Abstract
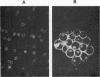
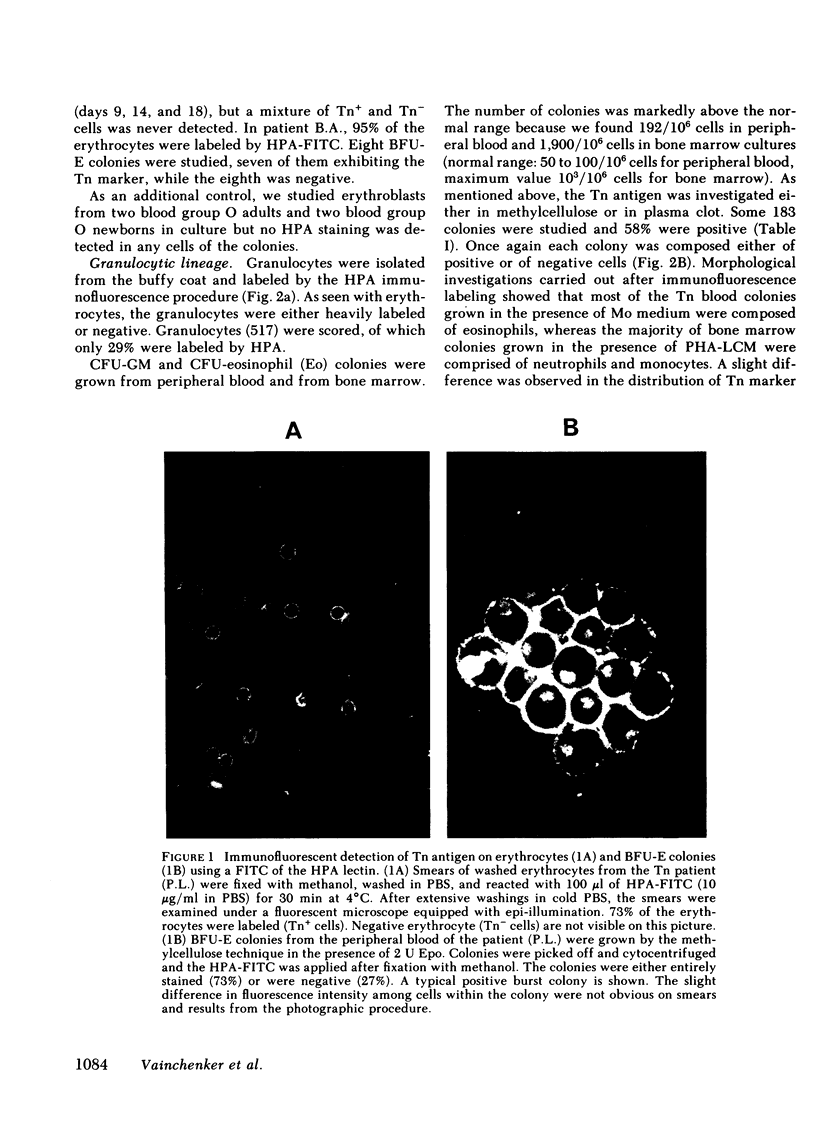
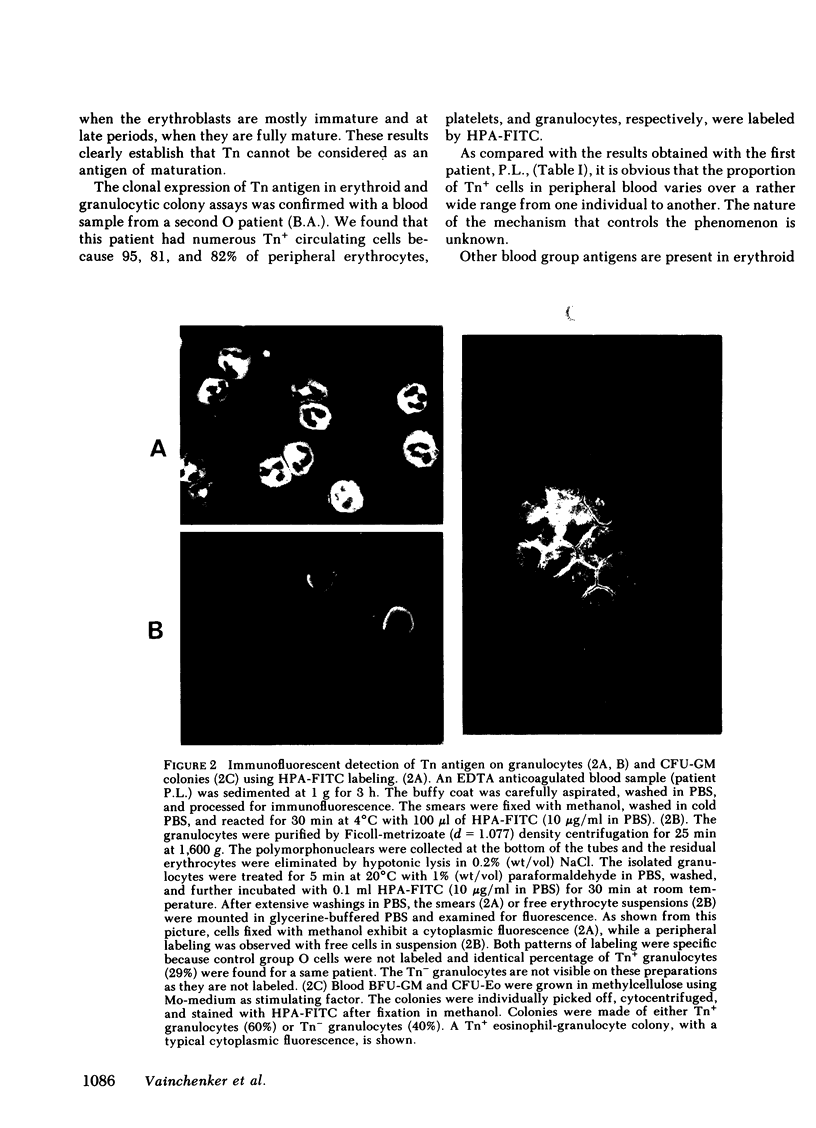
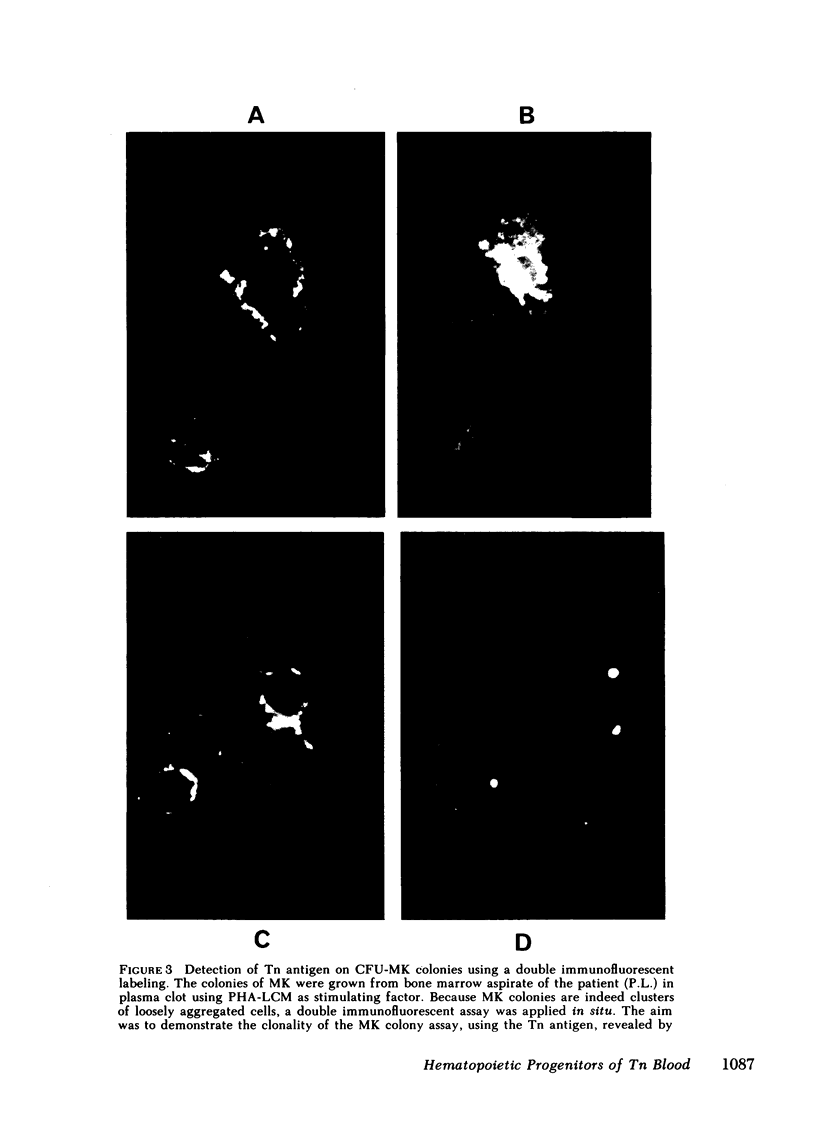
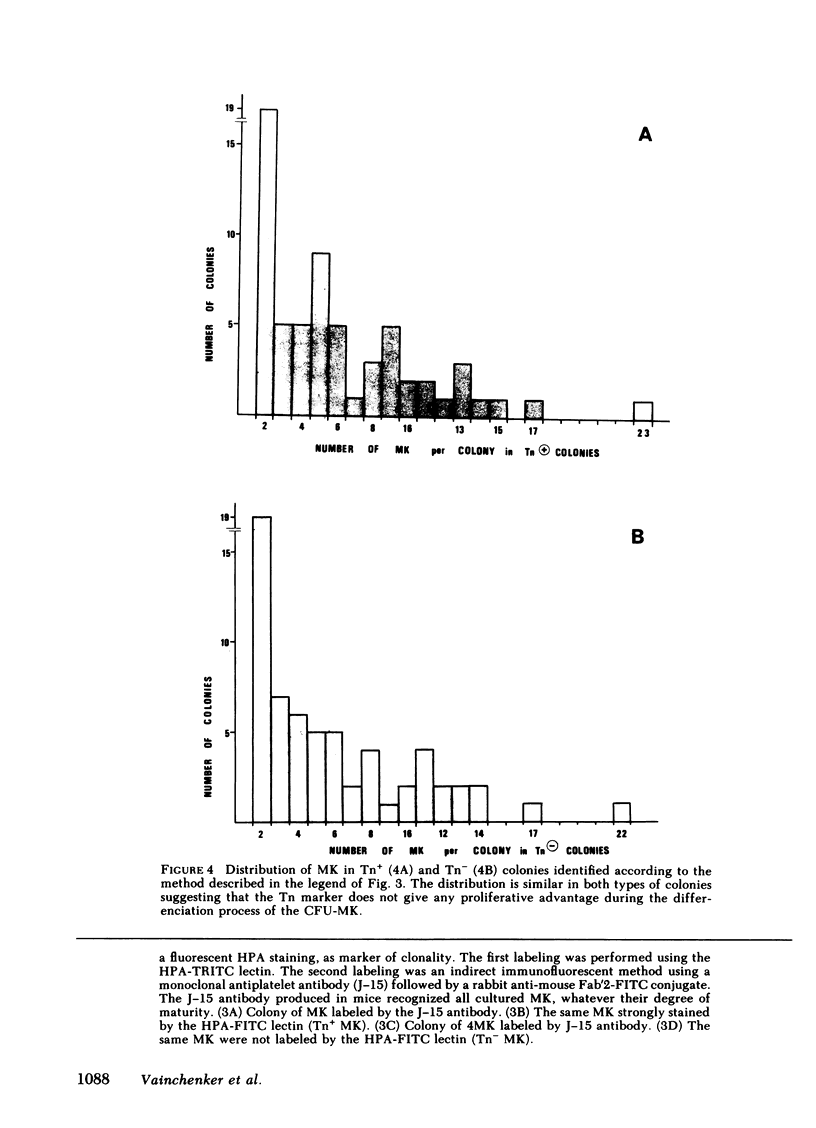
To evaluate whether exposure of Tn determinants at the surface of human erythrocytes, platelets, and granulocytes could arise from a somatic mutation in a hemopoietic stem cell, burst-forming unit erythroid (BFU-E) colonies, colony-forming unit granulocyte-macrophage (CFU-GM), and colony-forming unit-eosinophil (CFU-Eo) were grown from a blood group O patient with a typical Tn syndrome displaying two distinct populations (Tn+ and Tn-) of platelets, granulocytes, and erythrocytes. A large number of colonies was observed. Individual colonies were studied with a fluorescent conjugate of Helix pomatia agglutinin (HPA). A sizeable fraction of each of the erythroid and granulocytic colonies appeared to consist exclusively of either HPA-positive or HPA-negative cells, thereby demonstrating the clonal origin of those exhibiting the Tn marker. Similar results were obtained from a second patient. These findings establish that the HPA labeling of Tn cells is an accurate marker permitting assessment of the clonality of the human megakaryocyte (MK) colony assay. For the study of MK cultures a double-staining procedure using the HPA lectin and a monoclonal antiplatelet antibody (J-15) was applied in situ to identify all MK constituting a colony. Our results, obtained in studies of 133 MK colonies, provide definitive evidence that the human MK colony assay is clonal because all MK colonies were exclusively composed of Tn+ and Tn- MK. Furthermore, the distribution of MK within a single colony was shown to be seminormal with a mean at 6 MK, isolated MK typically being absent in culture.
