Abstract
The direct and interactive effects of climate change on host species and infectious disease dynamics are likely to initially manifest at latitudinal extremes. As such, Alaska represents a region in the United States for introspection on climate change and disease. Rabies is enzootic among arctic foxes (Vulpes lagopus) throughout the northern polar region. In Alaska, arctic and red foxes (Vulpes vulpes) are reservoirs for rabies, with most domestic animal and wildlife cases reported from northern and western coastal Alaska. Based on passive surveillance, a pronounced seasonal trend in rabid foxes occurs in Alaska, with a peak in winter and spring. This study describes climatic factors that may be associated with reported cyclic rabies occurrence. Based upon probabilistic modeling, a stronger seasonal effect in reported fox rabies cases appears at higher latitudes in Alaska, and rabies in arctic foxes appear disproportionately affected by climatic factors in comparison to red foxes. As temperatures continue a warming trend a decrease in reported rabid arctic foxes may be expected. The overall epidemiology of rabies in Alaska is likely to shift to increased viral transmission among red foxes as the primary reservoir in the region. Information on fox and lemming demographics, in addition to enhanced rabies surveillance among foxes at finer geographic scales, will be critical to develop more comprehensive models for rabies virus transmission in the region.
Keywords: climate change, rabies, red fox, arctic fox, epizootiology
Introduction
During the 20th century, temperatures increased throughout the western Arctic (Stafford et al., 2000). This region is predicted to be more susceptible to climate change, as global warming is projected to have a stronger influence at higher latitudes (Corell, 2005). For example, the average temperature of the Arctic increased from 4 °C to 7°C during the 20th century, a larger increase than observed in most other geographic areas (Hassol, 2004). Climate change of this magnitude may be expected to alter the spread and proliferation of zoonoses by affecting host population densities, home ranges, predator-prey relationships, and seasonal disease dynamics (Epstein, 1999). Climate change may also affect the timing and perpetuation of outbreaks, as well as competition among sympatric species (Preston et al., 2008). Although some studies revealed that climate variation affected occurrence of zoonotic vector borne diseases such as Giardia lamblia and Echinococcus multilocularis in the Arctic region (Berner et al., 2005, Rausch, 2003), many other zoonoses, including rabies, in the Arctic are not fully understood in terms of associations with progressive climate change (Hueffer et al., 2011, Parkinson & Butler, 2005).
Rabies is a disease caused by viruses in the family Rhabdoviridae, genus Lyssavirus. Rabies virus is the only Lyssavirus reported with multiple reservoirs in the order Carnivora, and has a circumpolar distribution among arctic foxes. Arctic fox rabies virus variants are enzootic in both arctic foxes (Vulpes lagopus) and red foxes (Vulpes vulpes) in Alaska, with a hypothesized three to four year epizootic cycle based on reported cases throughout the northwest and coastal portions of the state (Dieterich, 1981). In general, arctic foxes use two different habitat types: inland and coastal tundra, including pack ice (Fuglei & Ims, 2008). Arctic foxes also exhibit long distance movements and inhabit sea ice for extensive periods (Pamperin et al., 2008). Genetic connectivity between arctic fox populations using sea ice have been described between Svalbard and Russian populations suggesting intercontinental movment of this host species (Noren et al., 2011). To our knowledge no studies have been published concerning movement and home ranges of red foxes at high latitudes. Lemmings and other small rodents are the primary inland habitat food source of both arctic and red foxes. Rodent populations exhibit large cyclic fluctuations every 3 to 5 years, a primary driver of fox population dynamics (Roth, 2003, Hanski, 1995) and could drive the need for long distance foraging by arctic foxes. While this cyclic prey population affect red foxes equally, nothing is known about winter home ranges of red foxes at high latitudes in response to low food availability. In coastal areas, shorebirds provide the main food source, which is more stable than inland lemming populations. Marine foods sources of arctic foxes mainly consist of seal carcasses, primarily the pups of ringed seals (Pusa hispida), left by polar bears (Ursus maritimus) on pack ice, with the abundance of this food source associated with arctic fox densities (Roth, 2002, Smith, 1987, Hiruki & Stirling, 1989). Both seals and polar bears are vulnerable to the loss of sea ice which may impact the availability of this marine food source for artic foxes (Kovacs & Lydersen, 2008, Wiig et al., 2008). However, the effect of climate change on arctic and red fox ecology, and the epizootiology of rabies virus among these fox populations in the Arctic remains poorly understood.
We present an analysis of seasonal and climatic effects upon the reported occurrence of rabies in Alaska foxes. Our primary objectives are to identify climatic factors that may influence rabies dynamics in red and arctic foxes under a model of predicted climate change and to suggest appropriate directions for future research.
