp130F/F Il-6−/−, gp130F/F/Stat3+/− and control mice were sacrificed 4 weeks after aerosol infection with M. tuberculosis, and CFU per lung assessed (A). The CFU in lungs of individual mice and the median per group (n≥6) are depicted. Results are pooled from two independent experiments. Differences in CFU are significant (*p<0.05, ***p<0.001 Mann Whitney U test). A gross-pathology photograph of the lungs from gp130F/F, gp130F/F Il-6−/− and control mice 4 weeks after infection with M. tuberculosis is shown (B). Gp130F/F, gp130F/F Il-6−/− and control mice were sacrificed at 4 weeks after M. tuberculosis infection and the total RNA was extracted from lungs. The accumulation of Il-6 (C) and Il-12 p40 (D) transcripts was measured by real time PCR. The mean fold cytokine mRNA increase ± SEM in lungs from infected mice (n = 5 per group) are depicted. Differences with controls are significant (**p<0.01 Student t test). 2×106 CD90+ gp130F/F and control splenic T cells were inoculated i.v. into Rag1−/− mice. Two weeks after transfer, mice were infected via the aerosol route with M. tuberculosis. Mice were sacrificed 4 weeks after infection and the CFU in lungs determined. The median CFU (n≥10) in lungs, quartiles and the 99th percentiles are depicted (E). Results are pooled from two independent experiments. Differences in CFU are significant (**p<0.01, ***p<0.001 Mann Whitney U test). The CFU in lungs of gp130F/F bone marrow→WT and WT bone marrow→ WT radiation chimeric mice were measured one month after infection with M. tuberculosis. A box and whisker diagram showing the median CFU (n≥8), quartiles and the 99th percentiles is depicted (F). Differences in CFU are significant (***p<0.001 Mann Whitney U test).
Surprisingly, lungs and spleens from M. tuberculosis-infected Rag1 −/− mice transferred prior to infection with either gp130F/F or WT T cells contained similar bacterial levels, indicating that T cells play, if any, a redundant role in the gp130-mediated control of M. tuberculosis. Bacterial levels in mice transferred with WT or gp130F/F cells were lower than those of non-transferred controls (Figure 9E). Moreover, frequencies of γδ+ T cells in organs from WT or gp130F/F were similar (data not shown). Thus, the susceptibility of Socs3fl/fl lck cre mice to infection with M. tuberculosis is mediated by receptors other than gp130.
Since T cells did not account for the increased susceptibility of gp130F/F mice and gp130F/F mice were significantly more susceptible to M. tuberculosis than Socs3fl/fl LysM cre mice, we studied the relative contributions of hematopoietic and non-hematopoietic cell lineages to the susceptibility of gp130F/F mice to infection with M. tuberculosis. Reciprocal bone marrow (BM) radiation chimeras between WT and gp130F/F mice were generated by inoculation of BM cells into irradiated recipients. WT mice reconstituted with gp130F/F BM contained higher titers of M. tuberculosis in the lungs than those reconstituted with WT BM (sham chimeric mice), although differences in bacterial levels were notably lower than those observed in the gp130F/F mice (Figure 9F). Although gp130F/F recipients showed significant mortality after irradiation, the few survivors inoculated with WT BM showed very high bacterial levels, similar to those from non-irradiated gp130F/F mice (data not shown).
Thus, these data suggest that non-lymphoid, hematopoietic cells only partially account for the susceptibility of gp130F/F mice to M. tuberculosis and suggest a relevant role for non-hematopoietic cells in the high sensitivity of these mice to infection.
Discussion
In the present study, we demonstrated that SOCS3 expression in lymphoid and myeloid cell populations is essential for the resistance against M. tuberculosis in mice via distinct mechanisms. M. tuberculosis and BCG infections were potent stimuli for Socs3 expression in vivo and in myeloid cell populations in vitro.
In line with previous studies, we found that SOCS3 expression in macrophages was mediated by MyD88 and NF-κB [29]. By using Socs3fl/fl LysM cre mice, our data suggest that myeloid SOCS3 expression contributes to a timely CD4+ cell-dependent IFN-γ-secretion rather than to improved innate effector immune mechanisms by macrophages. The observation that CD4+ cell-depleted Socs3fl/fl LysM cre and control mice had similar bacterial loads supports this hypothesis. Moreover, SOCS3-deficient and control BMM as well as pulmonary macrophages showed comparable intracellular mycobacterial growth, and IFN-γ diminished bacterial numbers with equal efficiency in Socs3fl/fl LysM cre and WT macrophages, as also recently demonstrated for T. gondii [12]. However, whether a defective IFN-γ secretion either by antigen-specific CD4+ T cells, or alternatively by NKT or CD8+ T cells underlies the susceptibility of Socs3fl/fl LysM cre mice to infection with M. tuberculosis remains to be determined.
SOCS3-deficient BMM showed increased STAT3 activation and diminished secretion of TNF and IL-12 after infection with either M. tuberculosis or BCG. This confirms previous data showing reduced TNF and IL-12 release by Socs3fl/fl LysM cre and gp130F/F macrophages in response to LPS, when co-incubated with IL-6 [11], [30]. Moreover, constitutively active STAT3 has been found to inhibit Il-12 p40 mRNA accumulation in LPS-stimulated BMDC [31]. Even though the LysM promoter is primarily active in neutrophils and macrophages, LysM promoter activity in DCs has previously been shown [26]. Socs3fl/fl LysM cre BMDC showed limited IL-12 production in response to mycobacterial stimulation and Il-12 p40 levels were also reduced in the lungs of infected Socs3fl/fl LysM cre and gp130F/F mice. Importantly, we found that IFN-γ levels were diminished in lungs of Socs3fl/fl LysM cre at 16 days but not at later time points after infection with M. tuberculosis. This delay in the establishment of immune protective responses might underlie the increased susceptibility of Socs3fl/fl LysM cre mice. In support of this notion, the resistance of different mouse strains to M. tuberculosis is associated with the timing of IFN-γ responses [32]. Although we observed a reduced secretion of IFN-γ by NK-cells during in vitro co-culture with M. tuberculosis-infected Socs3fl/fl LysM cre and control splenic DCs, the NK cell involvement in the enhanced susceptibility of Socs3fl/fl LysM cre mice is unlikely since NK cells were not required for controlling mycobacterial infections [33], [34].