Comparison of the proportion of mature Tn+ cells in blood with their respective Tn+ progenitors has also shown that no proliferative advantage occurs after the commitment; because Tn polyagglutinability is an acquired disorder, then the expansion of the Tn+ clone must occur either during the proliferative stage of the pluripotent stem cell or during the commitment itself. This study therefore affords evidence that a blood group antigen plays a role in the differentiation of a pluripotent stem cell.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aye M. T., Seguin J. A., McBurney J. P. Erythroid and granulocytic colony growth in cultures supplemented with human serum lipoproteins. J Cell Physiol. 1979 May;99(2):233–238. doi: 10.1002/jcp.1040990210. [DOI] [PubMed] [Google Scholar]
- Aye M. T., Till J. E., McCulloch E. A. Interacting populations affecting proliferation of leukemic cells in culture. Blood. 1975 Apr;45(4):485–493. [PubMed] [Google Scholar]
- Baldwin M. L., Barrasso C., Ridolfi R. L. Tn-polyagglutinability associated with acute myelomonocytic leukemia. Am J Clin Pathol. 1979 Dec;72(6):1024–1027. doi: 10.1093/ajcp/72.6.1024. [DOI] [PubMed] [Google Scholar]
- Berger E. G., Kozdrowski I. Permanent mixed-field polyagglutinable erythrocytes lack galactosyltransferase activity. FEBS Lett. 1978 Sep 1;93(1):105–108. doi: 10.1016/0014-5793(78)80815-1. [DOI] [PubMed] [Google Scholar]
- Bird G. W. Erythrocyte serology of some malignant diseases. Blut. 1977 Sep 29;35(3):165–169. doi: 10.1007/BF00999456. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Shinton N. K., Wingham J. Persistent mixed-field polyagglutination. Br J Haematol. 1971 Oct;21(4):443–453. doi: 10.1111/j.1365-2141.1971.tb02705.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Wingham J., Pippard M. J., Hoult J. G., Melikian V. Erythrocyte membrane modification in malignant diseases of myeloid and lymphoreticular tissues. I. Tn-polyagglutination in acute myelocytic leukaemia. Br J Haematol. 1976 Jun;33(2):289–294. doi: 10.1111/j.1365-2141.1976.tb03540.x. [DOI] [PubMed] [Google Scholar]
- Bird G. W., Wingham J. The M, N and NVg receptors of Tn-erythrocytes. Vox Sang. 1974 Feb;26(2):171–175. doi: 10.1111/j.1423-0410.1974.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Andreu G., Cartron J., Bird G. W., Salmon C., Gerbal A. Demonstration of T-transferase deficiency in Tn-polyagglutinable blood samples. Eur J Biochem. 1978 Dec 1;92(1):111–119. doi: 10.1111/j.1432-1033.1978.tb12728.x. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Cartron J., Andreu G., Salmon C., Bird G. W. Selective deficiency of 3-beta-d-galactosyltransferase (T-transferase) in Tn-polyagglutinable erythrocytes. Lancet. 1978 Apr 22;1(8069):856–857. doi: 10.1016/s0140-6736(78)90197-6. [DOI] [PubMed] [Google Scholar]
- Cartron J. P., Nurden A. T. Galactosyltransferase and membrane glycoprotein abnormality in human platelets from Tn-syndrome donors. Nature. 1979 Dec 6;282(5739):621–623. doi: 10.1038/282621a0. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Bird G. W. Cryptic A-like receptor sites in human erythrocyte glycoproteins: proposed nature of Tn-antigen. Vox Sang. 1974;27(1):29–42. doi: 10.1111/j.1423-0410.1974.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Dahr W., Uhlenbruck G., Gunson H. H., Van Der Hart M. Molecular basis of Tn-polyagglutinability. Vox Sang. 1975;29(1):36–50. doi: 10.1111/j.1423-0410.1975.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dukes P. P., Izadi P., Ortega J. A., Shore N. A., Gomperts E. Inhibitory effects of interferon on mouse megakaryocytic progenitor cells in culture. Exp Hematol. 1980 Sep;8(8):1048–1056. [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Identification of megakaryocytes, macrophages, and eosinophils in colonies of human bone marrow containing neurtophilic granulocytes and erythroblasts. Blood. 1979 May;53(5):1023–1027. [PubMed] [Google Scholar]