Materials and Methods
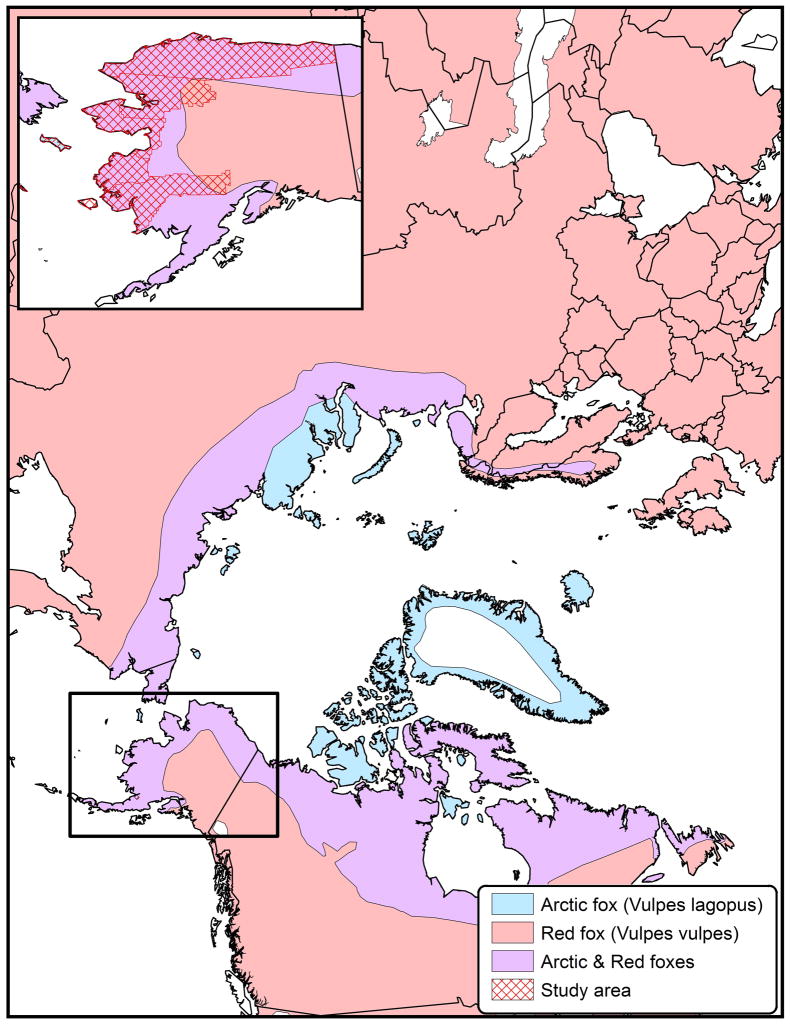
In Alaska, and throughout the United States, rabies surveillance is a passive laboratory-based system. Animals exhibiting clinical signs compatible with rabies which were involved in a potential exposure to a human or domestic animal are submitted to the Alaska State Virology Laboratory for rabies diagnosis. A smaller subset of animals submitted for rabies diagnosis included wildlife that displayed compatible illness, but were not involved in a human or domestic animals exposure, particularly in areas where rabies has not been confirmed or routinely reported in the past. A total of 772 rabid foxes were reported from 1980–2010. However, due to sparse human population and a harsh environment rabies is most certainly highly under reported. This study focused on six of the 27 boroughs or census areas in Alaska (North Slope, Northwest Arctic, Nome census area, Wade Hampton, Bethel census area, and Bristol Bay) (Figure 1), which accounted for 680 (88.1%) of the reported rabid foxes over that period.
Figure 1.
Geographic distribution of arctic and red foxes with study area
Climate data were obtained from the Alaska Climate Research Center, at the Geophysical Institute, University of Alaska Fairbanks. Five climate factors were extracted for each borough, including mean temperature (degrees Celsius), precipitation (cm), snowfall (cm), snow depth (cm) and temperature range (degrees Celsius, maximum temperature - minimum temperature) on a monthly basis from 1980 to 2010. Due to the lack of climate stations in Wade Hampton, climate data from Bethel were extrapolated based on geographical proximity and Wade Hampton census area cases were merged with cases reported from the Bethel Census area for analysis. Sea ice extent (the areal sum of sea ice covering the ocean where the percentage of sea ice covered area exceeds 15%) in the polar region was obtained from the Japan Aerospace Exploration Agency Earth Observation Research Center (JAXA/EORC).
We used a Poisson regression model to examine the patterns of reported rabid foxes with respect to climate factors. Data were aggregated by month and borough for analysis (n=1860). Parameters evaluated included mean temperature, precipitation, snowfall, snow depth, and temperature range (difference between maximum temperature and minimum temperature of each month), borough, and season. The alpha level was set at 0.05 and p-values were obtained by using a Wald chi-square test. Due to the passive nature of rabies surveillance being associated with a human or domestic animals exposure, the population density of each borough based on 2000 census data was used as an offset. Modeling was performed using SAS 9.2 and fit was evaluated using the deviance and Pearson chi-square of each model. Categorical variables for borough and season were evaluated to control for spatial and seasonal variations. Overdispersion was evaluated and not found to be a significant issue in the models. (Stokes et al., 2000).
To account for variations in rabies virus incubation period, which is estimated at approximately two weeks to three months in foxes (Steck & Wandeler, 1980, Baer, 1975), lag periods from one to three months were evaluated for the different climate variables. Final lag periods were chosen based on univariate analysis to select significant lag periods with the strongest association by fox species. These lag periods were then included in multivariate poisson models for final model selection. Variable interactions were assessed in the model between average temperature and other climate factors. Collinearity was evaluated using a variance inflation factor (VIF) and condition indices (CI) in the model (Belsley et al., 1980, Belsley, 1991). Final model selection was carried out by backwards selection. After evaluating a global model for all reported rabid foxes in Alaska, separate models were developed for red and arctic foxes, to evaluate potential differences in susceptibility to climate change, as judged by quantitative differences in the effect coefficients. Deviance R2 was calculated as described and presented for comparison of the variability in predicted values from the observed data between the different models (Cameron & Windmeijer, 1996).