TNF is of major importance in the control of M. tuberculosis [35]. Although TNF secretion by infected SOCS3-deficient macrophages is reduced, TNF expression in the lungs of M. tuberculosis-infected Socs3fl/fl LysM cre mice was not diminished, suggesting that a role for TNF in the susceptibility to infection of Socs3fl/fl LysM cre mice is unlikely.
The reduction of TNF and IL-12 levels observed in gp130F/F BMM was reversed when using gp130F/F Il-6 −/− cells, and addition of rIL-6 further diminished the release of IL-12 and TNF by either mycobacteria-infected, or Pam3CSK4-stimulated Socs3fl/fl LysM cre BMM, suggesting that in macrophages, SOCS3 allows proper TNF and IL-12 secretion by hampering an IL-6-mediated inhibition of the secretion of these cytokines.
Lungs from M. tuberculosis-infected Socs3fl/fl LysM cre and lck cre mice contained higher Il-6 mRNA levels than controls. However, neither macrophages nor T cells are likely to account for the elevated IL-6 levels in M. tuberculosis-infected SOCS3-deficient mice. Epithelial cells, fibroblasts and adipocytes have all been shown to secrete IL-6 in response to inflammatory stimuli [36], [37]. Whether non-hematopoietic cells are major IL-6 producers during M. tuberculosis infection remains to be investigated.
Socs3fl/fl lck cre mice showed a dramatically enhanced susceptibility to M. tuberculosis infection. However, SOCS3 expression in T cells was not required for the development of protective immune responses against M. tuberculosis in BCG-vaccinated mice. Thus, the requirement of SOCS3 in the control of mycobacterial infection depends on the mycobacterial species and on the immune status of the host.
Our results show a hitherto unknown role for SOCS3 controlling the frequency of γδ+ T cells in different organs before and during M. tuberculosis infection while frequencies of CD4+ or CD8+ T cells were not regulated by SOCS3. Moreover, γδ+ T cells impaired the transfer of protection by Socs3fl/fl lck cre CD4+T cells, suggesting that SOCS3 inhibits a non-redundant detrimental role of γδ+ T cells in the outcome of infection with M. tuberculosis. The detrimental activity of SOCS3-deficient γδ+ T cells contrasts with previous reports that have shown a minor role of WT γδ+ T cells in resistance to M. tuberculosis [38], [39].
SOCS3 can impair the secretion of IL-17 [16], [40]. The increased IL-17 mRNA and protein levels in Socs3fl/fl lck cre mice in M. tuberculosis-, but not in BCG-infected mice suggested that IL-17 levels might be causally associated to the increased susceptibility to M. tuberculosis infection. When γδ+ T cells but not CD4+ Socs3fl/fl lck cre T cells were adoptively transferred in Rag1 −/− mice, an increased IL-17 secretion by lung cells and impaired transfer of protection against M. tuberculosis was observed. The function of IL-17 during primary mycobacterial infections is controversial since only after high dose intratracheal infection mice deficient in IL-17 were reported to be unable to control M. tuberculosis infection [41]–[43]. On the other hand, IL-17 has been implicated to increase bacterial dissemination, recruitment of neutrophils and morbidity during infection with M. tuberculosis [44]–[46]. Socs3fl/fl lck cre mice showed elevated levels of neutrophil-derived molecules and necrotic granulomas during M. tuberculosis infection. Our results confirmed a previous report indicating that γδ+T cells dominate IL-17 production during M. tuberculosis infection [28]. SOCS3 also hampered the secretion of IL-17 by γδ+ T cells when incubated with infected DCs or their supernatants. However, SOCS3 did not impair the development of γδ+ T cells that are capable of secreting IL-17. Thus, the increased IL-17 levels in Socs3fl/fl lck cre mice are probably the consequence of both the increased numbers of γδ+ T cells and their unrestricted secretion of IL-17 in response to cytokines released by mycobacteria-stimulated DCs.
The increased susceptibility to M. tuberculosis of Socs3fl/fl lck cre mice was not associated to an impaired IFN-γ secretion by antigen-specific T cells, suggesting that SOCS3 is not required for IFN-γ secretion by T cells and that γδ+ T cells do not modulate IFN-γ secretion by αβ+ T cells.
Since SOCS3 regulates signalling via various receptors, we investigated whether signals mediated via the gp130 receptor account for the susceptibility to M. tuberculosis of SOCS3 conditional knockdown animals. We found that gp130F/F mice are highly susceptible to infection with M. tuberculosis. The susceptibility of gp130F/F is mediated by both IL-6-dependent as well as IL-6-independent signalling events, since gp130F/FIl-6 −/− mice showed lower bacterial load than gp130F/F mice but higher bacterial levels than controls. The cytokine responses of gp130F/F and Socs3fl/fl LysM cre macrophages to mycobacterial infections were similar suggesting that the protective role of SOCS3 in myeloid cells is dependent on gp130.
IL-1β and IL-23 have been shown to stimulate IL-17 production by γδ+ T cells [47], [48], but SOCS3 impairs IL-23 signalling [27]. Thus, IL-1 β and IL-23 might mediate the elevated IL-17 secretion in Socs3fl/fl lck cre T cells. Moreover, IL-23 signalling [49] as well as the increased frequency of γδ+ T cells in Socs3fl/fl lck cre mice are both independent of gp130 signalling (data not shown). Accordingly, T cells from highly susceptible gp130F/F mice transferred resistance to M. tuberculosis as previously shown for T. gondii infection [50]. Since gp130F/F were more susceptible to M. tuberculosis than Socs3fl/fl LysM cre mice, and lethally irradiated WT that were reconstituted with gp130F/F BM were more resistant to infection than gp130F/F or WT BM→ gp130F/F mice, we also suggest that gp130-dependent SOCS3-signalling in non-hematopoietic cells contributes to the control of infection with M. tuberculosis.