- Guilbert L. J., Iscove N. N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoietic cell cultures. Nature. 1976 Oct 14;263(5578):594–595. doi: 10.1038/263594a0. [DOI] [PubMed] [Google Scholar]
- Haynes C. R., Dorner I., Leonard G. L., Arrowsmith W. R., Chaplin H., Jr Persistent polyagglutinability in vivo unrelated to T-anigen activation. Transfusion. 1970 Mar-Apr;10(2):43–51. doi: 10.1111/j.1537-2995.1970.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Jiji R. M. Case report referred to in July 1979 issue of Blood article by Ness et al. "Tn polyagglutination preceding acute leukemia". Blood. 1979 Dec;54(6):1451–1451. [PubMed] [Google Scholar]
- Levin J., Levin F. C., Penington D. G., Metcalf D. Measurement of ploidy distribution in megakaryocyte colonies obtained from culture: with studies of the effects of thrombocytopenia. Blood. 1981 Feb;57(2):287–297. [PubMed] [Google Scholar]
- Lusis A. J., Quon D. H., Golde D. W. Purification and characterization of a human T-lymphocyte-derived granulocyte-macrophage colony-stimulating factor. Blood. 1981 Jan;57(1):13–21. [PubMed] [Google Scholar]
- Luzzatto L., Familusi J. B., Williams C. K., Junaid T. A., Rotoli B., Alfinito F. The PNH abnormality in myeloproliferative disorders: association of PNH and acute erythremic myelosis in two children. Haematologica. 1979 Feb;64(1):13–30. [PubMed] [Google Scholar]
- Mazur E. M., Hoffman R., Chasis J., Marchesi S., Bruno E. Immunofluorescent identification of human megakaryocyte colonies using an antiplatelet glycoprotein antiserum. Blood. 1981 Feb;57(2):277–286. [PubMed] [Google Scholar]
- Ness P. M., Garratty G., Morel P. A., Perkins H. A. Tn polyagglutination preceding acute leukemia. Blood. 1979 Jul;54(1):30–34. [PubMed] [Google Scholar]
- Oni S. B., Osunkoya B. O., Luzzatto L. Paroxysmal nocturnal hemoglobinuria: evidence for monoclonal origin of abnormal red cells. Blood. 1970 Aug;36(2):145–152. [PubMed] [Google Scholar]
- Prchal J. F., Adamson J. W., Steinmann L., Fialkow P. J. Human erythroid colony formation in vitro: evidence for clonal origin. J Cell Physiol. 1976 Nov;89(3):489–492. doi: 10.1002/jcp.1040890314. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Dacie J. V. Immune lysis of normal human and paroxysmal nocturnal hemoglobinuria (PNH) red blood cells. I. The sensitivity of PNH red cells to lysis by complement and specific antibody. J Clin Invest. 1966 May;45(5):736–748. doi: 10.1172/JCI105388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. W., Fialkow P. J., Dow L. W., Ernst C., Steinmann L. Unicellular or multicellular origin of human granulocyte-macrophage colonies in vitro. Blood. 1979 Dec;54(6):1395–1399. [PubMed] [Google Scholar]
- Springer G. F., Desai P. R. Human blood-group MN and precursor specificities: structural and biological aspects. Carbohydr Res. 1975 Mar;40(1):183–192. doi: 10.1016/s0008-6215(00)82680-4. [DOI] [PubMed] [Google Scholar]
- Stern M., Rosse W. F. Two populations of granulocytes in paroxysmal nocturnal hemoglobinuria. Blood. 1979 May;53(5):928–934. [PubMed] [Google Scholar]
- Strome J. E., McLeod D. L., Shreeve M. M. Evidence for the clonal nature of erythropoietic bursts: application of an in situ method for demonstrating centromeric heterochromatin in plasma cultures. Exp Hematol. 1978 May;6(5):461–467. [PubMed] [Google Scholar]
- Vainchenker W., Bouguet J., Guichard J., Breton-Gorius J. Megakaryocyte colony formation from human bone marrow precursors. Blood. 1979 Oct;54(4):940–945. [PubMed] [Google Scholar]