Results
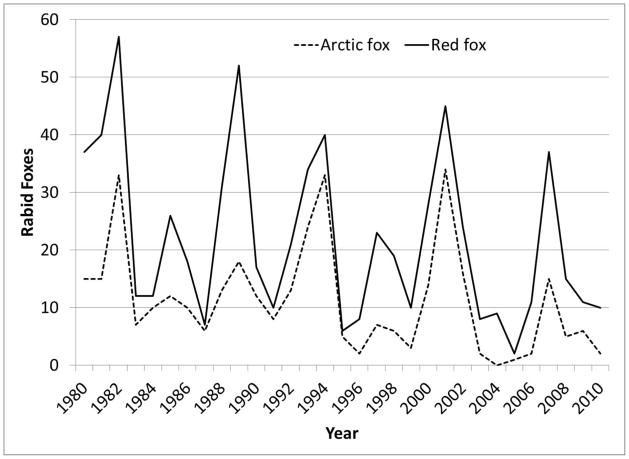
The annual number of reported rabid foxes was cyclical (Figure 2). Periodograms from finite Fourier transformation of reported monthly cases of rabies in red and arctic foxes showed a strong signal at 12 months, compatible with seasonal variation. In addition, secondary signals suggestive of an interepizootic cycle were observed at 3.1 and 3.8 years for red and arctic foxes respectively (data not shown). In total, 680 foxes submitted for rabies diagnosis from the study area were rabid with an approximately even divide between arctic foxes (n=349) and red foxes (n=331). Most rabid arctic foxes were reported from North Slope borough (n= 267, 76.5%) with the remainder distributed among the Northwest Arctic (n=14, 4%), Nome (n=38. 10.9%) and Bethel (n=30, 8.6%) boroughs/census areas. No rabid arctic foxes were reported from Bristol Bay borough. In contrast, red foxes were primarily reported from the Bethel/Wade Hampton area (n=138, 41.7%), with the remainder reported from: Nome (n=72, 21.8%), Northwest Arctic (n=58, 17.5%) and Bristol Bay (n=16, 4.8%) boroughs. Reports of rabid arctic and red foxes were correlated (r = 0.12, P<0.01).
Figure 2.
Number of reported rabid foxes in the study area, Alaska, 1980–2010
In the global Poisson model for interactions (Table 1), reported rabid foxes were negatively associated with mean temperature of 1 month prior (RR=0.90 for a 1° C increase) and precipitation of 2 months before (RR=0.92 for 1 cm increase) when controlling for seasonality and borough. There was a significant interaction between average temperature and temperature range of 1 month prior. No significant association was identified for snow depth in the final model.
Table 1.
Climate factors associated with reported rabid foxes, rate ratio, and 95% confidence intervals estimated by Poisson-regression models adjusted for overdispersion, Alaska, USA, 1980–2010
| Climate factors | time lag | Overall foxes | time lag | Arctic foxes | time lag | Red foxes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p-value | RR | 95% CI | B | p-value | RR | 95% CI | B | p-value | RR | 95% CI | |||||||
| Average temperature | 1 | −0.10 | <.01 | 0.90 | 0.87 | 0.94 | 1 | −0.13 | <.01 | 0.88 | 0.83 | 0.93 | 1 | −0.06 | <.01 | 0.95 | 0.93 | 0.96 |
| Precipitation | 2 | −0.08 | 0.02 | 0.92 | 0.87 | 0.96 | - | - | - | - | - | 2 | −0.10 | <.01 | 0.91 | 0.85 | 0.96 | |
| Snow depth | - | - | - | - | - | 3 | 0.01 | 0.02 | 1.01 | 1.00 | 1.02 | - | - | - | - | - | ||
| Temperature range | 1 | 0.03 | 0.63 | 1.03 | 0.94 | 1.12 | 1 | 0.09 | 0.31 | 1.09 | 0.92 | 1.30 | - | - | - | - | - | |
| Interaction | av*range | 0.01 | 0.03 | 1.01 | 1.00 | 1.01 | av*range | 0.01 | <.01 | 1.01 | 1.00 | 1.02 | - | - | - | - | - | |
| Season | ||||||||||||||||||
| Spring | 0.86 | 0.01 | 2.35 | 1.48 | 3.73 | 1.75 | <.01 | 5.75 | 2.33 | 14.14 | 0.34 | 0.34 | 1.41 | 0.81 | 2.45 | |||
| Summer | Ref | Ref | Ref | |||||||||||||||
| Fall | 0.80 | 0.01 | 2.22 | 1.42 | 3.44 | 1.96 | <.01 | 7.10 | 2.98 | 16.88 | 0.22 | 0.51 | 1.25 | 0.74 | 2.12 | |||
| Winter | 1.20 | <.01 | 3.33 | 2.09 | 5.31 | 2.58 | <.01 | 13.20 | 5.35 | 32.73 | 0.35 | 0.35 | 1.42 | 0.79 | 2.56 | |||
| Borough/census area | ||||||||||||||||||
| North Slope | 0.12 | 0.55 | 1.13 | 0.85 | 1.50 | 1.93 | <.01 | 6.90 | 4.32 | 11.04 | −1.64 | <.01 | 0.19 | 0.12 | 0.30 | |||
| Northwest Arctic | −0.94 | <.01 | 0.39 | 0.29 | 0.52 | −0.8 | 0.02 | 0.45 | 0.23 | 0.87 | −1.07 | <.01 | 0.34 | 0.25 | 0.48 | |||
| Nome | −0.39 | 0.03 | 0.68 | 0.53 | 0.86 | 0.24 | 0.36 | 1.27 | 0.78 | 2.08 | −0.75 | <.01 | 0.47 | 0.36 | 0.63 | |||
| Bethel/Wade Hampton | Ref | Ref | Ref | |||||||||||||||
| Bristol Bay | −4.01 | <.01 | 0.02 | 0.01 | 0.03 | No arctic fox cases reported | −3.81 | <.01 | 0.02 | 0.01 | 0.04 | |||||||
| Model fit statistics (deviance/df) | 0.99 | 0.51 | 0.68 | |||||||||||||||
| R2DEV | 0.52 | 0.62 | 0.38 | |||||||||||||||
RR: rate ratio
av: average temperature
range: temperature range
B: coefficient
R2 DEV: Deviance R2
-: Variable not included in model
Among species specific models, the arctic fox model showed a greater coefficient effect size compared to the global model and more pronounced seasonality (Table 1). Controlling for seasonality and borough, average temperature of 1 month prior was negatively associated with arctic fox cases (RR=0.88 for a 1° C increase). Snow depth was positively related with reported rabid arctic foxes (RR= 1.01 for 1 cm increase). The significant interaction between average temperature and temperature range of 1 month prior remained in the arctic fox model.