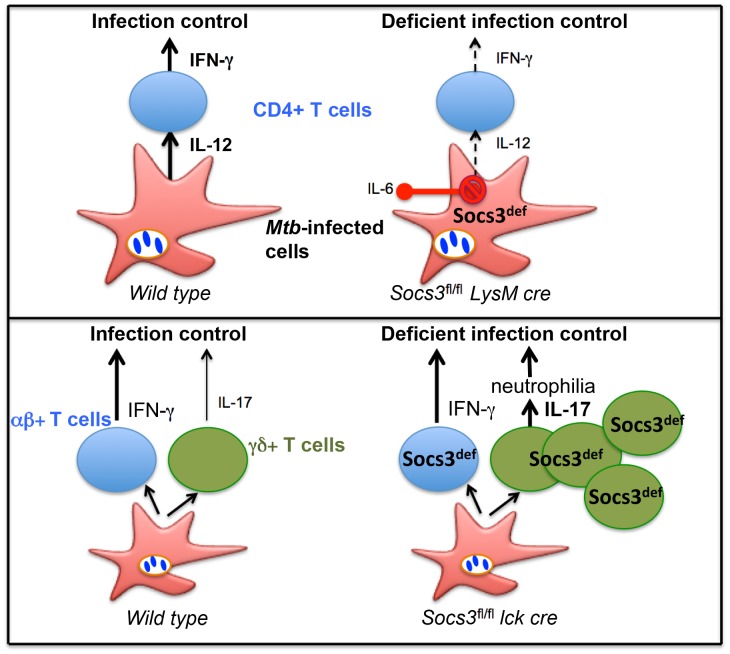
Collectively, our data indicate that the expression of SOCS3 either in myeloid or in T cells is essential for control of M. tuberculosis infection (Figure 10). SOCS3 mediates protection through inhibition of IL-6/gp-130 signalling in myeloid cells, while gp130-independent, SOCS3-mediated mechanisms in T cells contribute to the control of M. tuberculosis.
Figure 10. Proposed model of SOCS3-mediated roles during infection with M. tuberculosis.
SOCS3 expression in antigen-presenting cells prevented IL-6-mediated inhibition of IL-12 secretion SOCS3 expression in T cells reduces the frequency of γδ+ T cells in different organs and the secretion of IL-17 by γδ+ T cells in response to infection in a gp130-independent manner. Expression of Socs3 in myeloid and lymphoid cell populations is critical for a proper control of M. tuberculosis infection.
Material and Methods
Ethics statement
The animals were housed and handled at the Dept. of Microbiology, Tumor and Cell Biology and the Astrid Fagreus Laboratory, Karolinska Institute, Stockholm, according to directives and guidelines of the Swedish Board of Agriculture, the Swedish Animal Protection Agency, and the Karolinska Institute (djurskyddslagen 1988:534; djurskyddsförordningen 1988:539; djurskyddsmyndigheten DFS 2004:4). The study was performed under approval of the Stockholm North Ethical Committee on Animal Experiments permit number N302/10 and N487/11. Animals were housed under specific pathogen-free conditions.
Mice
Socs3fl/fl mice containing loxP-flanked Socs3 alleles have been described before [51]. For a T cell-specific deletion Socs3fl/fl mice were bred with transgenic lck cre mice [52] and for a myeloid-specific deletion with transgenic LysM cre mice [19]. Offsprings were genotyped as described [51] and Socs3fl/fl littermates negative for cre expression were used as controls for all experiments. Gp130F/F mice possess a homozygous substitution of tyrosine (Y)757 to phenylalanine (F) within the common IL-6 family receptor gp130 abrogating the SOCS3 binding site. Gp130F/F mice and their corresponding compound mutant homozygous null for IL-6 (gp130F/F Il-6 −/−) or heterozygous for STAT3 (gp130F/F Stat3 +/−) have been described previously [53], [54], all on a mixed C57Bl/6×129/Sv background which were used as controls. Rag1 −/− mice were generated by homologous recombination in embryonic stem cells [55] and crossed to C57Bl/6 background.
Infection and infectivity assay
BCG Montreal and M. tuberculosis Harlingen and H37Rv were grown in Middlebrook 7H9 (Difco, Detroit, MI) supplemented with albumin, dextrose, catalase and, for BCG cultures, 50 µg/ml hygromycin (Sigma, St. Louis, MO). BMM and BMDC were infected at the indicated multiplicity of infection (MOI) and after 4 hours cells were washed twice with PBS to remove extracellular bacteria. Mice were infected i.v. with 1×106 BCG or 250 M. tuberculosis Harlingen strain by aerosol using a nose-only exposure unit (In-tox Products, Uppsala, Sweden) [56]. A 15-ml suspension of 1×106 M. tuberculosis per ml was loaded into a nebulizer, and animals inhaled the bacteria aerosol for 20 min.
Bacteria were quantified on Middlebrook 7H11 agar containing 10% enrichment of oleic acid, albumin, dextrose, catalase, 5 µg of amphotericin B per ml and 8 µg/ml polymyxin B grown for 3 weeks at 37°C.
T cell transfer
Briefly, single-cell suspensions from spleens were selected for CD4+ or CD90+ T cells with magnetic beads (Miltenyi Biotech, Cologne, Germany) as specified by the manufacturer. When indicated, CD90+ cells were depleted of γδ+ cells by FACS sorting. 1–3×106 T cells were inoculated i.v. into Rag1 −/− mice. Two weeks after transfer, mice were infected with M. tuberculosis Harlingen.
T cell depletion
Mice were injected i. p. three consecutive days with 0.5 mg/mouse of Sepharose G affinity-purified anti-CD4 (GK1.5) antibody one week before infection. CD4-specific depletion was controlled in blood using flow analysis. Two weeks after the first injection, additional 0.5 mg/mouse anti-CD4 antibody was injected to maintain CD4+ cells depleted.
Immunization
Mice were immunized with 5×106 heat-killed BCG (60 min at 80°C) s. c. and boosted after 2 weeks with 2.5×106 heat-killed BCG. 4 weeks after the first injection, splenocytes from immunized and non-immunized mice were re-stimulated with 15 µg/ml PPD (Statens Seruminstitut, Copenhagen, Denmark) and supernatants collected after 72 h.
BCG immunization and M. tuberculosis challenge
Mice were immunized with 1×106 BCG i. v. and kept for 10 weeks before aerosol infection with M. tuberculosis together with non-immunized controls. Mice were sacrificed 4 weeks after M. tuberculosis strain Harlingen infection and bacterial loads were determined in the lungs.
Bone marrow chimera
Recipient C57Bl/6×129/Sv and gp130F/F were irradiated 2× with 550 rad and received 5×106 BM cell from either C57Bl/6×129/Sv or gp130F/F mice. Mice were kept for 3 weeks on antibiotics (Tribrissen in drinking water) and were infected with M. tuberculosis 8 weeks after transfer.
Generation of mouse bone marrow-derived macrophages
Bone marrow was extracted from tibia and femurs of mice and resuspended in Dulbecco's modified Eagle's medium (DMEM) containing glucose and supplemented with 2 mM L-glutamine, 10% FCS, 10 mM Hepes, 100 µg/ml streptomycin, 100 U/ml penicillin (all from Sigma), and 30% L929 cell-conditioned medium (as a source of macrophage-colony stimulating factor). Bone marrow cells were passed through a 70 µm cell strainer, plated and incubated for 6 days at 37°C, 5% CO2. Bone marrow-derived macrophage (BMM) cultures were then washed vigorously to remove non-adherent cells, trypsinized, counted and cultured for one day at 37°C in 24, 12 or 6 well plates. We have previously shown by immunofluorescence staining that these BMM are F4/80+, CD14+ and Mac-3+ [57].
Generation of mouse bone marrow-derived dendritic cells
Mouse bone marrow-derived dendritic cells (BMDC) were differentiated as previously described [58]. Briefly, bone marrow was extracted from tibia and femurs and cell suspensions cultured in RPMI-1640 medium containing 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin and 2 ng/ml GM-CSF (Peprotech, Rocky Hill, NJ). Fresh medium and cytokine were replaced after 3 days. After six days of culture, loosely adherent cells were harvested and seeded in concentrations for infection. In some cases, harvested cells were further selected for CD11c expression with magnetic beads (Miltenyi Biotech) before seeding.
Isolation of pulmonary macrophages
Pulmonary macrophages were isolated as previously described [59]. Briefly, lungs from Socs3fl/fl and Socs3fl/fl LysM cre mice were dissected, digested with 1.8 U/ml dispase for 60 min at RT, followed by digestion with DNase (both form Sigma) for 30 min at 37°C. After red blood cell lysis, hematopoietic CD45+ lung cells were positively enriched using magnetic beads (Miltenyi Biotech), and pulmonary macrophages selected by plastic-adherence. Forty-eight hours after seeding to culture plates, CD45+ adherent cells were washed four times with RPMI media.
Isolation of splenic dendritic cells
Splenic DCs were isolated as previously described [60]. Briefly, splenocyte suspensions were positively selected using anti-CD11c-coupled magnetic beads (Miltenyi Biotech). This protocol lead to a purity >95% and an approximate yield of 0.5–1×106 DCs per spleen.
In vitro stimulation of CD90+, CD4+ and γδ+ T cells
0.5×106 BMDC were seeded in 500 µl medium and infected with BCG (MOI2). After 24 h, supernatants were transferred on either CD90+, CD4+ or γδ+ T cells. All cells had been separated for CD90 with magnetic beads (Miltenyi Biotech) followed by flow cytometry-based sorting (for CD4+: CD3+ and CD4+, for γδ+: CD3+, CD4−, CD8−, γδ+ TCR). 72 h after transfer, supernatants were harvested and IFN-γ and IL-17 concentrations were determined by ELISA.
Real time PCR
Transcripts were quantified by real time PCR as previously described [56]. Hprt was used as a control gene to calculate the ΔCt values for individual samples. The relative amount of cytokine/Hprt transcripts was calculated using the 2−(ΔΔCt) method. These values were then used to calculate the relative expression of cytokine mRNA in uninfected and infected cells and tissues.
Cytokine determinations
Concentrations of cytokines in supernatants of stimulated cells were determined either by using cytometric bead array (CBA) mouse Th1/Th2/Th17 cytokine kit (BD Biosciences, San Jose, CA) or by enzyme-linked immunosorbent assays (ELISA) for IL-6, IFN-γ, TNF (BD Biosciences), IL-12 and IL-10 (eBioscience, San Diego, CA) and IL-17 (R&D systems, Minneapolis, MN) following the manufacturers' recommendations.
Flow cytometry and intracellular cytokine staining
Lungs were perfused with PBS through the heart before removal from mice. Following digestion with Collagenase D and DNase I, erythrocytes were lysed and single-cell suspensions prepared by filtering lung tissue through 70-µm nylon cell strainers. Single spleen cell suspensions were obtained by mechanical disruption, lysis of erythrocytes and straining over a 70-µm nylon mesh. Lung cells and splenocytes were stained for CD3, CD4, CD8 and γδ TCR (all eBioscience) or F4/80 and Gr1 (BD Biosciences) and fixed before acquisition.
For determination of IL-17-producing cells, lung cells were incubated with 50 ng/ml phorbol myristate acetate (PMA) and 2 µg/ml ionomycin (Sigma) in presence of brefeldin A (5 µg/ml) for 6 hours, stained with cell population-specific antibodies, fixed, permeabilized using leukocyte permeabilization reagent IntraPre (Immunotech, Marseille, France) and stained with anti-IL-17a (eBioscience).
CD11c+ splenic DCs were infected with M. tuberculosis H37Rv MOI5 for 4 hours, washed and cultured overnight with DX5+ NK cells separated from spleens with magnetic beads (Miltenyi Biotech). The next day, cells were treated with brefeldin A (5 µg/ml) for 4 hours, followed by a FACS stain for DX5 (PE, BD Biosciences) and intracellular IFN-γ (eBioscience).
Data were acquired in CyAn ADP flow cytometer (Beckman Coulter) and analyzed using FlowJo software (Tree star Inc., Ashland, OR).
Western blot
M. tuberculosis-infected and uninfected BMM were lysed and separated on 10% separating/5% stacking SDS-polyacrylamide gels. Samples were then transferred onto nitrocellulose membranes (BioRad, Hercules, CA) by electroblotting at 100 V, 250 mA for 80 min. Immunostaining was performed using polyclonal rabbit anti-phosphorylated (Tyr701) STAT3, total STAT3 (Cell signaling technology, Beverly, MA) or anti-actin (Sigma). Membranes were then washed and incubated with horse-radish peroxidase-conjugated polyclonal goat anti-rabbit immunoglobulin (DAKO) and developed using ECL-Plus (Amersham Biosciences, Buckinghamshire, UK) and photographed using a Fuji intelligent dark box II digital camera.