Compared to the global and arctic fox models, the red fox only model showed no significant seasonality. However, controlling for seasonality and borough, the average temperature of one month prior (RR=0.95 for 1° C increase) and precipitation 2 months prior (RR=0.91 for 1 cm increase) were both negatively associated with reported rabid red fox cases.
Total reported rabid foxes by month were correlated with mean Arctic sea ice extent during the period from 2002 to 2008 (r=0.80, P<0.01) (Figure 3). The time series for the total annual number of reported rabid foxes also appears similar to the annual percentage of area covered with sea ice for a 36 year time span (1972–2007) for the Southern Beaufort Sea, located in the northern region of the North Slope of Alaska (Wendler et al., 2010).
Figure 3.
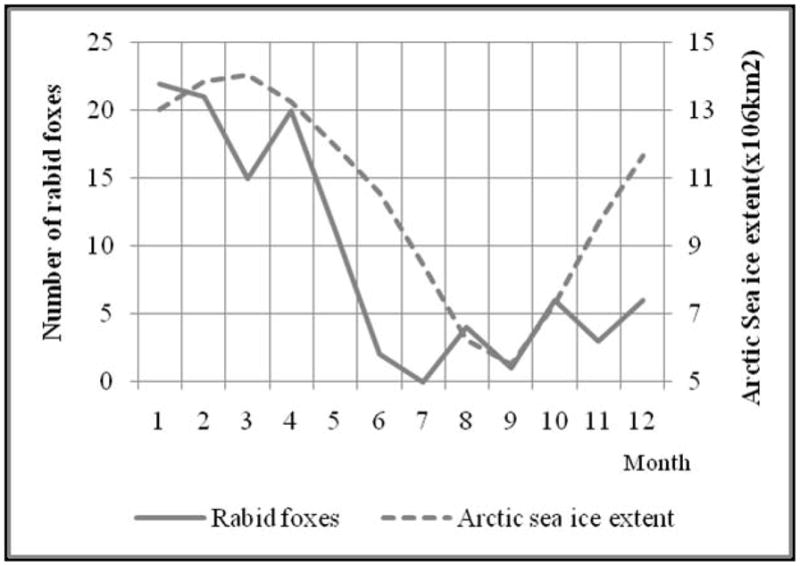
Number of rabid foxes and monthly mean arctic sea ice extent for the period 2002 – 2008
Discussion
Based on descriptive analysis, seasonal and geographical differences in reported rabid foxes were identified, although there were distinct differences between fox species. Model results suggested reporting of rabid arctic foxes was more likely to be affected by climate change than red foxes. Our findings indicate that the reported number of rabid foxes could be predicted by climate variation while controlling for seasonality and geographic variation. Specifically, average temperature and precipitation seem to be important factors in predicting relative incidence of both rabid arctic and red foxes. Snow depth and temperature range only affected reporting of rabid arctic foxes. That precipitation was negatively associated with reported rabid foxes in Alaska, could relate to less movement, resulting in reduced contact rates. Radio tracking studies would be required to confirm this trend. Furthermore, while the increasing average temperature trends have been consistent across Alaska the change in annual precipitation has varied by region which makes projections of the rabies dynamics among arctic foxes more difficult when utilizing short term climate change models for precipitation (Stafford et al., 2000).
If these correlations hold under a change in climate factors, a general warming trend may be expected to have a greater impact on the specialized arctic fox in comparison to red foxes, which are ecological generalists and have expanded their range northward in recent years. As climate changes, continued range expansion among red foxes may be expected in addition to epizootiological changes in rabies virus dynamics. Observed seasonality of rabies in foxes is likely associated with mating cycles and winter foraging habits (Mork & Prestrud, 2004). Arctic foxes typically mate between February and May, and give birth between April and July (Nowak & Walker, 1991). Reports of rabid arctic foxes may be lower during summer (June-August) and fall (September-November), as foxes travel less and remain closer to dens to rear young. Conversely, during winter (December-February), arctic foxes increase their home range to forage (Frafjord & Prestrud, 1992). Such life-history transitions may result in elevated contact rates during scavenging, particularly when young foxes begin to disperse from their natal dens (Rausch, 1972). Several researchers have concluded that rabies primarily affects juvenile foxes (Kantorovich, 1964, Secord et al., 1980, Ballard et al., 2001) because lack of experience regarding established territories and limited access to food under harsh environmental conditions may contribute to the frequency of antagonistic interactions. Based on our analysis, reports of rabid arctic foxes increased following colder months with wider temperature ranges. The arctic fox is a relatively small and weak competitor compared to the red fox (Hersteinsson & Macdonald, 1992). Arctic foxes may be expelled from their dens by red foxes (Linnell et al., 1999). In fact, arctic foxes may avoid breeding near red foxes, and locate their dens at higher elevations than red foxes, to avoid direct competition (Tannerfeldt et al., 2002). The long term implications of such behavior adaptations on disease dynamics are not understood.
Resource availability is considered a major factor for the northern advance of the red fox, while the arctic fox distribution at its southern range limit is constrained by interspecific competition (Hersteinsson & Macdonald, 1992). Competition is more likely to increase in winter because of reduced food sources, and has resulted in red fox predation on arctic foxes (Pamperin et al., 2006). Although detailed locations of fox dens were not available, rabid arctic foxes were mainly reported from the four northern boroughs/census areas of Alaska (i.e. North Slope, Northwest Arctic, Nome and Bethel). In comparison, rabid red foxes were primarily reported along the southwest coast of Alaska (Bethel), where they are also sympatric, at that scale, with arctic foxes. One reason for the observed distribution in coastal areas might be food availability. It is well known that inland food sources are unstable or cyclic in comparison to historic annually dependable marine food sources on sea ice (Fuglei & Ims, 2008). Maritime food sources for arctic foxes may become less available as sea ice extent decreases (Fuglei & Ims, 2008, Stirling & McEwan, 1975), which in turn should have a profound negative impact on arctic fox survival (Laidre et al., 2008), especially when inland food sources are scarce (Roth, 2003). Decreased sea ice extent appears to reduce the number of reported rabid foxes and may also limit long range movement of arctic foxes, potentially resulting in increased isolation of some populations. Such isolation would presumably lead to decreased opportunity for rabies transmission among more isolated populations. However, rabies within small, isolated fox populations could increase local rabies transmission and extirpation of spatially fragmented populations. In contrast, stable levels of sea ice facilitate longer range movement of arctic foxes, which could enhance rabies transmission when foxes interact antagonistically at limited food sources during the winter (Pamperin et al., 2008).
Prior phylogenetic analysis of arctic fox rabies virus variant lineages have suggested spatial segregation: Arctic-1 (viruses circulating in Ontario among red foxes and striped skunks), Arctic-2 (viruses circulating in north-eastern Siberia and western Alaska predominantly among arctic foxes), Arctic-3 (viruses circulating circumpolarly in Siberia, Alaska and Canada), and Arctic-4 (viruses isolated only in the south-western part of Alaska during 2006–2010). Based on phylogenetic reconstructions, the Arctic-4 group does not appear to have recently emerged. However, where these viruses circulated previously is unknown. The percentage identity of these viruses is high (98.6–99.2%) (Kuzmin et al., 2008). This together with evidence of large scale cross-continental movement of rabies virus (Mansfield et al., 2006) suggest circumpolar distribution and movement of arctic fox rabies virus variant lineages.
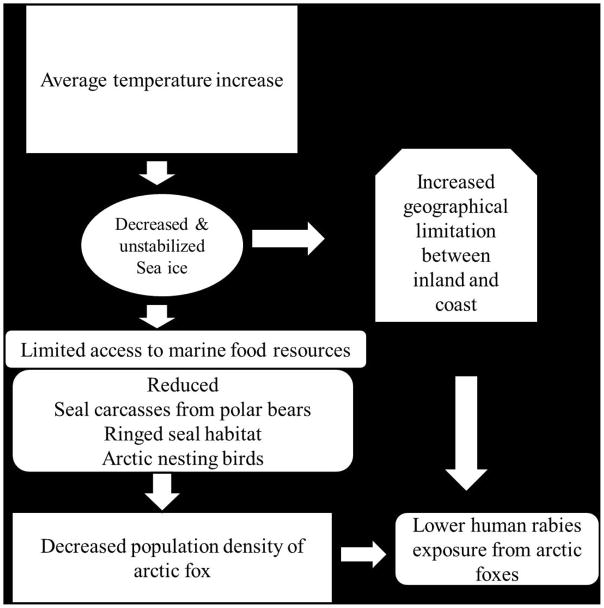
Based on the statistical model provided here and a review of current literature there appears to be sufficient evidence to suggest that rabies in arctic fox populations in northern Alaska will likely be affected by climate change to a greater extent than in red fox populations. This is supported by the greater effect size of the coefficients in the arctic fox model as well as the deviance R2 values which would suggest that the climate covariates explain a greater degree of the observed variation for arctic foxes compared to the final model selected for red foxes (Table 1). This would suggest a conceptual model where as average temperatures increase, a decrease in reported rabid arctic foxes is expected. If combined with an increasing monthly temperature range, this model also suggests this decrease will not be as dramatic and seasonality may become less pronounced (Figure 4). If this model holds under climate change and the potential for red foxes to continue to expand into areas predominantly occupied by arctic foxes, the arctic fox rabies virus variant may have the opportunity to perpetuate in red foxes in coastal Alaska as this species becomes established in the region (Kuzmin et al., 2008). As evidenced by phylogenetic analysis of rabid red foxes in Ontario, Canada; it appears that red foxes are susceptible and can efficiently transmit and perpetuate the arctic fox rabies virus variant (Rosatte et al., 2007). This observation raises concern due to the much broader distribution of red foxes in lower latitudes and potential for spread. Subsequent to the detection of fox rabies in the Northwest Territories in the late 1940s, this epizootic spread to all provinces in Canada, and eventually to the northeastern United States (Tabel et al., 1974). Establishment of the Arctic rabies virus variant in red fox populations in temperate southern Ontario had profound impacts on public and animal health (MacInnes et al., 2001). Through strategic use of oral rabies vaccination, arctic fox rabies has been nearly eliminated from southern Ontario (MacInnes et al., 2001, Rosatte et al., 2007). However, sporadic outbreaks of fox rabies have been detected in central Ontario. Genetic typing of isolates indicated that some outbreaks were the result of new introductions of the Arctic rabies virus variant from the north (Nadin-Davis et al., 1994, Nadin-Davis et al., 2006). Thus, attention is required for surveillance along the arctic-red fox interface and preparedness for a repeat incursion must be maintained across borders (Sterner et al., 2009).