Histopathological analysis
Formalin fixed left lungs of mice experimentally inoculated with M. tuberculosis were blocked on paraffin. From each lung sample 4 sections were obtained, one longitudinal along the long axis of the lobe and 3 across/transversal of the remaining piece of lung.
The blocks were processed and sections were stained with haematoxylin-eosin. All sections were interpreted by the same pathologist (D. G-W.) and scored semi-quantitatively, blinded to the variables of the experiment.
The following features were scored:
Lung area occupied with granulomas (% of the total area of the section)
Lung area free of lesions or area of healthy lung (% of the total area of the section)
Extension of necrosis, raging from 0: no necrosis observed to 4: extensive necrosis and necrotic centers with mineralization.
Supporting Information
Increased NO release in SOCS3-deficient BCG-infected BMM. Mouse BMM were infected with BCG (A, B). BMM were treated with the indicated concentrations of BAY-117082 1 h before BCG infection (B). Total RNA was isolated from Irf3−/−, MyD88−/− and WT (C57Bl/6) BMM at the indicated time points after infection. A MOI of 5∶1 was used all over. Real time PCR was used to obtain duplicate determinations of Socs3 and Hprt mRNA from triplicate samples for each group and time point. The mean fold Socs3 mRNA induction ± SEM is depicted. Differences with control BMM are significant (*p<0.05, **p<0.01, **p<0.001 Student t test). Nitrite concentrations in supernatants of Socs3fl/fl LysM cre and Socs3fl/fl (C) gp130F/F and WT (E) BMM were measured by Griess assay at the indicated time points after incubation with Pam3CSK4 or infection with BCG. The mean NO2 − concentration ± SEM in triplicate cultures per condition of one of two independent experiments is depicted (C, E). Differences with WT BMM are significant (**p<0.01 and ***p<0.001 Student t test). Total RNA was extracted from Socs3fl/fl LysM cre, Socs3fl/fl (D, G) gp130F/F and WT (F) BMM at the indicated times after infection with BCG or stimulation with Pam3CSK4. The relative accumulation of iNos (D, F), Cxcl10 (G) and Hprt was measured by real time PCR. The mean fold increase of cytokine mRNA ± SEM in triplicate cultures for each genotype and time point is depicted. Differences with WT BMM are significant (*p<0.05, **p<0.01 Student t test).
(EPS)
Diminished IL-6 and TNF-secretion by BCG-infected SOCS3-deficient BMM. IL-6 concentration was determined in supernatants of BCG-infected BMM (A, D) or peritoneal macrophages (B). The mean IL-6 in Socs3fl/fl and Socs3fl/fl LysM cre (A, B) and gp130F/F and WT (D) BMM ± SEM as determined by ELISA is depicted. Differences with control BMM are significant (*p<0.05 and ***p<0.001 Student t test). The mean levels of Il-6 mRNA in Socs3fl/fl and Socs3fl/fl LysM cre BMM either infected with BCG or treated with Pam3CSK4 were determined by real time PCR in triplicate independent cultures per condition and compared to non infected cultures (C). One representative of two independent experiments is shown. Differences with control BMM are significant (**p<0.01Student t test). The mean levels of Tnf mRNA in Socs3fl/fl and Socs3fl/fl LysM cre (E) and gp130F/F, gp130F/FIl6−/− and WT (G) BMM either infected with BCG or treated with Pam3CSK4 determined by real time PCR in triplicate independent cultures per condition compared to non infected cultures is depicted. Differences with control BMM are significant (**p<0.01Student t test). The mean TNF concentration in supernatants of BCG-infected gp130F/F, gp130F/FIl6−/− and WT BMM ± SEM as determined by ELISA in triplicate cultures per condition (F). Differences with control BMM are significant (**p<0.01Student t test).
(EPS)
Diminished IL-12-secretion by BCG-infected SOCS3-deficient BMM. The levels of Il-12 p40 mRNA were measured in triplicate cultures of BCG-infected gp130F/F, gp130F/F Il-6−/− and WT BMM (A). Differences with WT BMM are significant (*p<0.05, **p<0.01 Student t test). Isolated Socs3fl/fl LysM cre and Socs3fl/fl CD11c+ splenic DCs were infected with M. tuberculosis (MOI5) and cultured overnight with NK cells. The next day, cells were treated with brefeldin A, followed by FACS staining for DX5 and intracellular IFN-γ The mean percentage of IFN-γ+ NK cells ± is depicted (B). Differences with Socs3fl/fl splenic DC are significant (n = 5, *p<0.05, Student t test). Representative FACS contour plots from co-cultures of NK cells with either infected or uninfected Socs3fl/fl LysM cre and Socs3fl/fl splenic DCs are shown (C).
(EPS)
Immune response parameters of Socs3fl/fl LysM cre and Socs3fl/fl mice after infection with M. tuberculosis. In vivo depletion of CD4+ cells after inoculation with GK1.5 anti-mouse CD4 monoclonal antibodies. The FACS plots of CD3+ gated spleen cells from anti-CD4-treated or untreated Socs3fl/fl LysM cre and Socs3fl/fl mice 2.5 weeks after M. tuberculosis infection are shown (A). These plots are representative for 5 mice analysed per group. Socs3fl/fl LysM cre and Socs3fl/fl lung cells were stained for CD3, CD4, CD44 and CD62L. Representative FACS dot-plots of lung cells before or 2.5 weeks after M. tuberculosis infection in which staining for CD44 and CD62L on CD3+ CD4+ cells is shown (B). The mean frequency of CD44+/CD62L- cells within the CD3+CD4+ T cell population ± SEM (n = 5 per group) is displayed (C). Socs3fl/fl LysM cre and Socs3fl/fl mice were infected with M. tuberculosis via the aerosol route. Animals were sacrificed at the indicated time points after infection and the total RNA was extracted from lungs. The accumulation of Ifn-γ (D), Tnf (E) and iNos (F) transcripts was measured by real time PCR. The mean fold cytokine mRNA increase ± SEM in lungs from infected mice (n = at least 5 per group) was calculated. Differences with controls are significant (* p<0.05 Student t test). The mean frequency of FoxP3+ within CD4+ T cells in the pulmonary lymph nodes and lungs from mice (n = 5 mice per group) 6 weeks after infection with M. tuberculosis was determined by FACS (G).
(EPS)
T cell subpopulations in Socs3fl/fl lck cre mice. Lung and spleen cells obtained from Socs3fl/fl lck cre and Socs3fl/fl mice before or 2.5. weeks after infection with M. tuberculosis were stained for CD3, CD4 and CD8 (A–D). Mean frequencies of CD4+ (A, C) and CD8+ (B, D) CD3+ T cells in lungs (A, B) and spleens (C, D) ± SEM are shown.
(EPS)
Secretion of IL-6 in Socs3fl/fl lck cre mice during mycobacterial infection. M. tuberculosis-infected Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed at the indicated time points and the total RNA was extracted from lungs. The accumulation of Il-6 (A) transcripts was measured by real time PCR. The mean fold mRNA increase ± SEM in lungs from infected mice (n≥5) was calculated. Differences with controls are significant (*p<0.05 Student t test). Lung cell suspensions from Socs3fl/fl lck cre and Socs3fl/fl mice at 2.5 weeks after infection with M. tuberculosis were stimulated with 20 µg/ml PPD. The content of IL-6 (B) and IL-10 (C) in supernatants analysed by a cytokine bead array (CBA) 48 h after stimulation. The mean cytokine concentration ± SEM (n = 6 animals per group) is depicted. Levels of Il-6 mRNA expression was determined by real time PCR in lungs of Socs3fl/fl and Socs3fl/fl lck cre mice, before and 6 weeks after BCG infection (n = 6 per group) (D). Socs3fl/fl and Socs3fl/fl lck cre mice were immunized s.c. with 5×106 heat-killed BCG and boosted after 2 weeks with 2.5×106 heat-killed BCG. After 2 weeks, mice were sacrificed and splenocytes from immunized or non-immunized control mice were co-incubated or not with 20 µg/ml PPD for 72 h. The mean IL-6 concentration ± SEM (n = 6 animals per group) in lung cell supernatants was determined by ELISA (E).
(EPS)
Acknowledgments
We thank Antonio Rothfuchs for comments to the manuscript, Ms Berit Olsson, Elin Hildekrans and Helene Braxenholm for excellent technical assistance (all from Karolinska Institutet, Sweden), Ewa Westergren (SVA, Uppsala) for the preparation of histological slides.
Funding Statement
BJJ is supported by a Senior Medical Research Fellowship awarded by the Sylvia and Charles Viertel Foundation. This work was supported by the European Community 200732 HOMITB grant, the Karolinska Institutet, the Swedish Lung and Heart Foundation and the Swedish Research Council, as well as the Operational Infrastructure Support Program by the Victorian Government of Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2006) Global tuberculosis control. Surveillance, planning, financing. WHO report http://www.who.int/tb/publications/global_report/2006/download_centre/en/index.html.
- 2. Nathan CF, Murray HW, Wiebe ME, Rubin BY (1983) Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158: 670–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feng CG, Jankovic D, Kullberg M, Cheever A, Scanga CA, et al. (2005) Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol 174: 4185–4192. [DOI] [PubMed] [Google Scholar]
- 4. Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, et al. (2006) Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med 203: 1805–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper AM, Kipnis A, Turner J, Magram J, Ferrante J, et al. (2002) Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J Immunol 168: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 6. Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, et al. (2006) Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Sem Immunol 18: 347–361. [DOI] [PubMed] [Google Scholar]
- 7. Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7: 454–465. [DOI] [PubMed] [Google Scholar]
- 8.Carow B, Rottenberg ME (2011) “Suppressor of cytokine signalling” molecules in infection and inflammation. In: Mahin K, editor. Inflammatory Diseases Intech. pp. 279–306.
- 9. Marine JC, McKay C, Wang D, Topham DJ, Parganas E, et al. (1999) SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell 98: 617–627. [DOI] [PubMed] [Google Scholar]
- 10. Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, et al. (2001) Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci U S A 98: 9324–9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, et al. (2003) IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4: 551–556. [DOI] [PubMed] [Google Scholar]
- 12. Whitmarsh RJ, Gray CM, Gregg B, Christian DA, May MJ, et al. (2011) A critical role for SOCS3 in innate resistance to Toxoplasma gondii . Cell Host Microbe 10: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang R, Pauleau AL, Parganas E, Takahashi Y, Mages J, et al. (2003) SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol 4: 546–550. [DOI] [PubMed] [Google Scholar]
- 14. Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, et al. (2003) SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol 4: 540–545. [DOI] [PubMed] [Google Scholar]
- 15. Qin H, Wang L, Feng T, Elson CO, Niyongere SA, et al. (2009) TGF-β Promotes Th17 Cell Development through Inhibition of SOCS3. J Immunol 183: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, et al. (2006) Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 103: 8137–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, et al. (2006) Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med 203: 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, et al. (2011) IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144: 601–613. [DOI] [PubMed] [Google Scholar]
- 19. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 8: 265–277. [DOI] [PubMed] [Google Scholar]
- 20. Kimura A, Kinjyo I, Matsumura Y, Mori H, Mashima R, et al. (2004) SOCS3 is a physiological negative regulator for granulopoiesis and granulocyte colony-stimulating factor receptor signaling. J Biol Chem 279: 6905–6910. [DOI] [PubMed] [Google Scholar]
- 21. Croker BA, Metcalf D, Robb L, Wei W, Mifsud S, et al. (2004) SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity 20: 153–165. [DOI] [PubMed] [Google Scholar]
- 22. Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, et al. (2010) Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest 120: 1674–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, et al. (1997) Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci U S A 94: 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Underhill DM, Ozinsky A, Smith KD, Aderem A (1999) Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A 96: 14459–14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohtani T, Ishihara K, Atsumi T, Nishida K, Kaneko Y, et al. (2000) Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity 12: 95–105. [DOI] [PubMed] [Google Scholar]
- 26. Matsumura Y, Kobayashi T, Ichiyama K, Yoshida R, Hashimoto M, et al. (2007) Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J Immunol 179: 2170–2179. [DOI] [PubMed] [Google Scholar]
- 27. Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, et al. (2006) Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A 103: 8137–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lockhart E, Green AM, Flynn JL (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177: 4662–4669. [DOI] [PubMed] [Google Scholar]
- 29. Narayana Y, Balaji KN (2008) NOTCH1 up-regulation and signaling involved in Mycobacterium bovis BCG-induced SOCS3 expression in macrophages. J Biol Chem 283: 12501–12511. [DOI] [PubMed] [Google Scholar]