Figure 4.
Hypothetical model of climate change on human exposure to arctic fox rabies in Alaska
The models presented in this study represent an analysis of monthly aggregate rabies diagnostic reports based on passive surveillance, which does not permit broader generalization beyond the study areas or extrapolations to finer geographic scales. Furthermore, passive rabies surveillance is predicated on animal interactions with local human populations and domestic animals. While an offset to the Poisson model based on borough and census area population was controlled in the model, some reporting bias is likely in the model due to passive surveillance, a gradual decline in surveillance effort over the study period and seasonal variation in human activity may affect contact rates. We were also unable to evaluate the impact of factors such as fox and prey density on rabies incidence in Alaska as these data were unavailable. The same is true for movement of domestic animals, mainly dogs in the sparsely populated areas with endemic rabies. However, we believe that dogs serve mainly as a local vector of rabies that greatly increases possible human exposure and do not play an important role in maintaining rabies in Alaska.
However, classical mathematical models have been developed to examine the effect of ecological factors, such as habitat characteristics and fox density, as well as vaccination and other control practices such as depopulation, on rabies perpetuation and spread within red fox populations particularly in relation to oral rabies vaccination in Europe (Smith & Wilkinson, 2003, Voigt, 1985). These models may be adaptable to serve as a template for model development in the Arctic.
The findings of this study emphasize areas where research is needed to better evaluate and predict the dynamics of rabies epidemiology in this Arctic region under a model of continued climate change. Information on fox population densities and seasonal movement patterns will be critical to develop more comprehensive models which might better predict local rabies dynamics. The fox density at which rabies remained enzootic in southern Ontario was below the density at which rabies in European foxes was predicted to naturally die out (MacInnes et al., 2001), suggesting that factors affecting rabies perpetuation are complex and influenced by the local biological and physical environment. Improved understanding of prey population cycles (e.g., lemmings) and seals as a food source for arctic foxes will be important in the development of any future demographic models. Several studies have suggested that climate warming may extend the length of the lemming population cycle and reduce the maximum densities in eastern Greenland and Norway, but little information is available for lemming populations in Alaska (Kausrud et al., 2008, Gilg et al., 2009). In addition, a better understanding of interspecific interactions between arctic and red foxes will be important to future predictions. The generally low human population densities in Alaska may have affected the number of reported animals. Enhanced surveillance, consisting of active sampling and more accurate geographic information will be critical to develop predictive spatial models of rabies epidemiology in this region, as it is apparent that environmental perturbations and resulting pathogen-host shifts are likely.
Impacts.
A greater influence of global warming at latitudinal extremes is likely to result in the Arctic being more susceptible to climate change.
Climate change is expected to have an impact on the population dynamics of red (Vulpes vulpes) and arctic foxes (Vulpes lagopus) which will affect the local transmission dynamics of rabies. Arctic foxes are expected to be more adversely affected by climate change than red foxes.
Additional research, including active surveillance at a finer geographic scale, is necessary to better predict the dynamics of rabies epidemiology in the Arctic region under a model of continued climate change
Acknowledgments
The authors thank the Alaska Division of Public Health for their contribution in collecting and maintaining rabies surveillance data; Dustyn Palmer and Jessie Dyer, CDC Rabies program, for assistance with providing map figures, Ivan Kuzmin, CDC Rabies program for reviewing the manuscript; and Christine Fehlner-Gardiner, Canadian Food Inspection Agency Centre of Expertise for Rabies, for review of the manuscript. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of their institutions. Karsten Hueffer is supported by grants from the National Center for Research Resources (5P20RR016466-12) and the National Institute of General Medical Sciences (8 P20 GM103395-12) from the National Institutes of Health.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Baer GM. The natural history of rabies. Academic Press; New York: 1975. [Google Scholar]
- Ballard WB, Follmann EH, Ritter DG, Robards MD, Cronin MA. Rabies and canine distemper in an arctic fox population in Alaska. Journal of wildlife diseases. 2001;37:133–137. doi: 10.7589/0090-3558-37.1.133. [DOI] [PubMed] [Google Scholar]