- 30. El Kasmi KC, Holst J, Coffre M, Mielke L, de Pauw A, et al. (2006) General nature of the STAT3-activated anti-inflammatory response. J Immunol 177: 7880–7888. [DOI] [PubMed] [Google Scholar]
- 31. Hoentjen F, Sartor RB, Ozaki M, Jobin C (2005) STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood 105: 689–696. [DOI] [PubMed] [Google Scholar]
- 32. Maurya R, Kumar R, Prajapati VK, Manandhar KD, Sacks D, et al. (2010) Human visceral leishmaniasis is not associated with expansion or accumulation of Foxp3+ CD4 cells in blood or spleen. Parasite immunology 32: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, et al. (2006) NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis . J Immunol 177: 7086–7093. [DOI] [PubMed] [Google Scholar]
- 34. Junqueira-Kipnis AP, Kipnis A, Jamieson A, Juarrero MG, Diefenbach A, et al. (2003) NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol 171: 6039–6045. [DOI] [PubMed] [Google Scholar]
- 35. Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, et al. (1995) Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2: 561–572. [DOI] [PubMed] [Google Scholar]
- 36. Kim JS, Ryu MJ, Byun EH, Kim WS, Whang J, et al. (2011) Differential immune response of adipocytes to virulent and attenuated Mycobacterium tuberculosis . Microbes Infect 13: 1242–1251. [DOI] [PubMed] [Google Scholar]
- 37. Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, et al. (2008) Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29: 628–636. [DOI] [PubMed] [Google Scholar]
- 38. D'Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, et al. (1997) An anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis . J Immunol 158: 1217–1221. [PubMed] [Google Scholar]
- 39. Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ (2001) The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 193: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hill GR, Kuns RD, Raffelt NC, Don AL, Olver SD, et al. (2010) SOCS3 regulates graft-versus-host disease. Blood 116: 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aujla SJ, Dubin PJ, Kolls JK (2007) Th17 cells and mucosal host defense. Sem Immunol 19: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, et al. (2005) IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol 175: 788–795. [DOI] [PubMed] [Google Scholar]
- 43. Okamoto Yoshida Y, Umemura M, Yahagi A, O'Brien RL, Ikuta K, et al. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 184: 4414–4422. [DOI] [PubMed] [Google Scholar]
- 44. Desvignes L, Ernst JD (2009) Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. . Immunity 31: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Keller C, Hoffmann R, Lang R, Brandau S, Hermann C, et al. (2006) Genetically determined susceptibility to tuberculosis in mice causally involves accelerated and enhanced recruitment of granulocytes. Infect Immun 74: 4295–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Redford PS, Boonstra A, Read S, Pitt J, Graham C, et al. (2010) Enhanced protection to Mycobacterium tuberculosis infection in IL-10-deficient mice is accompanied by early and enhanced Th1 responses in the lung. Eur J Immunol 40: 2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, et al. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341. [DOI] [PubMed] [Google Scholar]
- 48. Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19: 93–129. [DOI] [PubMed] [Google Scholar]
- 49. Parham C, Chirica M, Timans J, Vaisberg E, Travis M, et al. (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol 168: 5699–5708. [DOI] [PubMed] [Google Scholar]
- 50. Silver JS, Stumhofer JS, Passos S, Ernst M, Hunter CA (2011) IL-6 Mediates the Susceptibility of Glycoprotein 130 Hypermorphs to Toxoplasma gondii. J Immunol 187: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, et al. (2003) IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4: 551–556. [DOI] [PubMed] [Google Scholar]
- 52. Takahama Y, Ohishi K, Tokoro Y, Sugawara T, Yoshimura Y, et al. (1998) Functional competence of T cells in the absence of glycosylphosphatidylinositol-anchored proteins caused by T cell-specific disruption of the Pig-a gene. Eur J Immunol 28: 2159–2166. [DOI] [PubMed] [Google Scholar]
- 53. Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, et al. (2002) Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med 8: 1089–1097. [DOI] [PubMed] [Google Scholar]
- 54. Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, et al. (2005) Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med 11: 845–852. [DOI] [PubMed] [Google Scholar]
- 55. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, et al. (1992) RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68: 869–877. [DOI] [PubMed] [Google Scholar]
- 56. Carow B, Qun Ye X, Gavier-Widen D, Bhuju S, Oehlmann W, et al. (2011) Silencing Suppressor of Cytokine Signaling-1 (SOCS1) in Macrophages Improves Mycobacterium tuberculosis Control in an Interferon-{gamma} (IFN-{gamma})-dependent Manner. J Biol Chem 286: 26873–26887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, et al. (2001) IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J Immunol 167: 6453–6461. [DOI] [PubMed] [Google Scholar]
- 58. Yang T, Stark P, Janik K, Wigzell H, Rottenberg ME (2008) SOCS-1 protects against Chlamydia pneumoniae-induced lethal inflammation but hampers effective bacterial clearance. J Immunol 180: 4040–4049. [DOI] [PubMed] [Google Scholar]
- 59. Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, et al. (2012) The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PloS one 7: e32125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, et al. (2009) Isolation of dendritic cells. Curr Protoc Immunol Chapter 3: Unit 3 7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased NO release in SOCS3-deficient BCG-infected BMM. Mouse BMM were infected with BCG (A, B). BMM were treated with the indicated concentrations of BAY-117082 1 h before BCG infection (B). Total RNA was isolated from Irf3−/−, MyD88−/− and WT (C57Bl/6) BMM at the indicated time points after infection. A MOI of 5∶1 was used all over. Real time PCR was used to obtain duplicate determinations of Socs3 and Hprt mRNA from triplicate samples for each group and time point. The mean fold Socs3 mRNA induction ± SEM is depicted. Differences with control BMM are significant (*p<0.05, **p<0.01, **p<0.001 Student t test). Nitrite concentrations in supernatants of Socs3fl/fl LysM cre and Socs3fl/fl (C) gp130F/F and WT (E) BMM were measured by Griess assay at the indicated time points after incubation with Pam3CSK4 or infection with BCG. The mean NO2 − concentration ± SEM in triplicate cultures per condition of one of two independent experiments is depicted (C, E). Differences with WT BMM are significant (**p<0.01 and ***p<0.001 Student t test). Total RNA was extracted from Socs3fl/fl LysM cre, Socs3fl/fl (D, G) gp130F/F and WT (F) BMM at the indicated times after infection with BCG or stimulation with Pam3CSK4. The relative accumulation of iNos (D, F), Cxcl10 (G) and Hprt was measured by real time PCR. The mean fold increase of cytokine mRNA ± SEM in triplicate cultures for each genotype and time point is depicted. Differences with WT BMM are significant (*p<0.05, **p<0.01 Student t test).