- Belsley DA. Conditioning diagnostics : collinearity and weak data in regression. Wiley; New York: 1991. p. 396. [Google Scholar]
- Belsley DA, Kuh E, Welsch RE. Regression diagnostics : identifying influential data and sources of collinearity. Wiley; New York: 1980. p. 292. [Google Scholar]
- Berner J, Symon C, Arris L, Heal OW. Arctic climate impact assessment. National Science Foundation (U.S.) and United States. National Oceanic and Atmospheric Administration; Cambridge University Press; Cambridge ; New York, N.Y: 2005. Arctic Climate Impact Assessment; p. v.p. 1042. [Google Scholar]
- Cameron AC, Windmeijer FAG. R-Squared measures for count data regression models with applications to health-care utilization. J Bus Econ Stat. 1996;14:209–220. [Google Scholar]
- Corell R. Arctic Climate Impact Assessment. Bulletin of hte American Meteorological Society. 2005;26:860–861. [Google Scholar]
- Dieterich RA. Alaskan Wildlife Diseases. University of Alaska; Fairbanks, AK; USA: 1981. [Google Scholar]
- Epstein PR. Perspectives: Medicine - Climate and Health. Science. 1999;285:347–348. doi: 10.1126/science.285.5426.347. [DOI] [PubMed] [Google Scholar]
- Frafjord K, Prestrud P. Home Range and Movements of Arctic Foxes Alopex-Lagopus in Svalbard. Polar Biology. 1992;12:519–526. [Google Scholar]
- Fuglei E, Ims RA. Global warming and effects on the Arctic fox. Sci Prog. 2008;91:175–191. doi: 10.3184/003685008X327468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilg O, Sittler B, Hanski I. Climate change and cyclic predator-prey population dynamics in the high Arctic. Global Change Biol. 2009;15:2634–2652. [Google Scholar]
- Hanski I. The Biology of Lemmings - Stenseth,Nc, Ims,Ra. Nature. 1995;376:306–306. [Google Scholar]
- Hassol SJ. Impacts of a warming Arctic: Arctic Climate Impact Assessment. Cambridge University Press; Cambridge, U.K: 2004. p. 139. [Google Scholar]
- Hersteinsson P, Macdonald DW. Interspecific Competition and the Geographical-Distribution of Red and Arctic Foxes Vulpes-Vulpes and Alopex-Lagopus. Oikos. 1992;64:505–515. [Google Scholar]
- Hiruki LM, Stirling I. Population-Dynamics of the Arctic Fox, Alopex-Lagopus, on Banks Island, Northwest Territories. Can Field Nat. 1989;103:380–387. [Google Scholar]
- Hueffer K, O’Hara TM, Follmann EH. Adaptation of mammalian host-pathogen interactions in a changing arctic environment. Acta veterinaria Scandinavica. 2011;53:17. doi: 10.1186/1751-0147-53-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantorovich RA. Natural Foci of a Rabies-Like Infection in the Far North. J Hyg Epidemiol Microbiol Immunol. 1964;104:100–110. [PubMed] [Google Scholar]
- Kausrud KL, Mysterud A, Steen H, Vik JO, Ostbye E, Cazelles B, Framstad E, Eikeset AM, Mysterud I, Solhoy T, Stenseth NC. Linking climate change to lemming cycles. Nature. 2008;456:93–U93. doi: 10.1038/nature07442. [DOI] [PubMed] [Google Scholar]
- Kovacs KM, Lydersen C. Climate change impacts on seals and whales in the North Atlantic Arctic and adjacent shelf seas. Sci Prog. 2008;91:117–150. doi: 10.3184/003685008X324010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin IV, Hughes GJ, Botvinkin AD, Gribencha SG, Rupprecht CE. Arctic and Arctic-like rabies viruses: distribution, phylogeny and evolutionary history. Epidemiology and Infection. 2008;136:509–519. doi: 10.1017/S095026880700903X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidre K, Stirling I, Lowry L, Wiig O, Heide-Jrgensen M, Ferguson S. Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecological Applications. 2008;18:97–125. doi: 10.1890/06-0546.1. [DOI] [PubMed] [Google Scholar]
- Linnell JDC, Strand O, Landa A. Use of dens by red Vulpes vulpes and arctic Alopex lagopus foxes in alpine environments: Can inter-specific competition explain the non-recovery of Norwegian arctic fox populations? Wildlife Biology. 1999;5:167–176. [Google Scholar]
- MacInnes CD, Smith SM, Tinline RR, Ayers NR, Bachmann P, Ball DG, Calder LA, Crosgrey SJ, Fielding C, Hauschildt P, Honig JM, Johnston DH, Lawson KF, Nunan CP, Pedde MA, Pond B, Stewart RB, Voigt DR. Elimination of rabies from red foxes in eastern Ontario. J Wildl Dis. 2001;37:119–132. doi: 10.7589/0090-3558-37.1.119. [DOI] [PubMed] [Google Scholar]
- Mansfield KL, Racloz V, McElhinney LM, Marston DA, Johnson N, Ronsholt L, Christensen LS, Neuvonen E, Botvinkin AD, Rupprecht CE, Fooks AR. Molecular epidemiological study of Arctic rabies virus isolates from Greenland and comparison with isolates from throughout the Arctic and Baltic regions. Virus Res. 2006;116:1–10. doi: 10.1016/j.virusres.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Mork T, Prestrud A. Arctic rabies - A review. Acta veterinaria Scandinavica. 2004;45:1–9. doi: 10.1186/1751-0147-45-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis SA, Casey GA, Wandeler AI. A molecular epidemiological study of rabies virus in central Ontario and western Quebec. J Gen Virol. 1994;75(Pt 10):2575–2583. doi: 10.1099/0022-1317-75-10-2575. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis SA, Muldoon F, Wandeler AI. Persistence of genetic variants of the arctic fox strain of Rabies virus in southern Ontario. Can J Vet Res. 2006;70:11–19. [PMC free article] [PubMed] [Google Scholar]
- Noren K, Carmichael L, Fuglei E, Eide NE, Hersteinsson P, Angerbjorn A. Pulses of movment across the sea ice: population connectiivty and temporal genetic structure in teh arctic fox. Oecologia. 2011;166:973–984. doi: 10.1007/s00442-011-1939-7. [DOI] [PubMed] [Google Scholar]
- Nowak RM, Walker EP. Walker’s mammals of the world. Johns Hopkins University Press; Baltimore: 1991. p. 1629. [Google Scholar]
- Pamperin NJ, Follmann EH, Person BT. Sea-ice use by arctic foxes in northern Alaska. Polar Biology. 2008;31:1421–1426. [Google Scholar]
- Pamperin NJ, Follmann EH, Petersen B. Interspecific killing of an arctic fox by a red fox at Prudhoe Bay, Alaska. Arctic. 2006;59:361–364. [Google Scholar]
- Parkinson AJ, Butler JC. Potential impacts of climate change on infectious diseases in the Arctic. Int J Circumpolar Health. 2005;64:478–486. doi: 10.3402/ijch.v64i5.18029. [DOI] [PubMed] [Google Scholar]
- Preston K, Rotenberry JT, Redak RA, Allen MF. Habitat shifts of endangered species under altered climate conditions: importance of biotic interactions. Global Change Biol. 2008;14:2501–2515. [Google Scholar]
- Rausch RL. Observations on some natural-focal zoonoses in Alaska. Arch Environ Health. 1972;25:246–252. doi: 10.1080/00039896.1972.10666170. [DOI] [PubMed] [Google Scholar]
- Rausch RL. Cystic echinococcosis in the Arctic and Sub-Arctic. Parasitology. 2003;127(Suppl):S73–85. doi: 10.1017/s0031182003003664. [DOI] [PubMed] [Google Scholar]
- Rosatte RC, Power MJ, Donovan D, Davies JC, Allan M, Bachmann P, Stevenson B, Wandeler A, Muldoon F. Elimination of arctic variant rabies in red foxes, metropolitan Toronto. Emerging Infectious Diseases. 2007;13:25–27. doi: 10.3201/eid1301.060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JD. Temporal variability in arctic fox diet as reflected in stable-carbon isotopes; the importance of sea ice. Oecologia. 2002;133:70–77. doi: 10.1007/s00442-002-1004-7. [DOI] [PubMed] [Google Scholar]
- Roth JD. Variability in marine resources affects arctic fox population dynamics. Journal of Animal Ecology. 2003;72:668–676. doi: 10.1046/j.1365-2656.2003.00739.x. [DOI] [PubMed] [Google Scholar]
- Secord DC, Bradley JA, Eaton RD, Mitchell D. Prevalence of Rabies Virus in Foxes Trapped in the Canadian Arctic. Canadian Veterinary Journal-Revue Veterinaire Canadienne. 1980;21:297–300. [PMC free article] [PubMed] [Google Scholar]
- Smith GC, Wilkinson D. Modeling control of rabies outbreaks in red fox populations to evaluate culling, vaccination, and vaccination combined with fertility control. Journal of wildlife diseases. 2003;39:278–286. doi: 10.7589/0090-3558-39.2.278. [DOI] [PubMed] [Google Scholar]
- Smith TG. The ringed seal, Phoca hispida, of the Canadian western arctic. Dept. of Fisheries and Oceans; Ottawa: 1987. p. 81. [Google Scholar]
- Stafford JM, Wendler G, Curtis J. Temperature and precipitation of Alaska: 50 year trend analysis. Theoretical and Applied Climatology. 2000;67:33–44. [Google Scholar]
- Steck F, Wandeler A. The epidemiology of fox rabies in Europe. Epidemiologic reviews. 1980;2:71–96. doi: 10.1093/oxfordjournals.epirev.a036227. [DOI] [PubMed] [Google Scholar]
- Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and Economics of Wildlife Oral Rabies Vaccination, Canada and the United States. Emerging Infectious Diseases. 2009;15:1176–1184. doi: 10.3201/eid1508.081061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling I, McEwan E. Caloric value of whole ringed seals (Phoca hispida) in relation to Polar Bear (Ursus maritimus) ecology and hunting behavior. Canadian Journal of Zoology. 1975;53:1021–1027. doi: 10.1139/z75-117. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. SAS Institute; Cary, NC: 2000. p. 626. [Google Scholar]
- Tabel H, Corner AH, Webster WA, Casey CA. History and epizootiology of rabies in Canada. The Canadian veterinary journal. 1974;15:271–281. [PMC free article] [PubMed] [Google Scholar]
- Tannerfeldt M, Elmhagen B, Angerbjorn A. Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia. 2002;132:213–220. doi: 10.1007/s00442-002-0967-8. [DOI] [PubMed] [Google Scholar]
- Voigt D, Tinline R, Broekhoven L. Population Dynamics of Rabies in Wildlife. Academic press; 1985. A spatial simulation model for rabies control. [Google Scholar]
- Wendler G, Shulski M, Moore B. Changes in the climate of the Alaskan North Slope and the ice concentration of the adjacent Beaufort Sea. Theoretical and Applied Climatology. 2010;99:67–74. [Google Scholar]
- Wiig O, Aars J, Born EW. Effects of climate change on polar bears. Sci Prog. 2008;91:151–173. doi: 10.3184/003685008X324506. [DOI] [PMC free article] [PubMed] [Google Scholar]