(EPS)
Diminished IL-6 and TNF-secretion by BCG-infected SOCS3-deficient BMM. IL-6 concentration was determined in supernatants of BCG-infected BMM (A, D) or peritoneal macrophages (B). The mean IL-6 in Socs3fl/fl and Socs3fl/fl LysM cre (A, B) and gp130F/F and WT (D) BMM ± SEM as determined by ELISA is depicted. Differences with control BMM are significant (*p<0.05 and ***p<0.001 Student t test). The mean levels of Il-6 mRNA in Socs3fl/fl and Socs3fl/fl LysM cre BMM either infected with BCG or treated with Pam3CSK4 were determined by real time PCR in triplicate independent cultures per condition and compared to non infected cultures (C). One representative of two independent experiments is shown. Differences with control BMM are significant (**p<0.01Student t test). The mean levels of Tnf mRNA in Socs3fl/fl and Socs3fl/fl LysM cre (E) and gp130F/F, gp130F/FIl6−/− and WT (G) BMM either infected with BCG or treated with Pam3CSK4 determined by real time PCR in triplicate independent cultures per condition compared to non infected cultures is depicted. Differences with control BMM are significant (**p<0.01Student t test). The mean TNF concentration in supernatants of BCG-infected gp130F/F, gp130F/FIl6−/− and WT BMM ± SEM as determined by ELISA in triplicate cultures per condition (F). Differences with control BMM are significant (**p<0.01Student t test).
(EPS)
Diminished IL-12-secretion by BCG-infected SOCS3-deficient BMM. The levels of Il-12 p40 mRNA were measured in triplicate cultures of BCG-infected gp130F/F, gp130F/F Il-6−/− and WT BMM (A). Differences with WT BMM are significant (*p<0.05, **p<0.01 Student t test). Isolated Socs3fl/fl LysM cre and Socs3fl/fl CD11c+ splenic DCs were infected with M. tuberculosis (MOI5) and cultured overnight with NK cells. The next day, cells were treated with brefeldin A, followed by FACS staining for DX5 and intracellular IFN-γ The mean percentage of IFN-γ+ NK cells ± is depicted (B). Differences with Socs3fl/fl splenic DC are significant (n = 5, *p<0.05, Student t test). Representative FACS contour plots from co-cultures of NK cells with either infected or uninfected Socs3fl/fl LysM cre and Socs3fl/fl splenic DCs are shown (C).
(EPS)
Immune response parameters of Socs3fl/fl LysM cre and Socs3fl/fl mice after infection with M. tuberculosis. In vivo depletion of CD4+ cells after inoculation with GK1.5 anti-mouse CD4 monoclonal antibodies. The FACS plots of CD3+ gated spleen cells from anti-CD4-treated or untreated Socs3fl/fl LysM cre and Socs3fl/fl mice 2.5 weeks after M. tuberculosis infection are shown (A). These plots are representative for 5 mice analysed per group. Socs3fl/fl LysM cre and Socs3fl/fl lung cells were stained for CD3, CD4, CD44 and CD62L. Representative FACS dot-plots of lung cells before or 2.5 weeks after M. tuberculosis infection in which staining for CD44 and CD62L on CD3+ CD4+ cells is shown (B). The mean frequency of CD44+/CD62L- cells within the CD3+CD4+ T cell population ± SEM (n = 5 per group) is displayed (C). Socs3fl/fl LysM cre and Socs3fl/fl mice were infected with M. tuberculosis via the aerosol route. Animals were sacrificed at the indicated time points after infection and the total RNA was extracted from lungs. The accumulation of Ifn-γ (D), Tnf (E) and iNos (F) transcripts was measured by real time PCR. The mean fold cytokine mRNA increase ± SEM in lungs from infected mice (n = at least 5 per group) was calculated. Differences with controls are significant (* p<0.05 Student t test). The mean frequency of FoxP3+ within CD4+ T cells in the pulmonary lymph nodes and lungs from mice (n = 5 mice per group) 6 weeks after infection with M. tuberculosis was determined by FACS (G).
(EPS)
T cell subpopulations in Socs3fl/fl lck cre mice. Lung and spleen cells obtained from Socs3fl/fl lck cre and Socs3fl/fl mice before or 2.5. weeks after infection with M. tuberculosis were stained for CD3, CD4 and CD8 (A–D). Mean frequencies of CD4+ (A, C) and CD8+ (B, D) CD3+ T cells in lungs (A, B) and spleens (C, D) ± SEM are shown.
(EPS)
Secretion of IL-6 in Socs3fl/fl lck cre mice during mycobacterial infection. M. tuberculosis-infected Socs3fl/fl lck cre and Socs3fl/fl mice were sacrificed at the indicated time points and the total RNA was extracted from lungs. The accumulation of Il-6 (A) transcripts was measured by real time PCR. The mean fold mRNA increase ± SEM in lungs from infected mice (n≥5) was calculated. Differences with controls are significant (*p<0.05 Student t test). Lung cell suspensions from Socs3fl/fl lck cre and Socs3fl/fl mice at 2.5 weeks after infection with M. tuberculosis were stimulated with 20 µg/ml PPD. The content of IL-6 (B) and IL-10 (C) in supernatants analysed by a cytokine bead array (CBA) 48 h after stimulation. The mean cytokine concentration ± SEM (n = 6 animals per group) is depicted. Levels of Il-6 mRNA expression was determined by real time PCR in lungs of Socs3fl/fl and Socs3fl/fl lck cre mice, before and 6 weeks after BCG infection (n = 6 per group) (D). Socs3fl/fl and Socs3fl/fl lck cre mice were immunized s.c. with 5×106 heat-killed BCG and boosted after 2 weeks with 2.5×106 heat-killed BCG. After 2 weeks, mice were sacrificed and splenocytes from immunized or non-immunized control mice were co-incubated or not with 20 µg/ml PPD for 72 h. The mean IL-6 concentration ± SEM (n = 6 animals per group) in lung cell supernatants was determined by ELISA (E).
(EPS)